Small Molecular Weight Aldose (d-Glucose) and Basic Amino Acids (l-Lysine, l-Arginine) Increase the Occurrence of PAHs in Grilled Pork Sausages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Validation
2.2. Effect of Carbohydrate Characteristics on PAHs Content
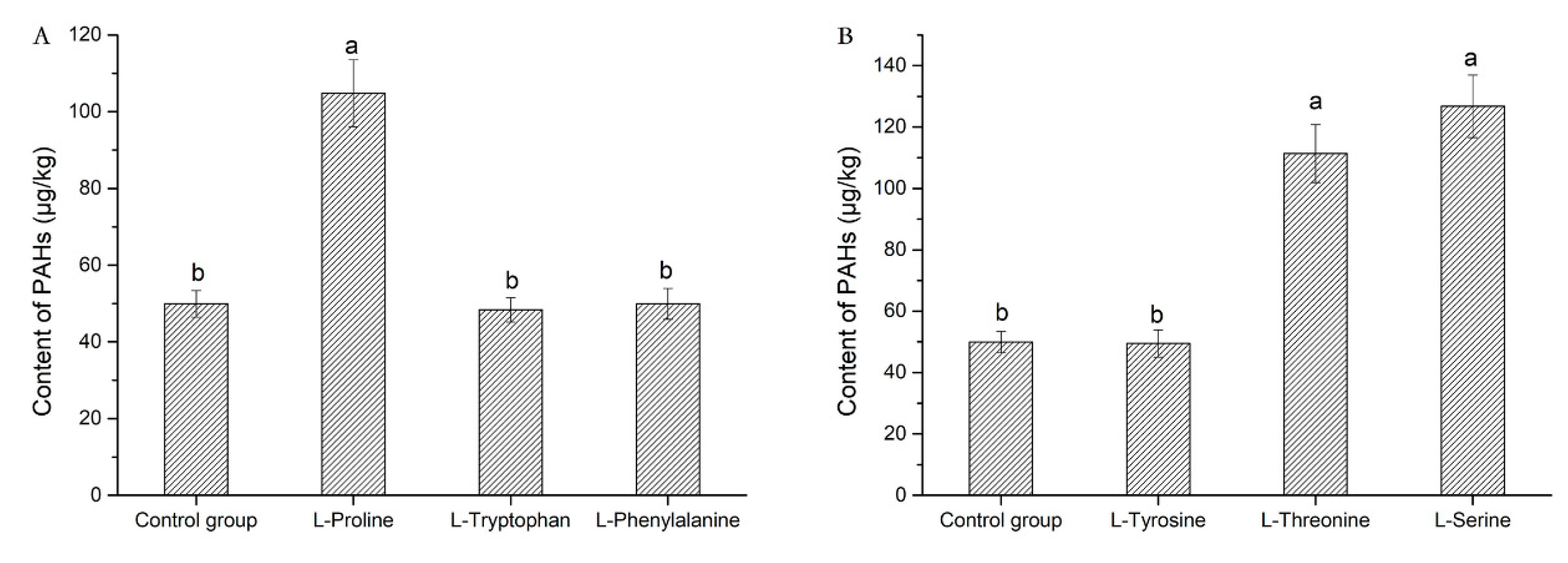
2.3. Effect of Amino Acid Properties on PAHs Content
2.4. Effect of Benzene Ring in Amino Acid on PAHs Content
3. Materials and Methods
3.1. Standards and Reagents
3.2. Sample Preparation
3.3. Grilling Tools and Cooking Procedures
3.4. Extraction and Clean-Up
3.5. HPLC Analysis of PAHs
3.6. Method Validation
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Iwegbue, C.M.; Onyonyewoma, U.A.; Bassey, F.I.; Nwajei, G.E. Concentrations and health risk of polycyclic aromatic hydrocarbons in some brands of biscuits in the nigerian market. Hum. Ecol. Risk Assess 2015, 21, 338–357. [Google Scholar] [CrossRef]
- Simon, R.; Gomez, J.A.; Von, H.C.; Wenzl, T.E. Results of a european inter-laboratory comparison study on the determination of eu priority polycyclic aromatic hydrocarbons (PAHs) in edible vegetable oils. Anal. Bioanal. Chem. 2008, 391, 1397–1408. [Google Scholar] [CrossRef]
- Authority, E.F. Findings of the efsa data collection on polycyclic aromatic hydrocarbons in food. Efsa J. 2007, 5, 3–55. [Google Scholar] [CrossRef]
- Alomirah, H.; Al-Zenki, S.; Al-Hooti, S.; Zaghloul, S.; Sawaya, W. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 2011, 22, 2028–2035. [Google Scholar] [CrossRef]
- Raj, A.; Prada, I.D.; Amer, A.A. A reaction mechanism for gasoline surrogate fuels for large polycyclic aromatic hydrocarbons. Combust. Flame. 2012, 159, 500–515. [Google Scholar] [CrossRef]
- Richter, H.; Howard, J.B. Formation of polycyclic aromatic hydrocarbons and their growth to sootda review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Mojica, M.; Francisco, M.A. The Diels-Alder cycloaddition reaction of substituted hemifullerenes with 1,3-butadiene: Effect of electron-donating and electron-withdrawing substituents. Molecules 2016, 21, 200. [Google Scholar] [CrossRef]
- Farhadian, A.; Jinap, S.; Abas, F.S. Determination of polycyclic aromatic hydrocarbons in grilled meat. Food Control 2010, 21, 606–610. [Google Scholar] [CrossRef]
- Saito, E.; Tanaka, N.; Miyazaki, A. Concentration and particle size distribution of polycyclic aromatic hydrocarbons formed by thermal cooking. Food Chem. 2014, 153, 285–291. [Google Scholar] [CrossRef]
- Santos, C.; Gomes, A. Polycyclic aromatic hydrocarbons incidence in Portuguese traditional smoked meat products. Food Chem. Toxicol. 2011, 49, 2343–2347. [Google Scholar] [CrossRef]
- Singh, L.; Agarwalauthor, T. Polycyclic aromatic hydrocarbons formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, J.; Tan, S. Effect of l-lysine on the physicochemical properties of pork sausage. Food Sci. Biotechnol. 2014, 23, 775–780. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.; Tan, S. Effects of l-arginine on physicochemical and sensory characteristics of pork sausage. Adv. J. Food Sci. Technol. 2014, 6, 660–667. [Google Scholar] [CrossRef]
- Phillip, F.; Britt, A.C.; Buchanan, C.V.; Owens, J.D.J. Glucose enhance the formation of nitrogen containing polycyclic aromatic compounds and polycyclic aromatic hydrocarbons in the pyrolysis of proline. Fuel 2004, 83, 1417–1432. [Google Scholar] [CrossRef]
- Ramesh, K.S.; Geoffrey, C. Formation of low molecular weight heterocycles and polycyclic aromatic compounds(PACs) in the pyrolysis of a-amino acids. J. Anal. Appl. Pyrolysis 2003, 66, 97–121. [Google Scholar] [CrossRef]
- Naknean, P.; Meenune, M. Factors affecting retention and release of flavour compounds in food carbohydrates. Int. Food Res. J. 2010, 17, 23–34. [Google Scholar] [CrossRef]
- Goubet, I.; Le, Q.J.; Voilley, A.J. Retention of aroma compounds by carbohydrates: Influence of their physicochemical characteristics and of their physical state. A review. J. Agric. Food. Chem. 1998, 46, 1981–1990. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Noel, D.C.; Moody, W.G. Textural and sensory properties of low-fat beef sausages with added water and polysaccharides as affected by pH and salt. J. Food Sci. 2010, 64, 5. [Google Scholar] [CrossRef]
- Appendix A to 40 CFR Part 423, 2010. United States Environmental Protection Agency (US-EPA). Available online: http://www.epa.gov/waterscience/methods/pollutants.htm (accessed on 1 July 2018).
- COMMISSION REGULATION (EU) No 835 2011 of 19 August 2011 Amending Regulation (EC) No 18812006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs. Available online: http://www.docin.com/p-275090873.html (accessed on 19 August 2018).
- Chen, B.H.; Lin, Y.S. Formation of polycyclic aromatic hydrocarbons during processing of duck meat. J. Agric. Food Chem. 1997, 45, 1394–1403. [Google Scholar] [CrossRef]
- Bansal, V.; Kim, K.H. Review of PAH contamination in food products and their health hazards. Environ. Inter. 2015, 84, 26–38. [Google Scholar] [CrossRef]
- Britt, P.F.; Buchanan, A.C.; Owens, C.V.; Todd, S.J. Formation of nitrogen containing polycyclic aromatic hydrocarbons from the co-pyrolysis of carbohydrates and amino acids. Div. Fuel Chem. 2002, 47, 400–403. [Google Scholar] [CrossRef]
- Kandhro, A.; Sherazi, S.T.; Mahesar, S.A.; Bhanger, M.I.; Younis, T.M. GC-MS quantification of fatty acid profile including trans fa in the locally manufactured margarines of pakistan. Food Chem. 2008, 109, 207–211. [Google Scholar] [CrossRef]
- Masuda, Y.; Mori, K.; Kuratsune, M. Polycyclic aromatic hydrocarbons formed by pyrolysis of carbohydrates, amino acids, and fatty acids. Gan 1967, 58, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; McGrath, W.; Geoffrey, C. Low temperature mechanism for the formation of polycyclic aromatic hydrocarbons from the pyrolysis of cellulose. J. Anal. Appl. Pyrolysis 2003, 66, 51–70. [Google Scholar] [CrossRef]
- Wanwisa, W.; Kanithaporn, V. Effects of oil types and pH on carcinogenic polycyclic aromatic hydrocarbons (PAHs) in grilled chicken. Food Control 2017, 79, 119–125. [Google Scholar] [CrossRef]
- Farhadian, A.; Jinap, S.; Hanifah, H.N.; Zaidul, I.S. Effects of meat preheating and wrapping on the levels of polycyclic aromatic hydrocarbons in charcoal-grilled meat. Food Chem. 2010, 124, 141–146. [Google Scholar] [CrossRef]
- Juan, C.; Zinedine, A.; Moltó, J.C.; Idrissi, L.; Mañes, J. Aflatoxins levels in dried fruits and nuts from rabat-salé area, morocco. Food Control 2008, 19, 849–853. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |
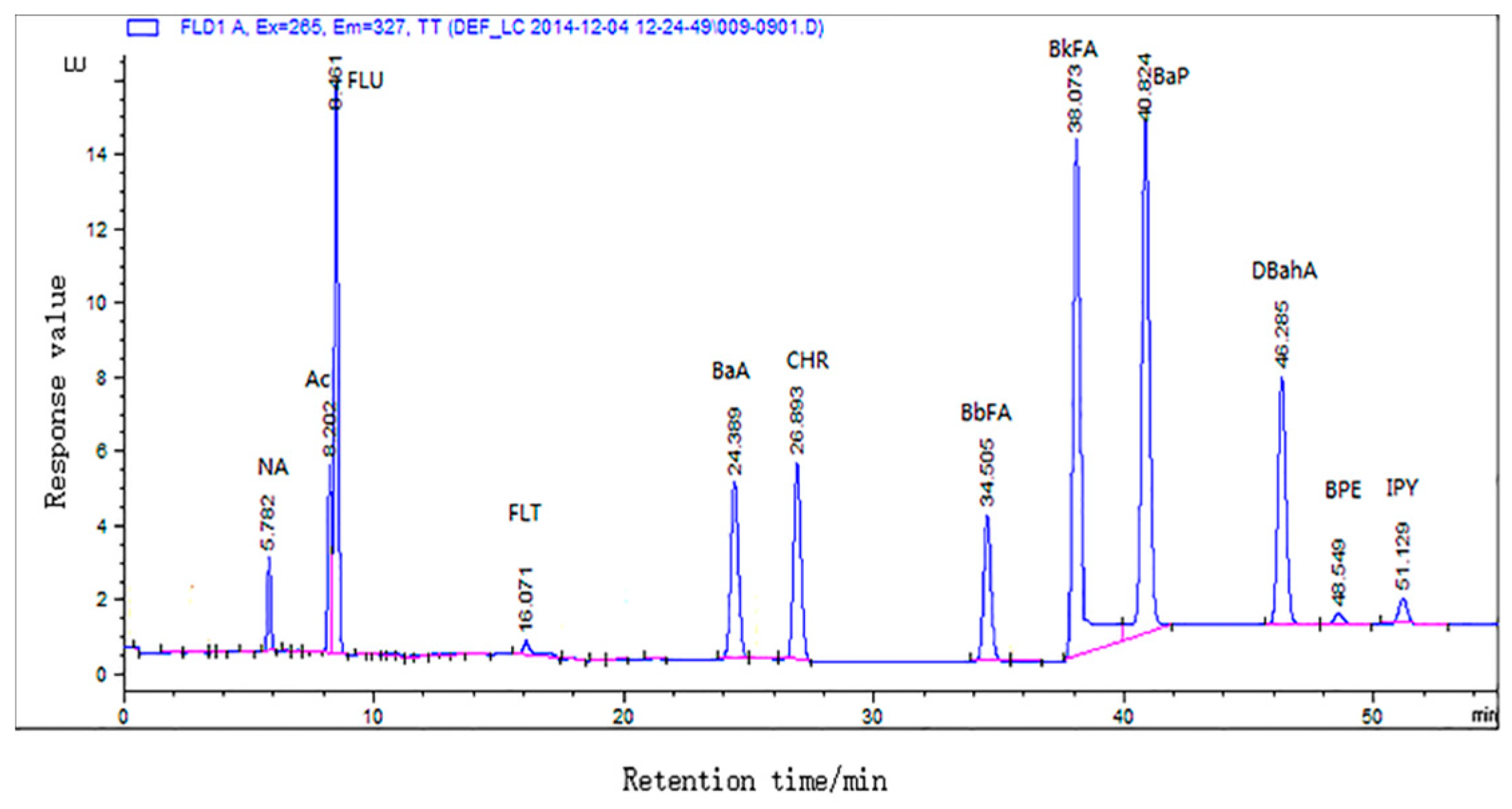
| PAHs | Linear Range (ng mL−1) | Correlation Coefficients (R2) | LOD (μg kg−1) | LOQ (μg kg−1) | Recovery a (%) | RSD b (%) |
|---|---|---|---|---|---|---|
| NA | 0.10–10.00 | 0.9999 | 0.05 | 0.17 | 105.78 | 1.23 |
| AC | 0.10–10.00 | 0.9996 | 0.18 | 0.60 | 93.46 | 1.46 |
| FLU | 0.20–10.00 | 0.9999 | 0.06 | 0.20 | 84.54 | 5.54 |
| FLT | 0.20–10.00 | 0.9993 | 0.16 | 0.53 | 83.83 | 7.89 |
| BaA | 0.25–5.00 | 0.9998 | 0.09 | 0.30 | 90.52 | 4.37 |
| CHR | 0.30–7.50 | 0.9999 | 0.10 | 0.91 | 92.56 | 3.42 |
| BbFA | 1.00–20.00 | 0.9995 | 0.06 | 0.20 | 96.74 | 1.90 |
| BkFA | 1.00–20.00 | 0.9999 | 0.08 | 0.27 | 95.33 | 2.05 |
| BaP | 0.20–10.00 | 0.9999 | 0.11 | 0.37 | 91.31 | 4.55 |
| DBahA | 0.10–10.00 | 0.9991 | 0.03 | 0.10 | 84.53 | 9.71 |
| BPE | 0.20–10.00 | 0.9998 | 0.04 | 0.20 | 88.34 | 4.56 |
| IPY | 0.30–10.00 | 0.9997 | 0.06 | 0.30 | 79.22 | 6.18 |
| Species | NA | Ac | FLU | FLT | BaA | CHR | BbFA | BkFA | DBahA | BPE | BaP | IPY | ∑PAH12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group | 17.93 ± 1.35 c | 12.99 ± 1.02 c | 2.79 ± 0.14 c | ND | 2.21 ± 0.16 c | 3.62 ± 0.10b c | ND | 7.52 ± 0.36 b | 0.71 ± 0.05 c | 1.25 ± 0.08 c | 0.88 ± 0.12 d | ND | 49.90 ± 3.46 c |
| d-Fructose | 18.36 ± 1.41 c | 11.15 ± 1.14 c | 3.13 ± 0.26 c | 1.06 ± 0.15 c | 3.04 ± 0.27 bc | 2.47 ± 0.16 d | ND | 6.01 ± 0.33 c | 1.04 ± 0.11 b | 1.37 ± 0.09 b | 1.26 ± 0.06 c | ND | 48.89 ± 4.05 c |
| d-Glucose | 34.25 ± 1.22 a | 23.45 ± 1.68 a | 7.66 ± 0.23 a | 4.24 ± 0.18 a | 7.41 ± 0.36 a | 6.75 ± 0.36 a | 0.47 ± 0.03 a | 13.76 ± 1.28 a | 3.16 ± 0.30 a | 3.07 ± 0.26 a | 5.59 ± 0.22 a | ND | 109.81 ± 6.49 a |
| 4-(α-d-Glucosido)-d-glucose | 25.17 ± 2.23 b | 17.42 ± 1.35 b | 5.06 ± 0.44 b | 2.31 ± 0.18 b | 4.01 ± 0.23 b | 4.48 ± 0.24 b | 0.29 ± 0.01 b | 8.47 ± 0.31 b | 1.35 ± 0.10 b | 1.54 ± 0.10 b | 3.46 ± 0.25 b | ND | 73.56 ± 5.67 b |
| Cellulose | 17.16 ± 1.27 c | 11.56 ± 1.06 c | 3.03 ± 0.21 c | ND | 2.44 ± 0.17 c | 3.53 ± 0.28 c | ND | 6.46 ± 0.31 c | 0.82 ± 0.03 bc | 1.39 ± 0.11 b | 1.12 ± 0.18 cd | ND | 47.51 ± 3.38 c |
| Species | NA | Ac | FLU | FLT | BaA | CHR | BbFA | BkFA | DBahA | BPE | BaP | IPY | ∑PAH12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group | 17.93 ± 1.35 de | 12.99 ± 1.02 cd | 2.79 ± 0.14 cd | ND | 2.21 ± 0.16 c | 3.62 ± 0.10 c | ND | 7.52 ± 0.36 c | 0.71 ± 0.05 c | 1.25 ± 0.08 cd | 0.88 ± 0.12 b | ND | 49.90 ± 3.46 d | |
| Non-polar amino acid | l-proline | 30.42 ± 2.26 c | 27.10 ± 1.54 b | 6.17 ± 0.33 b | 3.16 ± 0.18 a | 5.06 ± 0.27 ab | 7.83 ± 0.24 b | 1.01 ± 0.04 a | 17.43 ± 1.33 a | 1.64 ± 0.11 b | 2.73 ± 0.13 b | 2.23 ± 0.20 a | ND | 104.78 ± 8.72 b |
| l-tryptophan | 18.35 ± 1.42 de | 11.43 ± 1.24 d | 3.01 ± 0.26 c | ND | 1.88 ± 0.13 d | 3.59 ± 0.21 c | ND | 7.17 ± 0.45 c | 0.75 ± 0.07 c | 1.16 ± 0.11 d | 1.01 ± 0.16 b | ND | 48.35 ± 3.18 d | |
| l-phenylalanine | 16.77 ± 1.37 e | 13.21 ± 1.10 cd | 2.85 ± 0.24 cd | ND | 2.35 ± 0.17 c | 3.81 ± 0.26 c | ND | 8.02 ± 0.43 c | 0.67 ± 0.05 c | 1.36 ± 0.14 cd | 0.85 ± 0.11 b | ND | 49.89 ± 4.05 d | |
| Polar amino acid | l-tyrosine | 18.19 ± 1.52 de | 12.15 ± 1.13 d | 2.66 ± 0.22 d | ND | 1.99 ± 0.14 cd | 3.77 ± 0.28 c | ND | 7.86 ± 0.46 c | 0.69 ± 0.04 c | 1.22 ± 0.11 d | 0.94 ± 0.06 b | ND | 49.47 ± 4.44 d |
| l-threonine | 39.45 ± 3.26 b | 28.58 ± 2.19 ab | 6.82 ± 0.31 b | ND | 4.86 ± 0.20 b | 7.96 ± 0.30 b | 0.86 ± 0.03 a | 16.54 ± 1.42a | 1.56 ± 0.12 b | 2.75 ± 0.21 b | 1.96 ± 0.08 a | ND | 111.34 ± 9.43 b | |
| l-serine | 37.49 ± 3.33 b | 34.01 ± 2.64 a | 8.12 ± 0.36 a | 3.72 ± 0.20 a | 5.78 ± 0.26 ab | 9.47 ± 0.82 ab | 1.02 ± 0.01 a | 19.68 ± 1.54 a | 1.85 ± 0.11 b | 3.27 ± 0.20 a | 2.35 ± 0.17 a | ND | 126.76 ± 10.17 a | |
| Basic amino acid | l-lysine | 50.48 ± 2.57 a | 32.08 ± 1.88 a | 8.74 ± 023 a | ND | 8.76 ± 0.18 a | 12.15 ± 0.32 a | ND | 12.78 ± 1.14 b | 3.21 ± 0.08 a | 3.13 ± 0.10 a | 1.52 ± 0.05 ab | ND | 132.85 ± 6.13 a |
| l-arginine | 52.67 ± 3.36 a | 30.91 ± 2.49 a | 9.64 ± 0.33 a | ND | 7.26 ± 0.21 a | 11.61 ± 1.40 a | ND | 17.89 ± 1.42 a | 3.69 ± 0.33 a | 3.98 ± 0.36 a | 2.17 ± 0.14 a | ND | 139.82 ± 8.45 a | |
| Acidic amino acid | l-glutamic acid | 20.96 ± 3.61 d | 17.67 ± 2.20 c | 2.37 ± 0.27 d | ND | 5.45 ± 0.22 ab | 7.27 ± 0.31 b | ND | 10.17 ± 1.26 b | 1.62 ± 0.11 b | 1.86 ± 0.14 c | 2.09 ± 0.10 a | ND | 69.46 ± 9.27 c |
| l-aspartate acid | 22.76 ± 2.18 d | 15.37 ± 1.06 c | 3.54 ± 0.16 c | ND | 3.03 ± 0.17 bc | 4.59 ± 0.20 c | ND | 9.54 ± 0.96 bc | 2.92 ± 0.16 a | 1.58 ± 0.10 c | 1.14 ± 0.04 b | ND | 64.47 ± 3.47 c |
| Molecular Weight (g/mol) | pKa | logP | |
|---|---|---|---|
| d-Glucose | 180.16 | 12.43 (t = 18 °C) | −3.24 |
| d-Fructose | 180.16 | 12.06 (t = 18 °C) | −2.23 |
| 4-(α-d-Glucosido)-d-glucose | 342.30 | - | −5.03 |
| Cellulose | >50000 | - | - |
| l-Proline | 115.13 | 10.64 (t = 25 °C) | −2.54 |
| l-Tryptophan | 204.22 | 7.38 (t = 25 °C) | −1.06 |
| l-Phenylalanine | 165.19 | 1.24 (t = 25 °C) | −1.38 |
| l-Tyrosine | 181.19 | 2.20 (t = 25 °C) | −2.26 |
| l-Threonine | 119.12 | 5.60 (t = 25 °C) | −2.94 |
| l-Serine | 105.09 | 2.21 (t = 25 °C) | −3.07 |
| l-Lysine | 146.19 | 3.12 (t = 0 °C) | −3.05 |
| l-Arginine | 174.20 | 2.24 (t = 0 °C) | −4.20 |
| l-Glutamic acid | 147.13 | 2.23 (t = 0 °C) | −3.69 |
| l-Aspartic acid | 133.10 | 2.01 (t = 0 °C) | −3.89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, W.; Cai, K.-z.; Li, Y.-z.; Zhang, S.; Wang, Y.; Guo, J.; Chen, C.-g.; Xu, B.-c. Small Molecular Weight Aldose (d-Glucose) and Basic Amino Acids (l-Lysine, l-Arginine) Increase the Occurrence of PAHs in Grilled Pork Sausages. Molecules 2018, 23, 3377. https://doi.org/10.3390/molecules23123377
Nie W, Cai K-z, Li Y-z, Zhang S, Wang Y, Guo J, Chen C-g, Xu B-c. Small Molecular Weight Aldose (d-Glucose) and Basic Amino Acids (l-Lysine, l-Arginine) Increase the Occurrence of PAHs in Grilled Pork Sausages. Molecules. 2018; 23(12):3377. https://doi.org/10.3390/molecules23123377
Chicago/Turabian StyleNie, Wen, Ke-zhou Cai, Yu-zhu Li, Shuo Zhang, Yu Wang, Jie Guo, Cong-gui Chen, and Bao-cai Xu. 2018. "Small Molecular Weight Aldose (d-Glucose) and Basic Amino Acids (l-Lysine, l-Arginine) Increase the Occurrence of PAHs in Grilled Pork Sausages" Molecules 23, no. 12: 3377. https://doi.org/10.3390/molecules23123377
APA StyleNie, W., Cai, K.-z., Li, Y.-z., Zhang, S., Wang, Y., Guo, J., Chen, C.-g., & Xu, B.-c. (2018). Small Molecular Weight Aldose (d-Glucose) and Basic Amino Acids (l-Lysine, l-Arginine) Increase the Occurrence of PAHs in Grilled Pork Sausages. Molecules, 23(12), 3377. https://doi.org/10.3390/molecules23123377






