4-Thiazolidinone Derivatives as MMP Inhibitors in Tissue Damage: Synthesis, Biological Evaluation and Docking Studies
Abstract
:1. Introduction
2. Results and Discussion
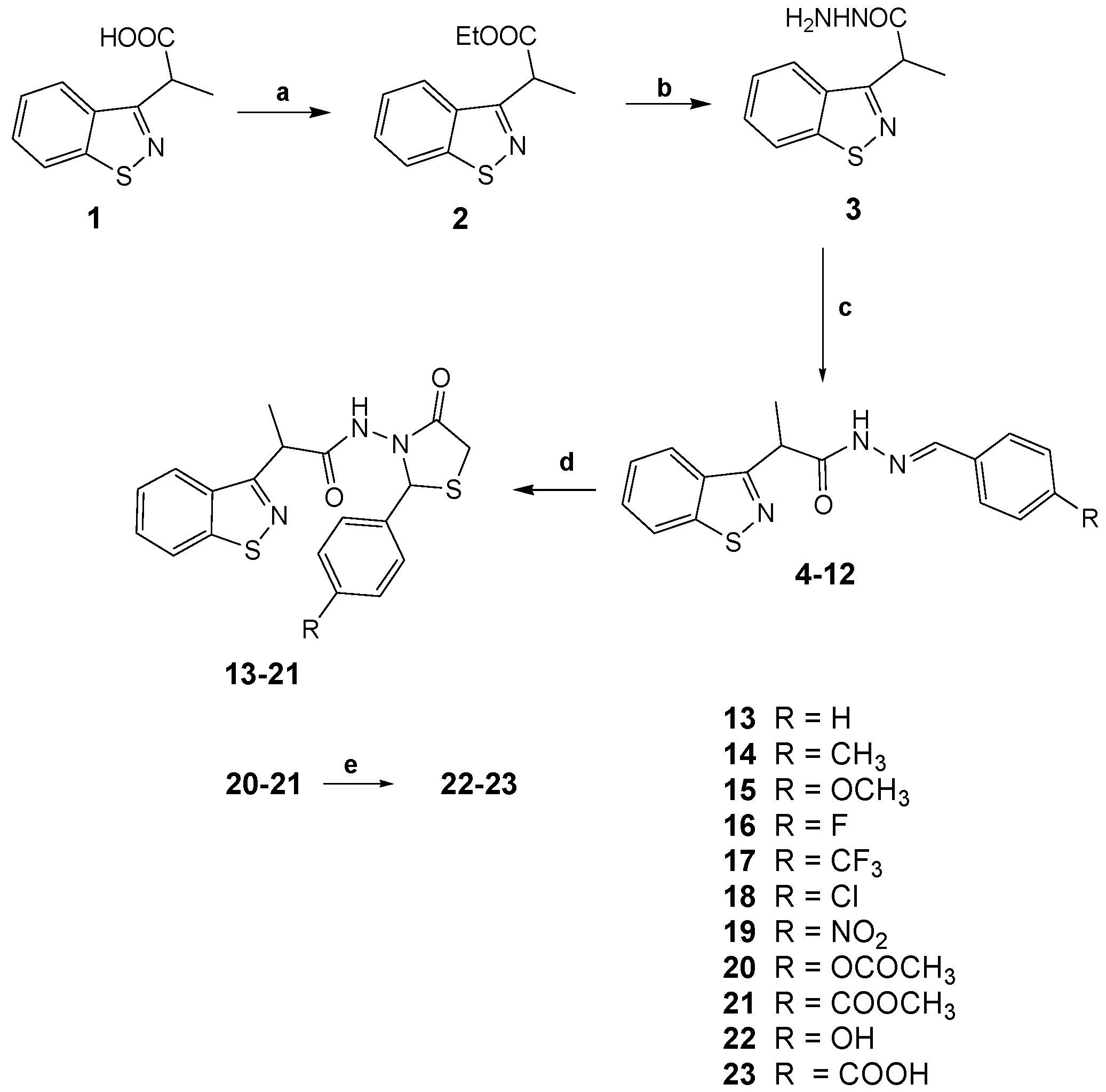
2.1. Synthesis
2.2. Biological Evaluation
2.2.1. Antioxidant Capacity
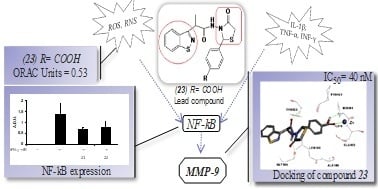
2.2.2. Inhibitory Activity on MMP-9
2.2.3. Cellular Assay
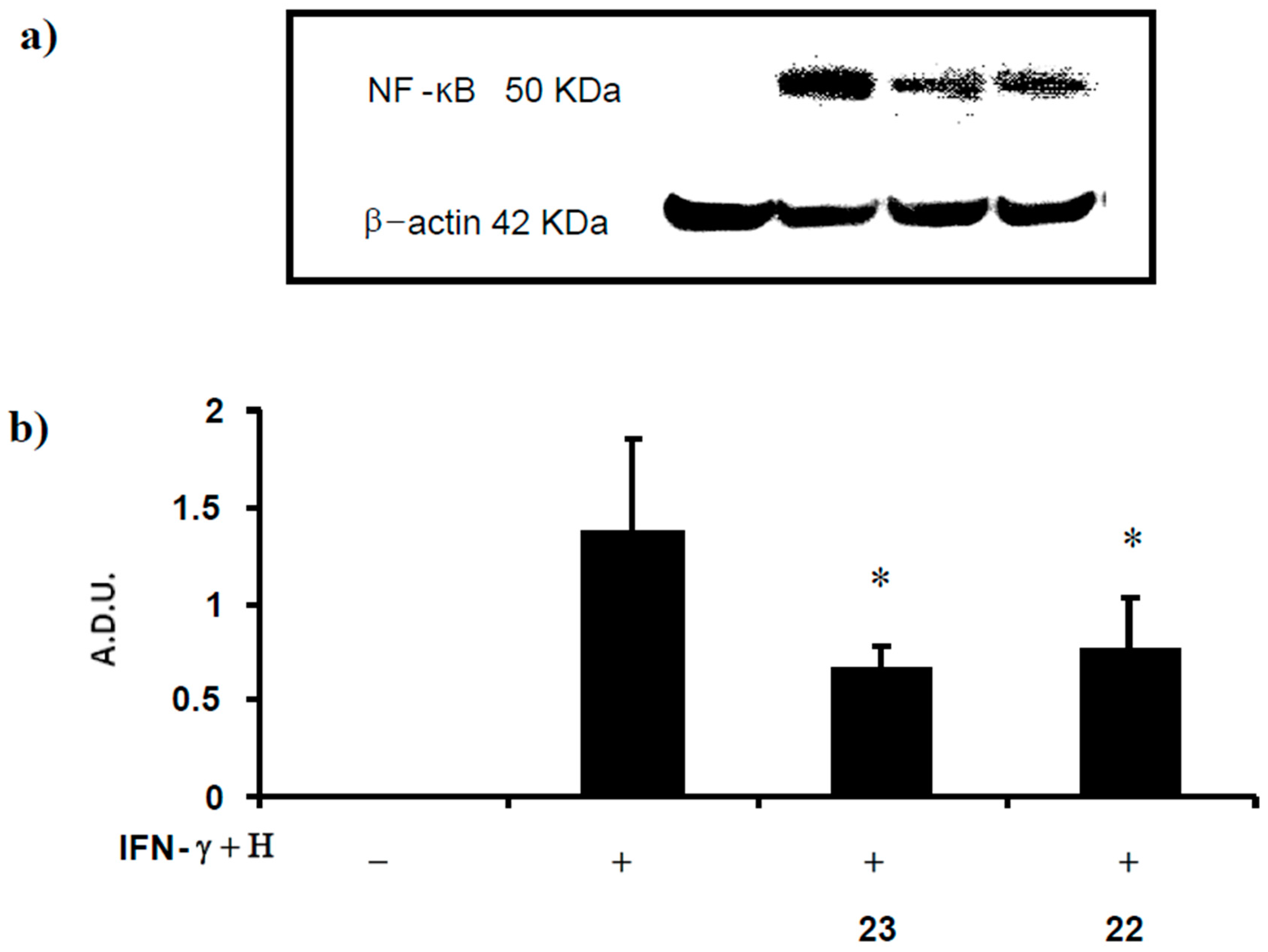
2.2.4. Determination of NF-κB Levels
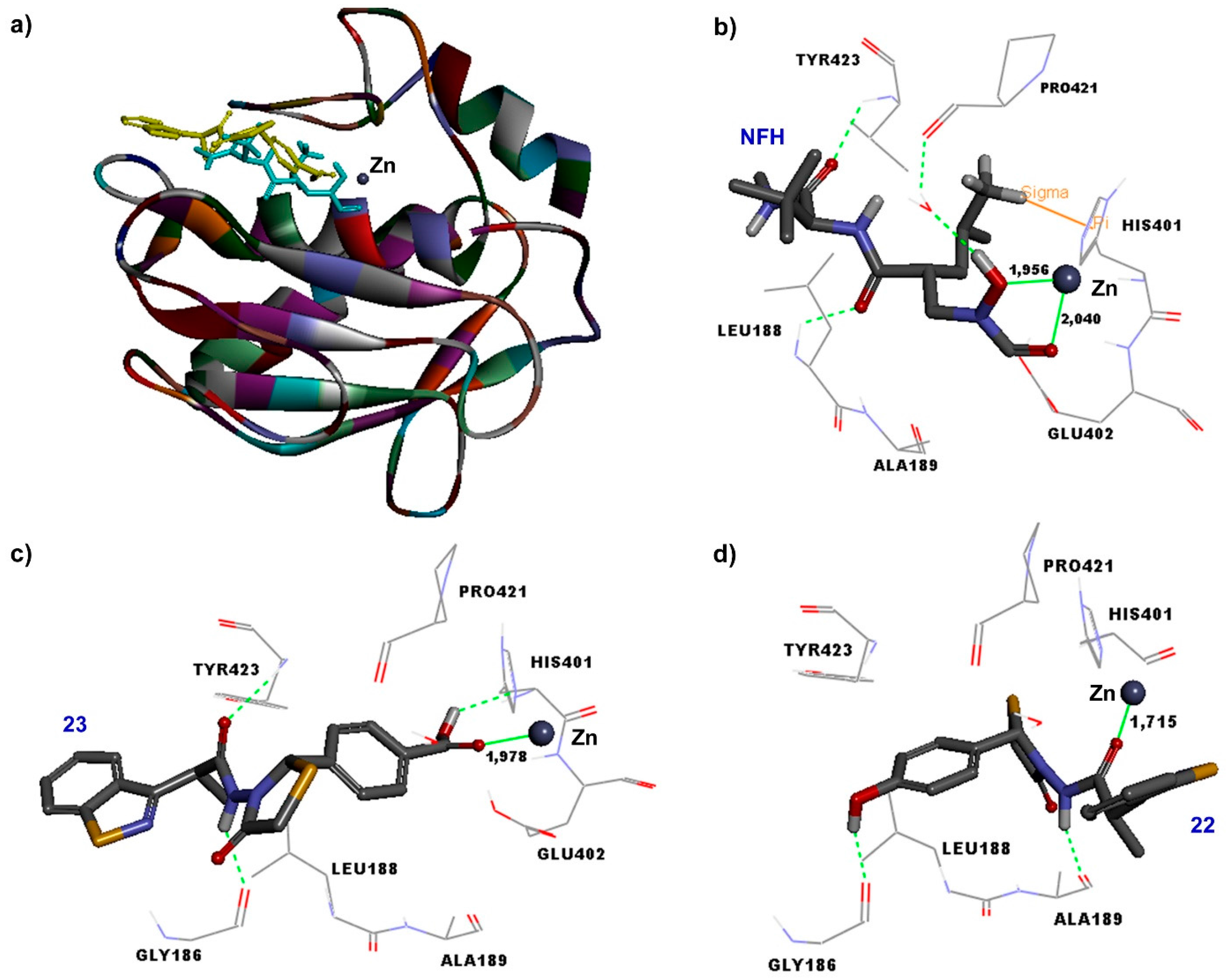
2.3. Docking Studies on MMP-9
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of 2-(1,2-Benzothiazol-3-yl)-N′-(phenylmethylidene)-propanehydrazides 4–12
3.2.2. General Procedure for the Synthesis of 2-(1,2-Benzothiazol-3-yl)-N-(4-oxo-2-phenyl-1,3-thiazolidin-3-yl)Propanamides 13–19
3.2.3. Synthesis of 3-{[2-(1,2-Benzothiazol-3-yl)propanoyl]amino}-1,3-thiazolidin-4-ones 20–21
3.2.4. Synthesis of 2-(1,2-Benzothiazol-3-yl)-1,3-thiazolidin-4-one Target Derivatives 22–23
3.3. Oxygen Radical Absorbance Capacity (ORAC) Assays
3.4. MMP-9 Fluorimetric Assay
3.5. Keratinocyte Cultures and Treatments
3.6. Cell Viability Assay
3.7. Western Blot Analysis
3.8. Statistical Analysis
3.9. DockingStudies
3.9.1. Preparation of the Enzyme
3.9.2. Preparation of Ligands
3.9.3. Molecular Docking
3.9.4. Analysis of Results
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Simopoulos, A.P.; Milner, J., 3rd. Congress of the International Society of Nutrigenetics/Nutrigenomics (ISSN) Bethesda, Maryland, USA, 21–23 October. J. Nutrigenet. Nutrige. 2009, 2, 189–224. [Google Scholar] [CrossRef]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A.M. Natural Antioxidant Polyphenols On Inflammation Management: Anti-glycation Activity Vs Metalloproteinases Inhibition. Crit. Rev. Food Sci. 2016, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Yang, C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed. Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; van den Steen, P.E.; Sang, Q.X.A.; Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. 2007, 6, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Tallant, C.; Marrero, A.; Gomis-Rüth, F.X. Matrix metalloproteinases: Fold and function of their catalytic domains. BBA Mol. Cell Res. 2010, 1803, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.P. Quantitative structure-activity relationship studies on zinc-containing metalloproteinase inhibitors. Chem. Rev. 2007, 107, 3042–3087. [Google Scholar] [CrossRef] [PubMed]
- Vicini, P.; Incerti, M.; Cardile, V.; Garufi, F.; Ronsisvalle, S.; Panico, A.M. Benzo[d]isothiazol-3-yl-benzamidines: A class of protective agents on culture of Human cartilage and chondrocytes stimulated by IL-1β. ChemMedChem 2007, 2, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Amadasi, A.; Cozzini, P.; Incerti, M.; Duce, E.; Fisicaro, E.; Vicini, P. Molecular modeling of binding between amidinobenzisothiazoles with antidegenerative activity on cartilage and matrix metalloproteinase-3. Bioorg. Med. Chem. 2007, 15, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.; Vicini, P.; Incerti, M.; Cardile, V.; Gentile, B.; Ronsisvalle, G. AmidinoBenzisotiazole derivatives with antidegenerative activity on cartilage. IL Farmaco 2002, 57, 671–672. [Google Scholar] [CrossRef]
- Verma, A.; Saraf, S.K. 4-thiazolidinone A biologically active scaffold. Eur. J. Med. Chem. 2008, 43, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.M.; Vicini, P.; Geronikaki, A.; Incerti, M.; Cardile, V.; Crasci, L.; Mesina, L.; Ronsisvalle, S. Heteroarylimino-4-thiazolidinones asinihibitors of cartilagedegradation. Bioorg. Med. Chem. 2011, 39, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Incerti, M.; Vicini, P.; Geronikaki, A.; Elefteriou, P.; Tsagkadouras, A.; Zoumpolakis, P.; Fotakis, C.; Ciric, A.; Glamocljia, J.; Sokovic, M. New n-(2-phenyl-4-oxo-1,3-thiazolidin-3-yl)-1,2-benzothiazole-3-carboxamides and acetamidesasantimicrobial agents. MedChemComm 2017, 8, 2142–2154. [Google Scholar] [CrossRef]
- Bordi, F.; Mor, M.; Plazzi, P.V.; Silva, C.; Morini, G.; Impicciatore, M.; Barocelli, E.; Chiavarini, M. 4-(1,2-benzisothiazol-3yl-)alkanoic and phenylalcanoic acids: Synthesis and anti-inflammatory, analgesic and antipyretic activities. IL Farmaco 1992, 47, 551–565. [Google Scholar] [PubMed]
- Vitali, T.; Lugari Mangia, M.T. Phytotoxic activity of 2-methyl-2(1,2-benzisothiazol-3-yl) acetic acid derivatives. AteneoParmense. Acta Nat. 1973, 9, 29–37. [Google Scholar]
- Giméneza, R.; Oriola, L.; Piñolb, M.; Serrano, J.L.; Viñualesa, A.I.; Fisherc, T.; Stumpe, J. Synthesis and Properties of 2-Phenylbenzoxazole-Based Luminophores for in situ Photopolymerized Liquid-Crystal Films Helvetica. Acta Chim. 2006, 89, 304–319. [Google Scholar] [CrossRef]
- Kato, T.; Saito, N.; Kashimura, K.; Shinohara, M.; Kurahashi, T.; Taniguchi, K. Germination and Growth Inhibitors from Wheat (Triticum aestivumL.) Husks. J. Agric. Food Chem. 2002, 50, 6307–6312. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Kampoli, A.M.; Tousoulis, D.; Papageorgiou, N.; Antoniades, C.; Androulakis, E.; Tsiamis, E.; Stefanadis, C. Matrix metalloproteinases in acute coronary syndromes: Current perspectives. Curr. Top. Med. Chem. 2012, 12, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Calderone, V.; Fragai, M.; Luchinat, C.; Nativi, C.; Richichi, B.; Roelens, S. A High-Affinity Carbohydrate-Containing Inhibitor of Matrix Metalloproteinases. Chem. Med. Chem. 2006, 1, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Cardile, V.; Libra, M.; Caggia, S.; Frasca, G.; Umezawa, K.; Stivala, F.; Mazzarino, M.C.; Bevelacqua, Y.; Coco, M.; Malaponte, G. Dehydroxymethylepoxyquinomicin, a novel nuclear factor-kappaB inhibitor, prevents inflammatory injury induced by interferon-gamma and histamine in NCTC 2544 keratinocytes. Clin. Exp. Pharmacol. Physiol. 2010, 37, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Discovery Studio 3.5, Accelrys Inc.: San Diego, CA, USA, 2012.
- Whittaker, M.; Floyd, C.D.; Brown, P.; Gearing, A.J. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem. Rev. 1999, 99, 2735–2776. [Google Scholar] [CrossRef] [PubMed]
- Puerta, D.T.; Cohen, S.M. A bioinorganic perspective on matrix metalloproteinase inhibition. Curr. Top. Med. Chem. 2004, 4, 1551–1573. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Nagata, N. Metaleligand interactions: An analysis of zinc binding groups using the Protein Data Bank. Med. Chem. Eur. J. 2012, 51, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.M. Geometry of metal-ligand interactions in proteins. Acta Crystallogr. D 2001, 57, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Rowsel, L.S.; Hawtin, P.; Minshull, C.A.; Jepson, H.; Brockbank, S.M.V.; Barratt, D.G.; Slater, A.M.; McPheat, W.L.; Waterson, D.; Henneyand, A.M.; et al. Crystal Structure of Human MMP-9 in Complex with a Reverse Hydroxamate Inhibitor. J. Mol. Biol. 2002, 319, 173–181. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Crascì, L.; Vicini, P.; Incerti, M.; Cardile, V.; Avondo, S.; Panico, A. 2-Benzisothiazolylimino-5-benzylidene-4-thiazolidinones as protective agents against cartilage destruction. Bioorg. Med. Chem. 2015, 23, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.M.; Vicini, P.; Massimo, G.; Cardile, V.; Gentile, B.; Avondo, S.; Vittorio, F.; Ronsisvalle, G. Protective effects of benzisothiazolylamidines on IL-1β induced alterations in human articular chondrocyte metabolism. Inflammation 2004, 28, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Robertson, D.H.; Brooks, C.L.; Vieth, M. Detailed analysis of grid-based molecular docking: A case study of CDOCKER—A CHARMm-based MD docking algorithm. J. Comput. Chem. 2003, 24, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compounds | ORAC UNITs a | |
|---|---|---|
| 13 | R=-H | 0.61 ± 0.14 * |
| 14 | R=-CH3 | 0.40 ± 0.06 * |
| 15 | R=-OCH3 | 0.66 ± 0.12 * |
| 16 | R=-F | 0.46 ± 0.20 * |
| 17 | R=-CF3 | 0.27 ± 0.06 * |
| 18 | R=-Cl | 0.47 ± 0.23 * |
| 19 | R=-NO2 | 0.40 ± 0.17 * |
| 22 | R=-OH | 1.24 ± 0.09 * |
| 23 | R=-COOH | 0.53 ± 0.17 * |
| Compounds | IC50 Value (µM) | |
|---|---|---|
| 13 | R=-H | 7.99 ± 0.65 * |
| 14 | R=-CH3 | 9.01 ± 0.45 * |
| 15 | R=-OCH3 | 6.82 ± 0.95 * |
| 16 | R=-F | 17.15 ± 1.92 * |
| 17 | R=-CF3 | n.a. |
| 18 | R=-Cl | 16.12 ± 1.23 * |
| 19 | R=-NO2 | 22.05 ± 1.42 * |
| 22 | R=-OH | 0.30 ± 0.05 * |
| 23 | R=-COOH | 0.04 ± 0.01 * |
| NNGH ^ | 0.0065 ± 0.00025 |
| Compound | Interacted Residues * | Binding Energies |
|---|---|---|
| 13 | Gly186, Leu187, Leu188 (1.66 Å), Ala189, His190, Ala191, Tyr393, Val398, His401, Glu402 (2.40 Å), His405, His411, Pro421, Met422, Tyr423 (1.89 Å) | −22.10 |
| 14 | Gly186, Leu187, Leu188 [b] (1.92 Å), Ala189, Tyr393, Leu397, Val398, His401 [a], Glu402, Leu418, Tyr420, Pro421 (2.30 Å), Met422, Tyr423, Arg424 | −21.30 |
| 15 | Gly186, Leu187, Leu188 (2.19 Å), Ala189 (2.13 Å), His190, Tyr393, Leu397, Val398, His401 [a], Glu402, Leu418, Tyr420, Pro421, Met422, Tyr423 (2.28 Å), Arg424 | −22.33 |
| 16 | Gly186, Leu187, Leu188 (1.73 Å), Ala189 (2.18 Å), His190, Ala191, Tyr393, Val398, His401, Glu402 (2.44), His405, Pro421, Met422, Tyr423 (1.48 Å) | −17.50 |
| 17 | Phe110, Glu111, Leu187, Leu188, Ala189, His190, Ala191 (2.49 Å) (water mediated), Val398, His401 [a], Glu402, His405, His411, Tyr420, Pro421, Met422, Tyr423, Zn | −24.19 |
| 18 | Phe110, Glu111, Gly186, Leu187, Leu188, Ala189, His190, Ala191 (2.37 Å) (water mediated) His401, Glu402, His405, His411, Pro421, Met422, Tyr423, Zn | −32.33 |
| 19 | Gly186, Leu187, Leu188, Ala189 (2.24 Å), His190, Val398, His401, Glu402, His405, His411, Pro421, Met422, Tyr423 | −18.63 |
| 22 | Phe110, Glu111, Gly186 (1.75 Å), Leu187, Leu188, Ala189 (2.12 Å), His190, Ala191, Val398, His401, Glu402, His405, His411, Pro421, Met422, Tyr423, Zn | −24.70 |
| 23 | Asp185, Gly186 (2.03 Å), Leu187, Leu188, Tyr393, His401 (2.26 Å), Glu402, His411, Pro421, Met422, Tyr423 (2.35 Å), Zn | −24.30 |
| NFH | Gly186 (2.32), Leu187, Leu188 (1.92 Å), Ala189, His190, Ala191, Tyr393, Val398, His401 [b], Glu402, His405, His411, Tyr420, Pro421 (2.04 Å) (water mediated), Met422, Tyr423 (2.15 Å), Zn | −70.88 |
| NNGH | Gly186, Leu187, Leu188 (2.17 Å), Ala189 (2.19 Å), His190, Ala191, Leu397, Val398, His401 [a], Glu402 (1.34 Å), His405, His411, Leu418, Tyr420, Pro421, Met422, Tyr423, Arg424, Zn | −47.76 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Incerti, M.; Crascì, L.; Vicini, P.; Aki, E.; Yalcin, I.; Ertan-Bolelli, T.; Cardile, V.; Graziano, A.C.E.; Panico, A. 4-Thiazolidinone Derivatives as MMP Inhibitors in Tissue Damage: Synthesis, Biological Evaluation and Docking Studies. Molecules 2018, 23, 415. https://doi.org/10.3390/molecules23020415
Incerti M, Crascì L, Vicini P, Aki E, Yalcin I, Ertan-Bolelli T, Cardile V, Graziano ACE, Panico A. 4-Thiazolidinone Derivatives as MMP Inhibitors in Tissue Damage: Synthesis, Biological Evaluation and Docking Studies. Molecules. 2018; 23(2):415. https://doi.org/10.3390/molecules23020415
Chicago/Turabian StyleIncerti, Matteo, Lucia Crascì, Paola Vicini, Esin Aki, Ismail Yalcin, Tugba Ertan-Bolelli, Venera Cardile, Adriana Carol Eleonora Graziano, and Annamaria Panico. 2018. "4-Thiazolidinone Derivatives as MMP Inhibitors in Tissue Damage: Synthesis, Biological Evaluation and Docking Studies" Molecules 23, no. 2: 415. https://doi.org/10.3390/molecules23020415
APA StyleIncerti, M., Crascì, L., Vicini, P., Aki, E., Yalcin, I., Ertan-Bolelli, T., Cardile, V., Graziano, A. C. E., & Panico, A. (2018). 4-Thiazolidinone Derivatives as MMP Inhibitors in Tissue Damage: Synthesis, Biological Evaluation and Docking Studies. Molecules, 23(2), 415. https://doi.org/10.3390/molecules23020415