Curcumin Analog CH-5 Suppresses the Proliferation, Migration, and Invasion of the Human Gastric Cancer Cell Line HGC-27
Abstract
:1. Introduction
2. Results
2.1. CH-5 Reduces Cell Viability and Induces Apoptosis in a Gastric Cancer Cell Line
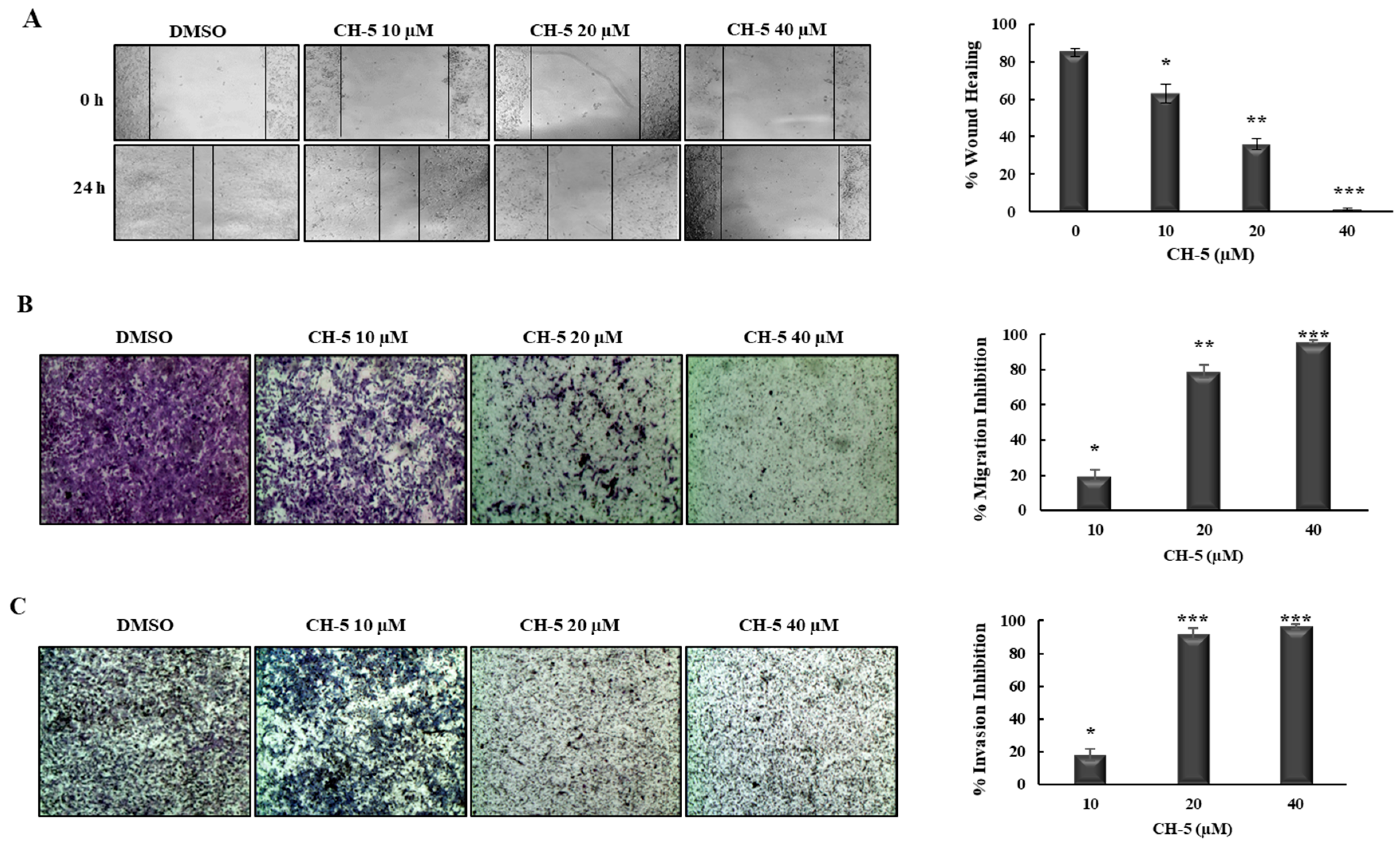
2.2. CH-5 Decreases Migration and Invasion in a Gastric Cancer Cell Line
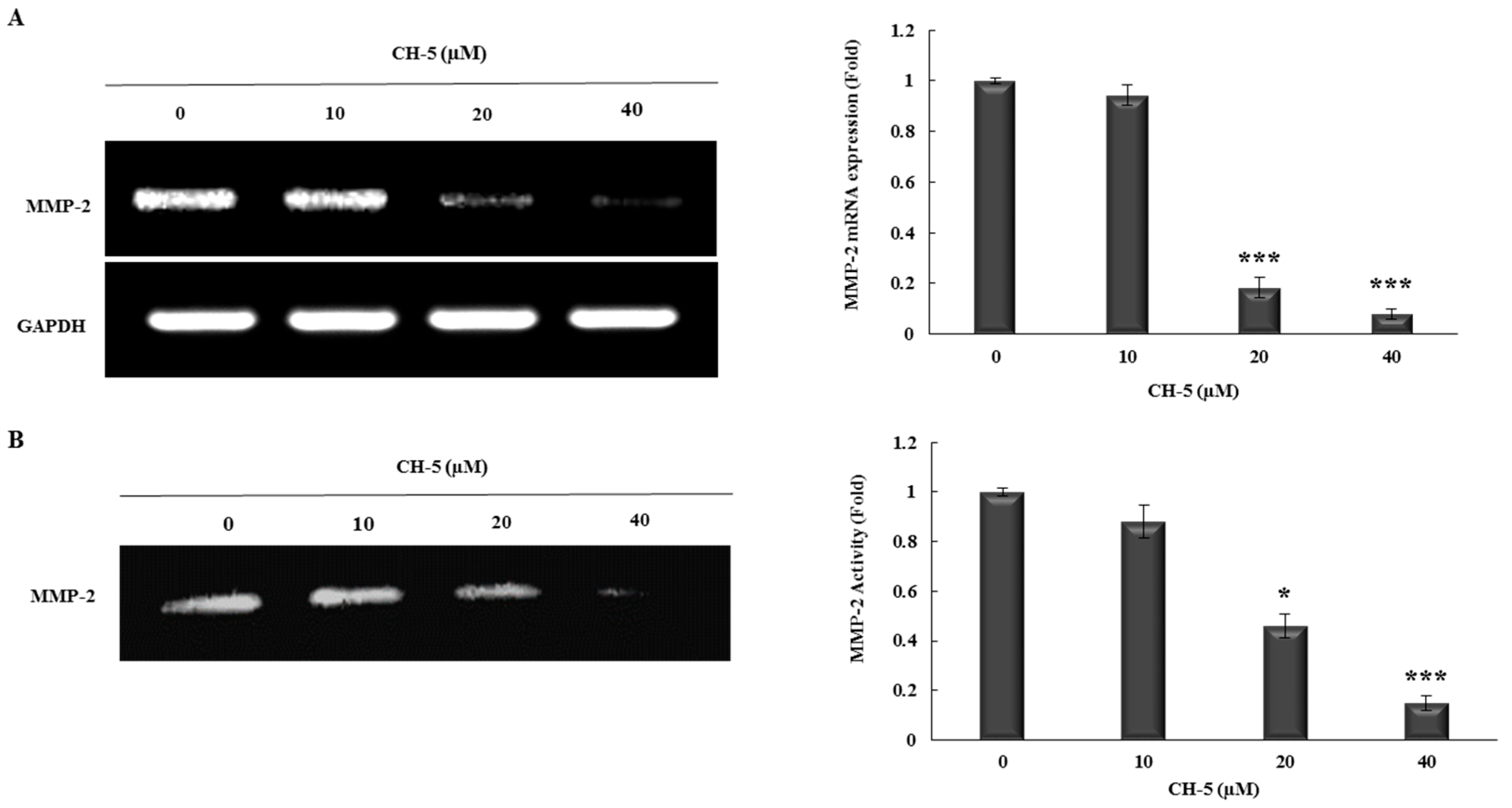
2.3. CH-5 Reduces the Transcriptional Levels and Activity of MMP-2
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Chemicals
4.2. Cell Viability Assay
4.3. Apoptosis Assay
4.4. Caspase 3 Assay
4.5. Wound-Healing Assay
4.6. Transwell Assay
4.7. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.8. Gelatin Zymography
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fock, K.M. Review article: The epidemiology and prevention of gastric cancer. Aliment. Pharmacol. Ther. 2014, 40, 250–260. [Google Scholar] [CrossRef] [PubMed]
- D’Ugo, D.; Persiani, R.; Zoccali, M.; Cananzi, F.; Vigorita, V.; Mazzeo, P.; Tufo, A.; Biondi, A. Surgical issues after neoadjuvant treatment for gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 315–319. [Google Scholar] [PubMed]
- Zuo, Q.F.; Cao, L.Y.; Yu, T.; Gong, L.; Wang, L.N.; Zhao, Y.L.; Xiao, B.; Zou, Q.M. Microrna-22 inhibits tumor growth and metastasis in gastric cancer by directly targeting mmp14 and snail. Cell Death Dis. 2015, 6, e2000. [Google Scholar] [CrossRef] [PubMed]
- Loffek, S.; Schilling, O.; Franzke, C.W. Series “matrix metalloproteinases in lung health and disease”: Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and timps. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Gu, W.J.; Wang, C.Z.; Ji, X.J.; Mu, Y.M. Matrix metalloproteinase-9 and -2 and tissue inhibitor of matrix metalloproteinase-2 in invasive pituitary adenomas: A systematic review and meta-analysis of case-control trials. Medicine 2016, 95, e3904. [Google Scholar] [CrossRef] [PubMed]
- Sariahmetoglu, M.; Crawford, B.D.; Leon, H.; Sawicka, J.; Li, L.; Ballermann, B.J.; Holmes, C.; Berthiaume, L.G.; Holt, A.; Sawicki, G.; et al. Regulation of matrix metalloproteinase-2 (mmp-2) activity by phosphorylation. FASEB J. 2007, 21, 2486–2495. [Google Scholar] [CrossRef] [PubMed]
- Kargozaran, H.; Yuan, S.Y.; Breslin, J.W.; Watson, K.D.; Gaudreault, N.; Breen, A.; Wu, M.H. A role for endothelial-derived matrix metalloproteinase-2 in breast cancer cell transmigration across the endothelial-basement membrane barrier. Clin. Exp. Metastasis 2007, 24, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, K.; Fukamachi, H.; Komori, K.; Ohtake, Y.; Nonaka, T.; Sakamoto, T.; Shiomi, T.; Okada, Y.; Itoh, T.; Itohara, S.; et al. Stroma-derived matrix metalloproteinase (mmp)-2 promotes membrane type 1-mmp-dependent tumor growth in mice. Cancer Res. 2007, 67, 4311–4319. [Google Scholar] [CrossRef] [PubMed]
- Monig, S.P.; Baldus, S.E.; Hennecken, J.K.; Spiecker, D.B.; Grass, G.; Schneider, P.M.; Thiele, J.; Dienes, H.P.; Holscher, A.H. Expression of mmp-2 is associated with progression and lymph node metastasis of gastric carcinoma. Histopathology 2001, 39, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Jung, J.J.; Jung, M.; Kim, T.S.; Park, C.H.; Lim, S.J.; Jeung, H.C.; Cheol, H.; Chung, H.C.; Rha, S.Y. Mmp-2 as a putative biomarker for carcinomatosis in gastric cancer. Hepatogastroenterology 2011, 58, 2015–2019. [Google Scholar] [CrossRef] [PubMed]
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Wozniak, K.; Nabavi, S.F.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H.; Nabavi, S.M. Molecular targets of curcumin for cancer therapy: An updated review. Tumour Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Chakrabarti, J.; Banerji, A.; Chatterjee, A.; Das, B.R. Curcumin, a potential inhibitor of mmp-2 in human laryngeal squamous carcinoma cells hep2. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.L.; Chang, J.M.; Chong, I.W.; Hung, Y.L.; Chen, Y.H.; Huang, W.T.; Kuo, H.F.; Hsieh, C.C.; Liu, P.L. Curcumin inhibits lin-28a through the activation of miRNA-98 in the lung cancer cell line a549. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Fan, C.C.; Chen, Y.A.; Cheng, C.W.; Sung, Y.J.; Hsu, C.P.; Kao, T.Y. Curcumin inhibits invasiveness and epithelial-mesenchymal transition in oral squamous cell carcinoma through reducing matrix metalloproteinase 2, 9 and modulating p53-e-cadherin pathway. Integr. Cancer Ther. 2015, 14, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Kasinski, A.L.; Du, Y.; Thomas, S.L.; Zhao, J.; Sun, S.Y.; Khuri, F.R.; Wang, C.Y.; Shoji, M.; Sun, A.; Snyder, J.P.; et al. Inhibition of IκB kinase-nuclear factor-κB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol. Pharmacol. 2008, 74, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.T.; Seba, V.; Silva, G.; Torrezan, G.S.; Polaquini, C.R.; Pinholato, V.C.; Baek, S.J.; Fachin, A.L.; Regasini, L.O.; Marins, M. The curcumin analog ch-5 exerts anticancer effects in human osteosarcoma cells via modulation of transcription factors p53/sp1. Manuscript in preparation.
- Lima, F.T. Cytotoxic and Epigenetic Activity of Diphenylpentanoids Analogs of Curcumin in Human and Canine Osteosarcoma Cells; University of Ribeirão Preto: Ribeirão Preto, Brazil, 2017. [Google Scholar]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [PubMed]
- Hunter, K.W.; Crawford, N.P.S.; Alsarraj, J. Mechanisms of metastasis. Breast Cancer Res. 2008, 10 (Suppl. 1), S2. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Chakrabarti, J.; Mitra, A.; Chatterjee, A. Effect of curcumin on gelatinase a (MMP-2) activity in B16F10 melanoma cells. Cancer Lett. 2004, 211, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Li, Q.; Yin, P.; Dong, Y.; Shi, H.; Wang, L.; Ji, G.; Xie, J.; Wu, D. Curcumin induces apoptosis in human gastric carcinoma ags cells and colon carcinoma ht-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress. Apoptosis 2013, 18, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yao, D.; Guo, L.; Teng, L. Curcumin suppresses gastric cancer by inhibiting gastrin-mediated acid secretion. FEBS Open Bio 2017, 7, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Zheng, Y.; Jiao, D.M.; Chen, F.Y.; Hu, H.Z.; Wu, Y.Q.; Song, J.; Yan, J.; Wu, L.J.; Lv, G.Y. Curcumin inhibits lung cancer cell migration and invasion through rac1-dependent signaling pathway. J. Nutr. Biochem. 2014, 25, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Cai, J.; Zhang, N.; Wu, J.; Yuan, J.; Li, J.; Li, M. Sp1 is upregulated in human glioma, promotes mmp-2-mediated cell invasion and predicts poor clinical outcome. Int. J. Cancer 2012, 130, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.C.; Tseng, W.L.; Shiea, J.; Chang, H.C. Skp2 overexpression increases the expression of mmp-2 and mmp-9 and invasion of lung cancer cells. Cancer Lett. 2010, 288, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.Y.; Leung, E.P.; Mak, N.K.; Wong, R.N. A simplified method for quantifying cell migration/wound healing in 96-well plates. J. Biomol. Screen. 2010, 15, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.H.; Nie, X.; Liu, H.Y.; Fang, Y.M.; Zhao, Y.; Xia, L.X. TMPyP4 promotes cancer cell migration at low doses, but induces cell death at high doses. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.; Teixeira Lima, F.; Seba, V.; Mendes Lourenço, A.L.; Lucas, T.G.; De Andrade, B.V.; Torrezan, G.S.; Polaquini, C.R.; Garcia, M.E.; Couto, L.B.; et al. Curcumin Analog CH-5 Suppresses the Proliferation, Migration, and Invasion of the Human Gastric Cancer Cell Line HGC-27. Molecules 2018, 23, 279. https://doi.org/10.3390/molecules23020279
Silva G, Teixeira Lima F, Seba V, Mendes Lourenço AL, Lucas TG, De Andrade BV, Torrezan GS, Polaquini CR, Garcia ME, Couto LB, et al. Curcumin Analog CH-5 Suppresses the Proliferation, Migration, and Invasion of the Human Gastric Cancer Cell Line HGC-27. Molecules. 2018; 23(2):279. https://doi.org/10.3390/molecules23020279
Chicago/Turabian StyleSilva, Gabriel, Felipe Teixeira Lima, Viviane Seba, Ana Laura Mendes Lourenço, Thaise Graminha Lucas, Bianca Vieira De Andrade, Guilherme Silva Torrezan, Carlos Roberto Polaquini, Marcelo Engracia Garcia, Lucélio Bernardes Couto, and et al. 2018. "Curcumin Analog CH-5 Suppresses the Proliferation, Migration, and Invasion of the Human Gastric Cancer Cell Line HGC-27" Molecules 23, no. 2: 279. https://doi.org/10.3390/molecules23020279
APA StyleSilva, G., Teixeira Lima, F., Seba, V., Mendes Lourenço, A. L., Lucas, T. G., De Andrade, B. V., Torrezan, G. S., Polaquini, C. R., Garcia, M. E., Couto, L. B., Bestetti, R. B., De Castro França, S., Fachin, A. L., Regasini, L. O., & Marins, M. (2018). Curcumin Analog CH-5 Suppresses the Proliferation, Migration, and Invasion of the Human Gastric Cancer Cell Line HGC-27. Molecules, 23(2), 279. https://doi.org/10.3390/molecules23020279





