Yacon (Smallanthus sonchifolius Poepp. & Endl.) as a Novel Source of Health Promoting Compounds: Antioxidant Activity, Phytochemicals and Sugar Content in Flesh, Peel, and Whole Tubers of Seven Cultivars
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Dry Matter Content
2.2. Glucose, Fructose and Sucrose Content
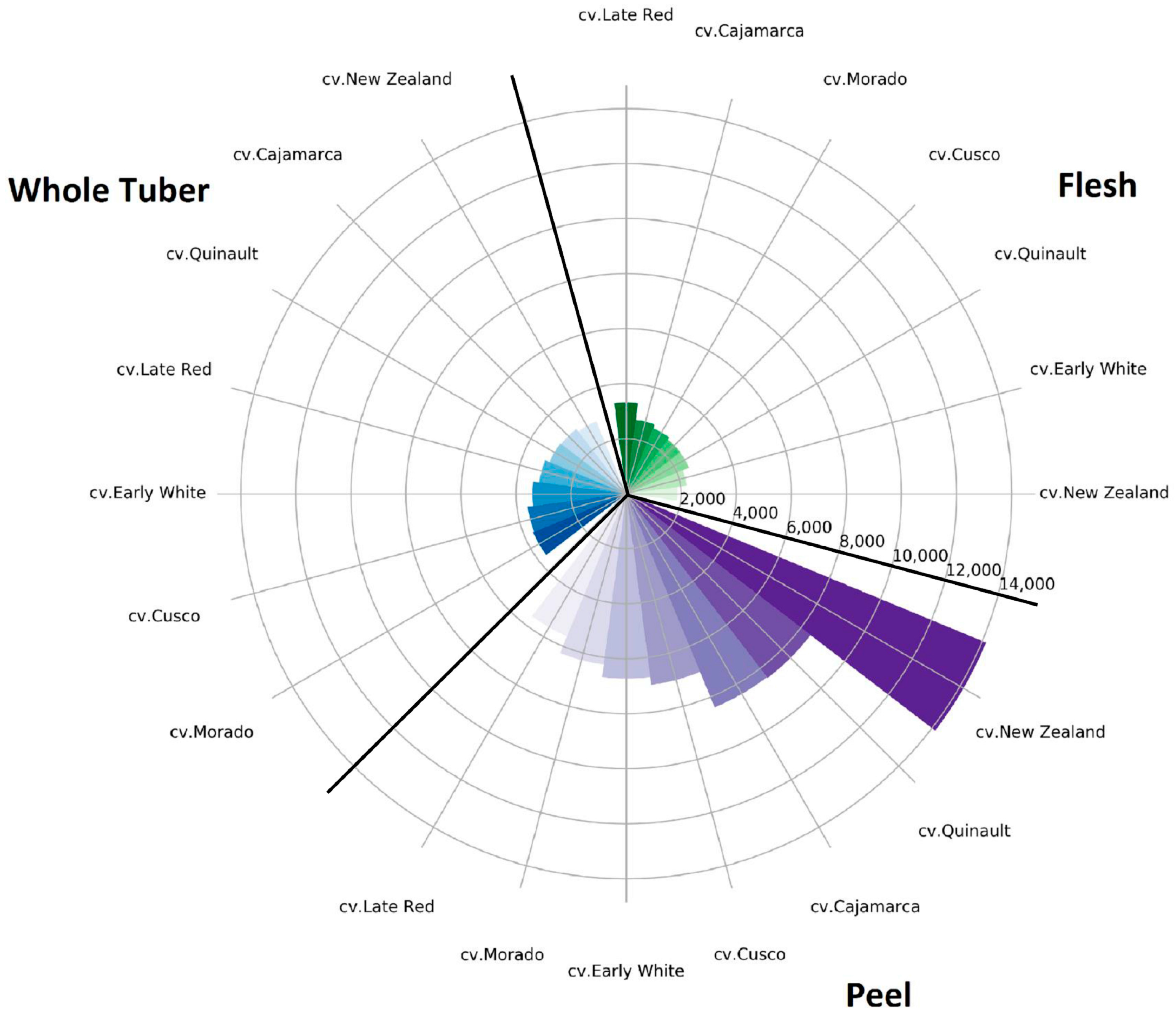
2.3. TPC
2.4. TFC
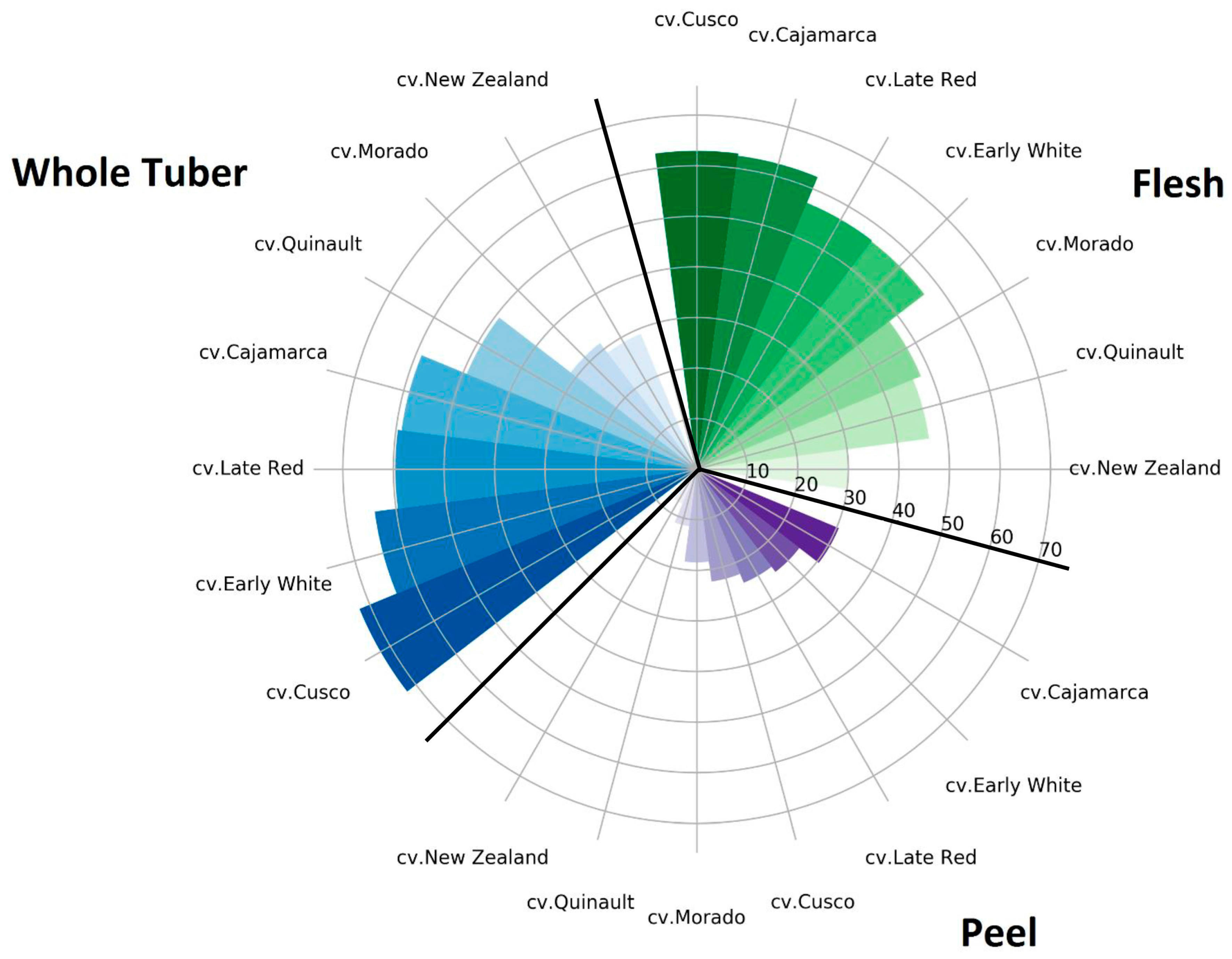
2.5. Antioxidant Activity
2.5.1. ABTS Radical Scavenging Activity
2.5.2. DPPH Radical Scavenging Activity
2.5.3. FRAP
2.6. Classification of Yacon Cultivars according to Their TPC and Total Sugar Content
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Total Dry Matter Content
3.4. Determination of Glucose, Fructose, and Sucrose Content
3.5. Extraction of Phytochemicals
3.5.1. TPC
3.5.2. TFC
3.5.3. Determination of Antioxidant Activity
ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) Diammonium Salt) Radical Scavenging Activity
DPPH (2,2-Diphenyl-1-picrylhydrazyl) Radicals Scavenging Activity
FRAP
3.6. Statistical Analysis Of Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, V.; Carvalho, I.S. A review of the health benefit potentials of herbal plant infusions and their mechanism of actions. Ind. Crops Prod. 2015, 65, 247–258. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2017, 1–18. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Paula, H.A.; Abranches, M.V.; de Luces Fortes Ferreira, C.L. Yacon (Smallanthus sonchifolius): A food with multiple functions. Crit. Rev. Food Sci. Nutr. 2015, 55, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Fernández, E.C.; Orsák, M. Yacon [Smallanthus sonchifolia (Poepp. et Endl.) H. Robinson] chemical composition and use—A review. Plant Soil Environ. 2003, 49, 283–290. [Google Scholar]
- Caetano, B.F.R.; de Moura, N.A.; Almeida, A.P.S.; Dias, M.C.; Sivieri, K.; Barbisan, L.F. Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides. Nutrients 2016, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Ito, O.; Yasuyoshi, S.; Ikarashi, T.; Minamisawa, K.; Kubota, M.; Tsukihashi, T.; Asami, T. Composition of storage carbohydrate in tubers of yacon (Polymnia sonchifolia). J. Soil Sci. Plant Nutr. 1990, 36, 167–171. [Google Scholar] [CrossRef]
- Delgado, G.T.C.; Tamashiro, W.M.D.S.C.; Junior, M.R.M.; Pastore, G.M. Yacon (Smallanthus sonchifolius): A functional food. Plant Foods Hum. Nutr. 2013, 68, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ojansivu, I.; Ferreira, C.L.; Salminen, S. Yacon, a new source of prebiotic oligosaccharides with a history of safe use. Trends Food Sci. Technol. 2011, 22, 40–46. [Google Scholar] [CrossRef]
- Dionísio, A.P.; de Carvalho-Silva, L.B.; Vieira, N.M.; de Souza Goes, T.; Wurlitzer, N.J.; de Fatima Borges, M.; de Brito, E.S.; Ionta, M.; de Figueiredo, R.W. Cashew-apple (Anacardium occidentale L.) and yacon (Smallanthus sonchifolius) functional beverage improve the diabetic state in rats. Food Res. Int. 2015, 77, 171–176. [Google Scholar]
- Fernández, E.C.; Rajchl, A.; Lachman, J.; Čížková, H.; Kvasnička, F.; Kotíková, Z.; Milella, L.; Voldřich, M. Impact of yacon landraces cultivated in the Czech Republic and their ploidy on the short-and long-chain fructooligosaccharides content in tuberous roots. LWT Food Sci. Technol. 2013, 54, 80–86. [Google Scholar] [CrossRef]
- Genta, S.; Cabrera, W.; Habib, N.; Pons, J.; Carillo, I.M.; Grau, A.; Sánchez, S. Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin. Nutr. 2009, 28, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Scheid, M.M.A.; Genaro, P.S.; Moreno, Y.M.F.; Pastore, G.M. Freeze-dried powdered yacon: Effects of FOS on serum glucose, lipids and intestinal transit in the elderly. Eur. J. Nutr. 2014, 53, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Betalleluz-Pallardel, I.; Chirinos, R.; Aguilar-Galvez, A.; Noratto, G.; Pedreschi, R. Prebiotic effects of yacon (Smallanthus sonchifolius Poepp. & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem. 2012, 135, 1592–1599. [Google Scholar] [PubMed]
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic compound contents and antioxidant activity in plants with nutritional and/or medicinal properties from the Peruvian Andean region. Ind. Crops Prod. 2013, 47, 145–152. [Google Scholar] [CrossRef]
- Sousa, S.; Pinto, J.; Rodrigues, C.; Gião, M.; Pereira, C.; Tavaria, F.; Malcata, F.X.; Gomes, A.; Pacheco, M.B.; Pintado, M. Antioxidant properties of sterilized yacon (Smallanthus sonchifolius) tuber flour. Food Chem. 2015, 188, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Caballero, M.; Herbas, A.; Carballo, S. Antioxidants in yacon products and effect of long term storage. Food Sci. Technol. 2012, 32, 432–435. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Recovery of antioxidant phenolics from white vinification solid by-products employing water/ethanol mixtures. Bioresour. Technol. 2007, 98, 2963–2967. [Google Scholar] [PubMed]
- Contreras-Calderón, J.; Calderón-Jaimes, L.; Guerra-Hernández, E.; García-Villanova, B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Michailidis, Z.; Pantelidis, G. Peel and flesh antioxidant content and harvest quality characteristics of seven apple cultivars. Sci. Hortic. 2008, 115, 149–153. [Google Scholar] [CrossRef]
- Mattila, P.; Hellström, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007, 20, 152–160. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Yusoff, N.A.M.; Eldeen, I.M.; Seow, E.M.; Sajak, A.A.B.; Ooi, K.L. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.). J. Food Compos. Anal. 2011, 24, 1–10. [Google Scholar] [CrossRef]
- Da Silva, M.D.F.G.; Dionísio, A.P.; Carioca, A.A.F.; Adriano, L.S.; Pinto, C.O.; de Abreu, F.A.P.; Wurlitzer, N.J.; Araújo, I.M.; dos Santos Garruti, D.; Pontes, D.F. Yacon syrup: Food applications and impact on satiety in healthy volunteers. Food Res. Int. 2017, 100, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Parussolo, G.; Busatto, R.T.; Schmitt, J.; Pauletto, R.; Schons, P.F.; Ries, E.F. Synbiotic ice cream containing yacon flour and Lactobacillus acidophylus NCFM. LWT Food Sci. Technol. 2017, 82, 192–198. [Google Scholar] [CrossRef]
- Tormena, M.M.L.; de Medeiros, L.T.; de Lima, P.C.; Possebon, G.; Fuchs, R.H.B.; Bona, E. Application of multi-block analysis and mixture design with process variable for development of chocolate cake containing yacon (Smallanthus sonchifolius) and maca (Lepidium meyenii). J. Sci. Food Agric. 2017, 97, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.R.; Teixeira, M.C.; Saczk, A.A.; Barcelos, M.D.F.P.; Oliveira, M.F.D.; Abreu, W.C.D. Total antioxidant activity of yacon tubers cultivated in Brazil. Ciênc. Agrotec. 2016, 40, 596–605. [Google Scholar]
- Hole, C.C.; Barnes, A.; Thomas, T.H.; Scott, P.A.; Rankin, W.E.F. Dry Matter Distribution between the Shoot and Storage Root of Carrot (Daucus carota L.) I. Comparison of Varieties. Ann. Bot. 1983, 51, 175–187. [Google Scholar] [CrossRef]
- Kleinkopf, G.E.; Westermann, D.T.; Dwelle, R.B. Dry matter production and nitrogen utilization by six potato cultivars. Agron. J. 1981, 73, 799–802. [Google Scholar]
- Grau, A.; Rea, J. Yacon. Smallanthus sonchifolius (Poepp. & Endl.) H. Robinson. In Andean Roots and Tubers: Ahipa, Arracacha, Maca and Yacon; Hermann, M., Heller, J., Eds.; International Plant Genetics Research Institute: Rome, Italy, 1997; p. 235. ISBN 92-9043-351-5. [Google Scholar]
- Hermann, M.; Freire, I.; Pazos, C. Compositional diversity of the yacon storage root. Impact Chang. World Program Rep. 1997, 98, 425–432. [Google Scholar]
- Graefe, S.; Hermann, M.; Manrique, I.; Golombek, S.; Buerkert, A. Effects of post-harvest treatments on the carbohydrate composition of yacon roots in the Peruvian Andes. Field Crops Res. 2004, 86, 157–165. [Google Scholar] [CrossRef]
- Lachman, J.; Fernandez, E.C.; Viehmannova, I.; Šulc, M.; Eepkova, P. Total phenolic content of yacon (Smallanthus sonchifolius) rhizomes, leaves, and roots affected by genotype. N. Z. J. Crop Hortic. Sci. 2007, 35, 117–123. [Google Scholar] [CrossRef]
- Scher, C.F.; de Oliveira Rios, A.; Noreña, C.P.Z. Hot air drying of yacon (Smallanthus sonchifolius) and its effect on sugar concentrations. Int. J. Food Sci. Technol. 2009, 44, 2169–2175. [Google Scholar] [CrossRef]
- Simonovska, B.; Vovk, I.; Andrenšek, S.; Valentová, K.; Ulrichová, J. Investigation of phenolic acids in yacon (Smallanthus sonchifolius) leaves and tubers. J. Chromatogr. A 2003, 1016, 89–98. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Lewis, C.E.; Walker, J.R.; Lancaster, J.E.; Sutton, K.H. Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Coloured cultivars of Solanum tuberosum L. J. Sci. Food Agric. 1998, 77, 45–57. [Google Scholar] [CrossRef]
- Khajehei, F.; Niakousari, M.; Seidi Damyeh, M.; Merkt, N.; Claupein, W.; Graeff-Hoenninger, S. Impact of Ohmic-Assisted Decoction on Bioactive Components Extracted from Yacon (Smallanthus sonchifolius Poepp.) Leaves: Comparison with Conventional Decoction. Molecules 2017, 22, 2043. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, E.F.; de Souza Leone, R.; Ellendersen, L.N.; Masson, M.L. Phenolic profile and antioxidant activity of extracts of leaves and flowers of yacon (Smallanthus sonchifolius). Ind. Crops Prod. 2014, 62, 499–506. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Reyes, L.F.; Miller, J.C.; Cisneros-Zevallos, L. Environmental conditions influence the content and yield of anthocyanins and total phenolics in purple-and red-flesh potatoes during tuber development. Am. J. Potato Res. 2004, 81, 187–193. [Google Scholar] [CrossRef]
- Yan, X.; Suzuki, M.; Ohnishi-Kameyama, M.; Sada, Y.; Nakanishi, T.; Nagata, T. Extraction and Identification of Antioxidants in the Roots of Yacon (Smallanthus sonchifolius). J. Agric. Food Chem. 1999, 47, 4711–4713. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Tierno, R.; López, A.; Riga, P.; Arazuri, S.; Jarén, C.; Benedicto, L.; Ruiz de Galarreta, J.I. Phytochemicals determination and classification in purple and red fleshed potato tubers by analytical methods and near infrared spectroscopy. J. Sci. Food Agric. 2016, 96, 1888–1899. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Achaerandio, I.; Pujolà, M. Classification of potato cultivars to establish their processing aptitude. J. Sci. Food Agric. 2016, 96, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Kolb, N.; Herrera, J.L.; Ferreyra, D.J.; Uliana, R.F. Analysis of sweet diterpene glycosides from Stevia rebaudiana: Improved HPLC method. J. Agric. Food Chem. 2001, 49, 4538–4541. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Attree, R.; Du, B.; Xu, B. Distribution of phenolic compounds in seed coat and cotyledon, and their contribution to antioxidant capacities of red and black seed coat peanuts (Arachis hypogaea L.). Ind. Crops Prod. 2015, 67, 448–456. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Total Dry Matter | Fructose Content | Glucose Content | Sucrose Content | Total Phenolic Content | Total Flavonoid Content | ABTS Radical Scavenging Activity | DPPH Radical Scavenging Activity | Ferric Reducing Antioxidant Power | |
|---|---|---|---|---|---|---|---|---|---|
| Tuber part | p = 0.0003 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| Cultivar | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| Cultivar.Tuber part | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| Cultivar | Total Dry Matter Content (g 100 g−1 FW) | ||
|---|---|---|---|
| Flesh | Peel | Whole Tuber | |
| Cajamarca | 9.83 Db ± 0.40 | 11.77 BCa ± 0.11 | 10.21 Eb ± 0.41 |
| Cusco | 10.29 Db ± 0.32 | 12.51 Ba ± 0.44 | 10.17 Eb ± 0.63 |
| Early White | 12.77 BCa ± 0.62 | 10.20 DEc ± 0.12 | 11.67 Db ± 0.20 |
| Late Red | 14.13 ABa ± 0.62 | 12.58 Bb ± 0.67 | 14.69 Ba ± 0.27 |
| Morado | 15.13 Ab ± 0.41 | 15.68 Aab ± 0.24 | 16.93 Aa ± 1.08 |
| New Zealand | 12.94 BCa ± 0.61 | 10.80 CDb ± 0.26 | 13.30 Ca ± 0.64 |
| Quinault | 11.84 Ca ± 0.86 | 9.39 Eb ± 0.84 | 11.30 DEa ± 0.21 |
| Cultivar | Fructose (g 100 g−1 DW) | Glucose (g 100 g−1 DW) | Sucrose (g 100 g−1 DW) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Flesh | Peel | Whole Tuber | Flesh | Peel | Whole Tuber | Flesh | Peel | Whole Tuber | |
| Cajamarca | 8.54 Ba ± 0.42 | 0.37 Cc ± 0.13 | 7.35 Cb ± 0.24 | 9.35 Aa ± 0.36 | 0.18 Cc ± 0.00 | 8.40 Ab ±0.25 | 44.59 Ba± 0.74 | 29.89 Ab ± 1.35 | 43.12 Ca ± 1.05 |
| Cusco | 10.64 Ab ± 0.42 | 0.50 Cc ± 0.13 | 16.99 Ba ± 0.60 | 6.65 Ca ± 0.29 | 0.47 Bb ± 0.02 | 6.88 Ba ± 0.25 | 45.62 Ba± 0.98 | 21.00 Cb ± 1.26 | 48.31 Ba ± 3.10 |
| Early White | 2.14 Db ± 0.00 | 0.83 Bc ± 0.00 | 3.41 Ea ± 0.24 | 1.06 Eb ± 0.00 | 0.29 Cc ± 0.12 | 2.07 Da± 0.12 | 53.54 Ab± 0.98 | 24.45 Bc ± 0.00 | 58.74 Aa ± 2.01 |
| Late Red | 4.01 Ca ± 0.91 | 0.04 Dc ± 0.00 | 1.24 Fb ± 0.15 | 0.18 Fa ± 0.00 | 0.17 Ca ± 0.02 | 0.29 Ea ± 0.12 | 52.79 Ab ±3.92 | 24.09 Bc ± 0.42 | 58.08 Aa ± 1.21 |
| Morado | 1.63 Da ± 0.30 | 0.04 Db ± 0.00 | 0.17 Gb ± 0.15 | 0.51 Fa ± 0.12 | 0.24 Ca ± 0.00 | 0.45 Ea ± 0.00 | 45.85 Ba ± 0.84 | 18.26 Dc ± 0.87 | 31.00 Db ± 1.05 |
| New Zealand | 3.69 Cb ± 0.12 | 0.30 Cc ± 0.00 | 4.39 Da ± 0.15 | 5.33 Da ± 0.24 | 1.04 Ac ± 0.06 | 4.73 Cb ± 0.13 | 21.00 Da ± 1.81 | 6.00 Eb ± 0.77 | 19.81 Ea ± 0.47 |
| Quinault | 10.83 Ab ± 0.37 | 3.09 Ac ± 0.14 | 21.55 Aa ± 0.74 | 8.32 Ba ± 0.17 | 0.18 Cc ± 0.00 | 5.09 Cb ± 0.23 | 27.15 Ca ± 0.93 | 8.10 Fc ± 0.78 | 22.58 Eb ± 1.14 |
| Cultivar | Total Phenolic Content (mg GAE 100 g−1 DW) | Total Flavonoid Content (mg RE 100 g−1 DW) | ||||
|---|---|---|---|---|---|---|
| Flesh | Peel | Whole Tuber | Flesh | Peel | Whole Tuber | |
| Cajamarca | 2710.39 Bb ± 69.06 | 8402.94 Ba ± 221.45 | 2964.93 CDb ± 95.77 | 2726.80 Bb ± 120.81 | 16,494.91 Ba ± 324.01 | 3304.86 CDb ± 353.80 |
| Cusco | 2477.75 Cb ± 60.79 | 7007.29 Ca ± 475.86 | 3608.79 Ab ± 72.98 | 2247.73 BCc ± 221.44 | 12,959.57 Ca ± 214.57 | 4645.10 ABb ± 126.29 |
| Early White | 2213.73 Dc ± 38.59 | 6720.50 Ca ± 200.95 | 3397.55 ABb ± 61.64 | 2303.99 Bc ± 559.50 | 11,541.16 CDa ± 276.43 | 4147.11 ABCDb ± 70.96 |
| Late Red | 3307.51 Ab ± 21.84 | 5602.23 Da ± 46.87 | 3181.52 BCb ± 83.97 | 4142.02 Ab ± 471.98 | 9814.18 Da ± 1096.11 | 4365.77 ABCb ± 312.05 |
| Morado | 2571.95 BCc ± 31.95 | 6260.79 CDa ± 75.74 | 3656.37 Ab ± 74.60 | 2621.66 Bc ± 264.06 | 9670.46 Da ± 454.18 | 4959.37 Ab ± 340.51 |
| New Zealand | 1855.89 Ec ± 3.55 | 14,144.53 Aa ± 45.34 | 2845.05 Db ± 0.87 | 1041.69 Cc ± 25.36 | 25,488.31 Aa ± 554.02 | 3221.47 Db ± 354.17 |
| Quinault | 2470.21 Cc ± 9.29 | 8439.17 Ba ± 159.98 | 3005.57 CDb ± 83.59 | 2886.14 Bc ± 124.93 | 12,627.69 Ca ± 183.34 | 3593.64 BCDb ± 142.03 |
| Cultivar | ABTS Radicals Scavenging Activity (mM TE 100 g−1 DW) | DPPH Radical Scavenging Activity (mg AAE 100 g−1 DW) | FRAP (mM Fe2+ 100 g−1 DW) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Flesh | Peel | Whole Tuber | Flesh | Peel | Whole Tuber | Flesh | Peel | Whole Tuber | |
| Cajamarca | 376.40 Da ± 0.79 | 262.29 Cb ± 0.14 | 371.48 ABCa ± 3.50 | 1498.64 Aa ± 3.06 | 1513.45 Aa ± 9.88 | 1473.92 Ba ± 16.20 | 17,495.62 Bc ± 178.98 | 28,222.52 ABa ± 73.17 | 19,762.35 Eb ± 55.30 |
| Cusco | 384.81 Ca ± 0.13 | 266.19 Bc ± 0.40 | 356.16 Db ± 0.52 | 1498.85 Ab ± 3.88 | 1541.74 Aa ± 7.97 | 1507.47 ABb ± 0.12 | 15,521.66 Cc ± 59.38 | 28,450.95 Aa ± 35.15 | 24,508.18 Bb± 184.62 |
| Early White | 397.30 Ba ± 1.84 | 264.68 BCc ± 0.04 | 369.43 BCb ± 1.27 | 1498.28 Aa ± 28.81 | 1509.07 Aa ± 3.47 | 1511.84 ABa ± 2.35 | 10,745.21 Ec ± 85.78 | 28,271.57 ABa ± 97.07 | 21,967.91 Db ± 92.47 |
| Late Red | 366.81 Eb ± 0.74 | 293.58 Ac ± 0.98 | 374.68 ABa ± 1.55 | 1520.51 Aa ± 8.54 | 1503.97 Aa ± 10.55 | 1540.98 Aa ± 13.76 | 24,393.48 Ab ± 141.37 | 28,040.22 Ba ± 116.32 | 22,852.36 Cc ± 34.32 |
| Morado | 392.25 Ba ± 1.27 | 261.98 Cc ± 1.25 | 372.28 ABCb ± 1.78 | 1526.70 Aab ± 2.22 | 1507.48 Ab ± 5.29 | 1540.91 Aa ± 11.34 | 15,137.52 Cc ± 19.02 | 27,959.12 Ba ± 137.14 | 25,418.22 Ab ± 78.64 |
| New Zealand | 407.62 Aa ± 2.77 | 262.18 Cc ± 1.82 | 377.23 Ab ± 0.43 | 976.98 Bb ± 103.18 | 1515.62 Aa ± 1.22 | 1513.12 ABa ± 1.96 | 6343.02 Fc ± 74.17 | 28,122.04 ABa ± 89.46 | 17,020.23 Gb± 60.65 |
| Quinault | 393.00 Ba ± 2.53 | 263.34 BCc ± 0.03 | 367.05 Cb ± 0.83 | 1510.69 Aa ± 47.79 | 1507.32 Aa ± 25.72 | 1529.75 Aa ± 9.86 | 14,145.80 Dc ± 73.92 | 28,231.91 ABa ± 40.48 | 18,959.50 Fb ± 50.17 |
| Cultivar | Peel Color | Flesh Color |
|---|---|---|
| Cajamarca | tan | white |
| Cusco | tan | white |
| Early White | tan | white |
| Late Red | red, tan | orange, yellow |
| Morado | purple | white |
| New Zealand | purple, tan | white |
| Quinault | white, tan | white |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khajehei, F.; Merkt, N.; Claupein, W.; Graeff-Hoenninger, S. Yacon (Smallanthus sonchifolius Poepp. & Endl.) as a Novel Source of Health Promoting Compounds: Antioxidant Activity, Phytochemicals and Sugar Content in Flesh, Peel, and Whole Tubers of Seven Cultivars. Molecules 2018, 23, 278. https://doi.org/10.3390/molecules23020278
Khajehei F, Merkt N, Claupein W, Graeff-Hoenninger S. Yacon (Smallanthus sonchifolius Poepp. & Endl.) as a Novel Source of Health Promoting Compounds: Antioxidant Activity, Phytochemicals and Sugar Content in Flesh, Peel, and Whole Tubers of Seven Cultivars. Molecules. 2018; 23(2):278. https://doi.org/10.3390/molecules23020278
Chicago/Turabian StyleKhajehei, Forough, Nikolaus Merkt, Wilhelm Claupein, and Simone Graeff-Hoenninger. 2018. "Yacon (Smallanthus sonchifolius Poepp. & Endl.) as a Novel Source of Health Promoting Compounds: Antioxidant Activity, Phytochemicals and Sugar Content in Flesh, Peel, and Whole Tubers of Seven Cultivars" Molecules 23, no. 2: 278. https://doi.org/10.3390/molecules23020278
APA StyleKhajehei, F., Merkt, N., Claupein, W., & Graeff-Hoenninger, S. (2018). Yacon (Smallanthus sonchifolius Poepp. & Endl.) as a Novel Source of Health Promoting Compounds: Antioxidant Activity, Phytochemicals and Sugar Content in Flesh, Peel, and Whole Tubers of Seven Cultivars. Molecules, 23(2), 278. https://doi.org/10.3390/molecules23020278





