Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole
Abstract
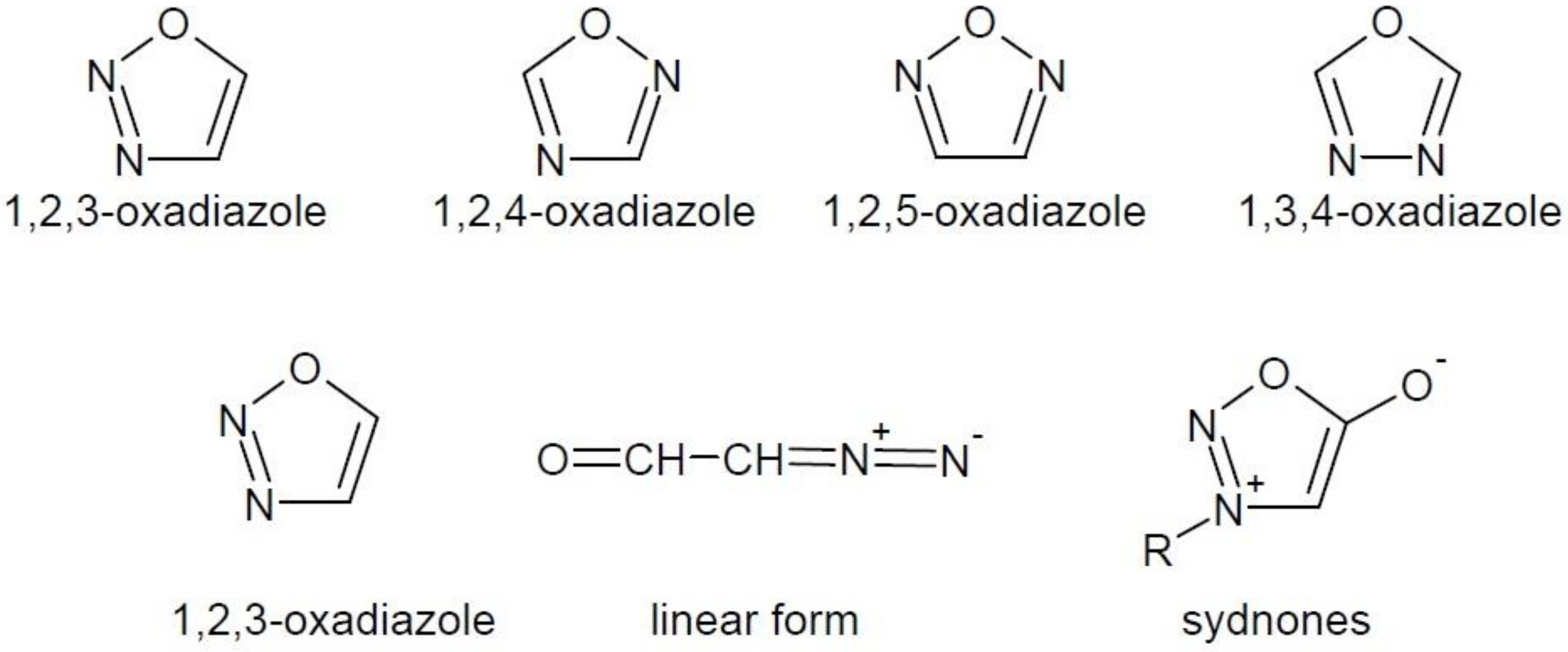
1. Introduction
2. Anti-Proliferative Effects of 1,3,4-Oxadiazole Derivatives
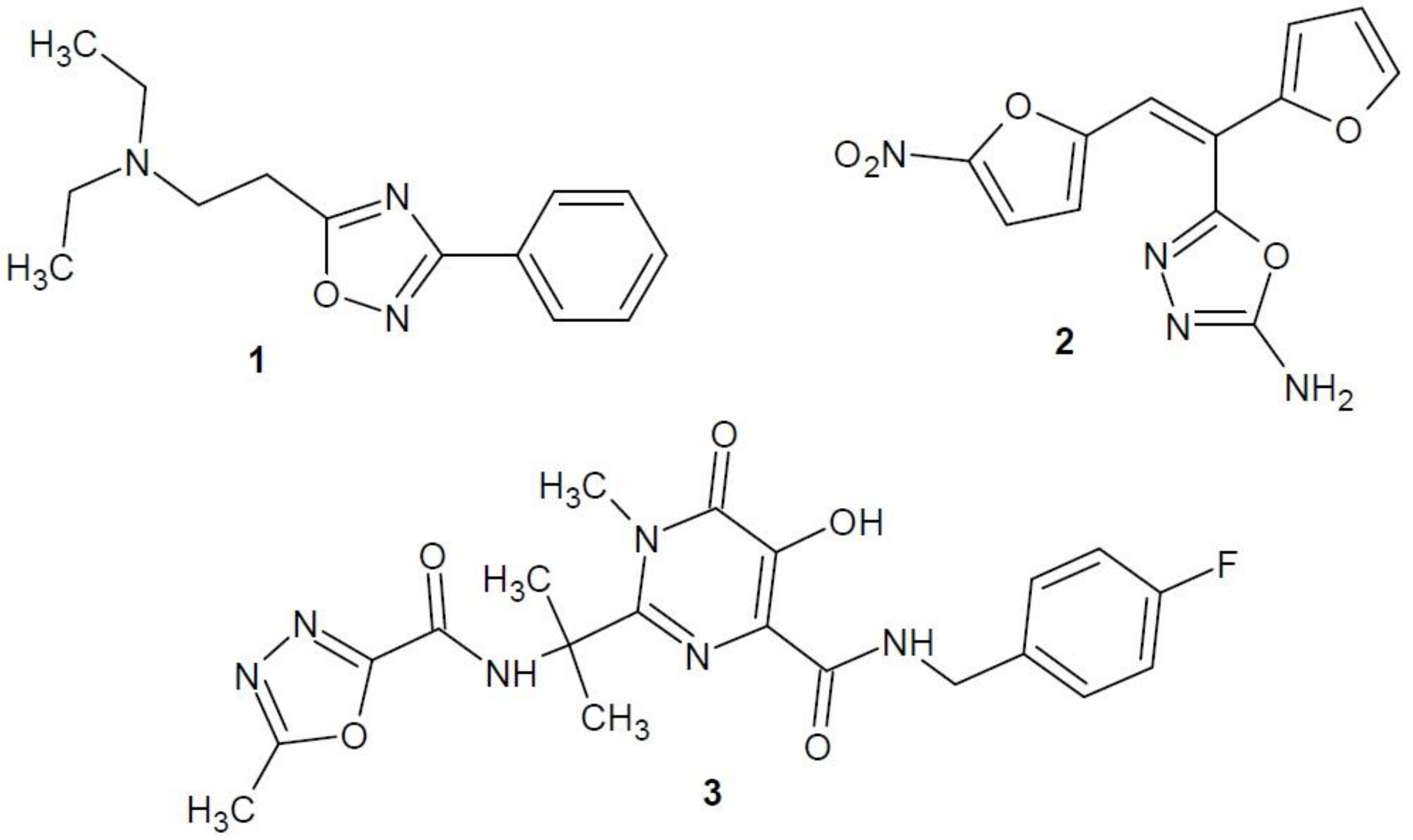
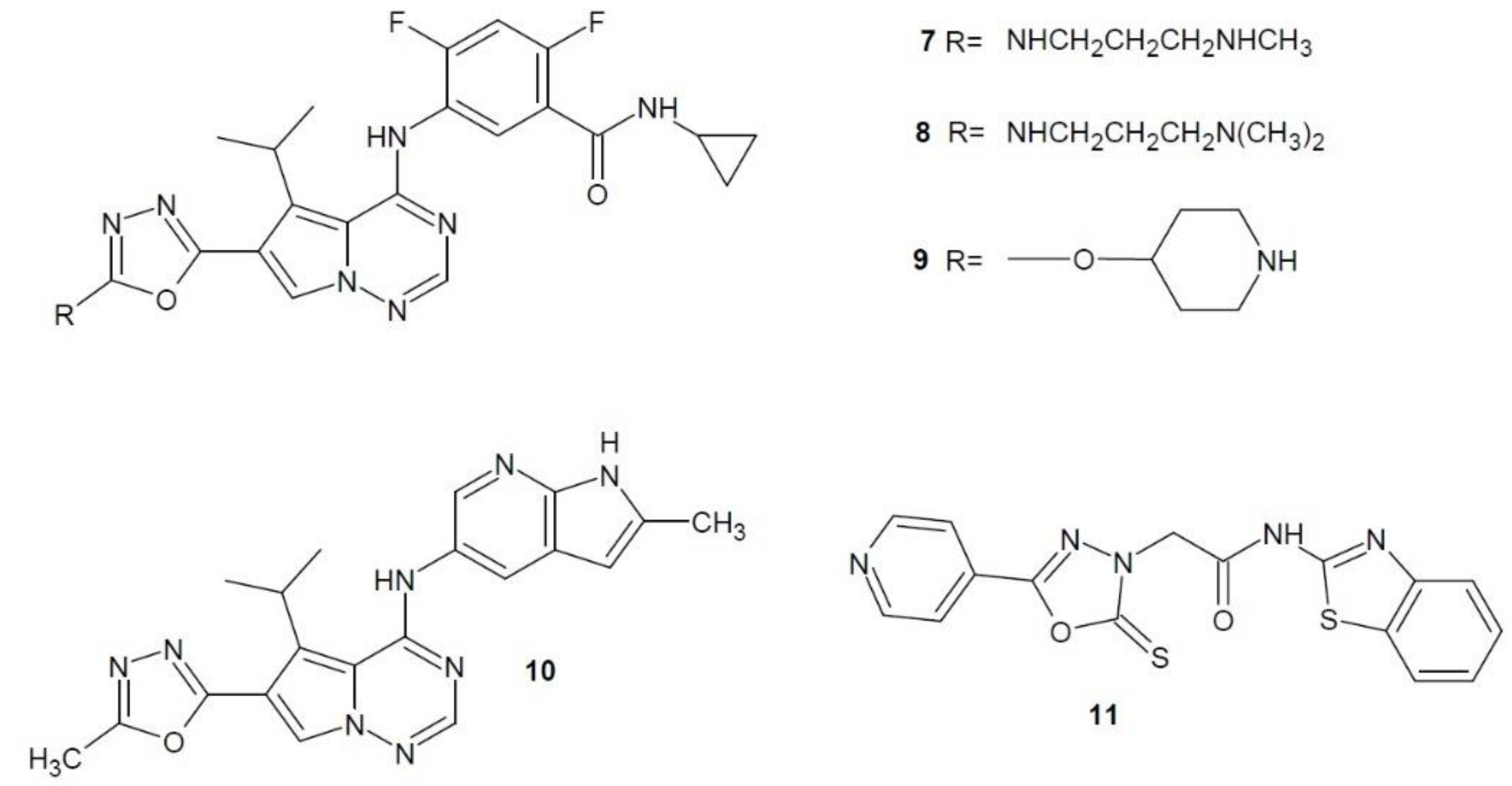
2.1. Epidermal Growth Factor Receptor Inhibitors
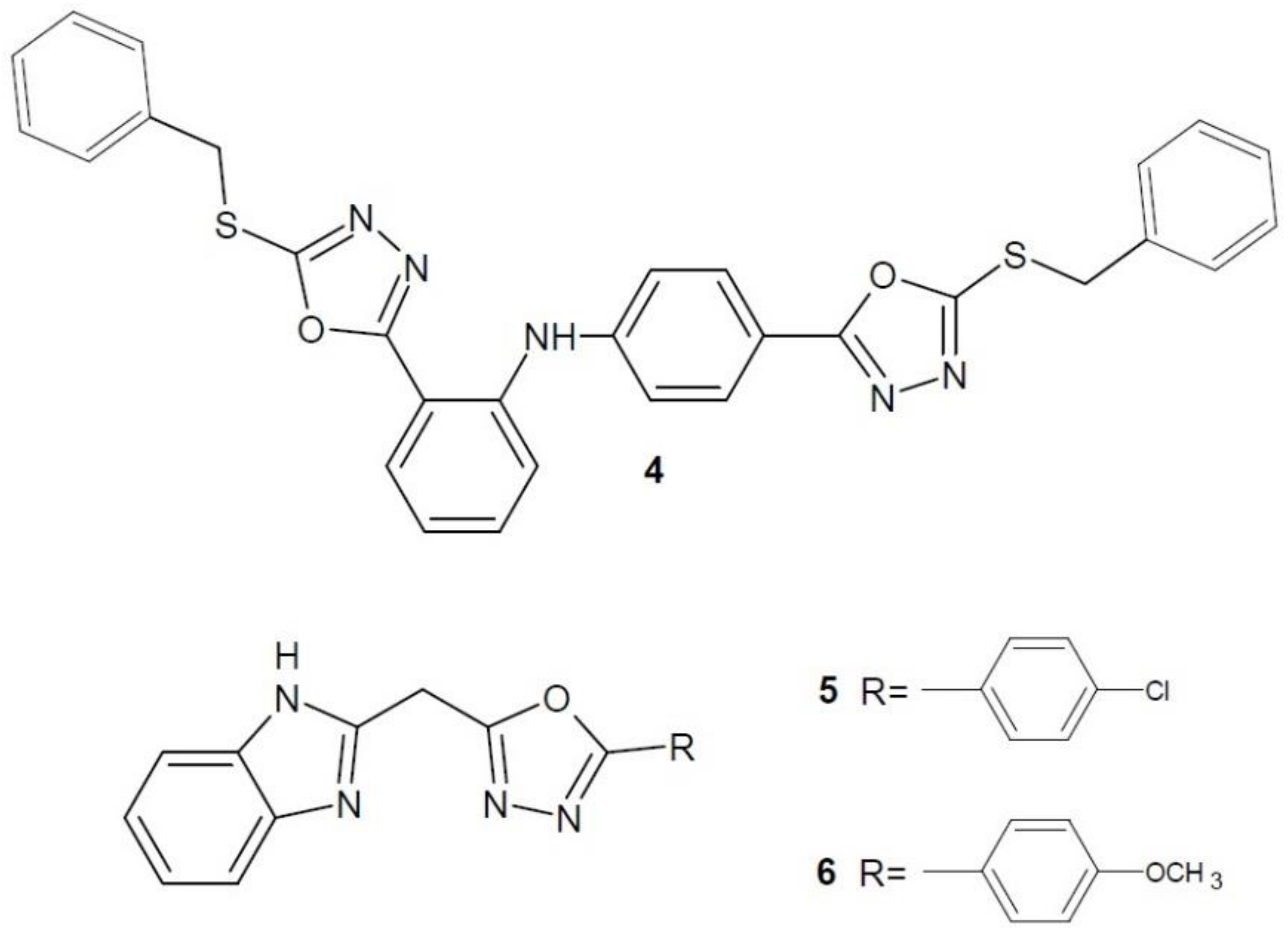
2.2. Vascular Endothelial Growth Factor Receptor Inhibitors
2.3. Endothelin Receptor Antagonists
2.4. Focal-Adhesion Kinase Inhibitors
2.5. Histone Deacetylase Inhibitors
2.6. Methionine Aminopeptidase Inhibitors
2.7. NF-κB Inhibitors
2.8. Poly(ADP-ribose) Polymerase Inhibitors
2.9. Telomerase Inhibitors
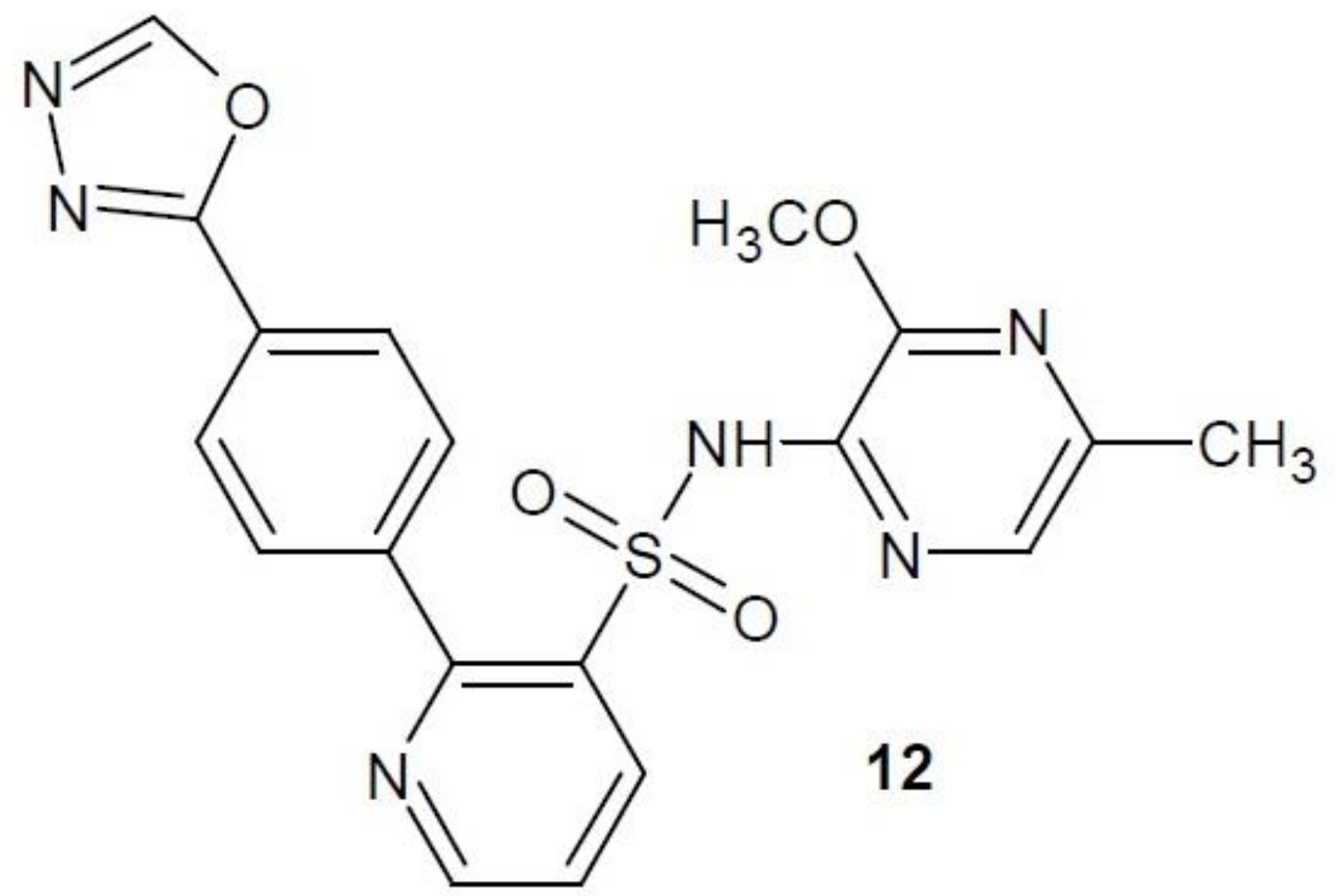
2.10. Thymidine Phosphorylase Inhibitors
2.11. Thymidylate Synthase Inhibitors
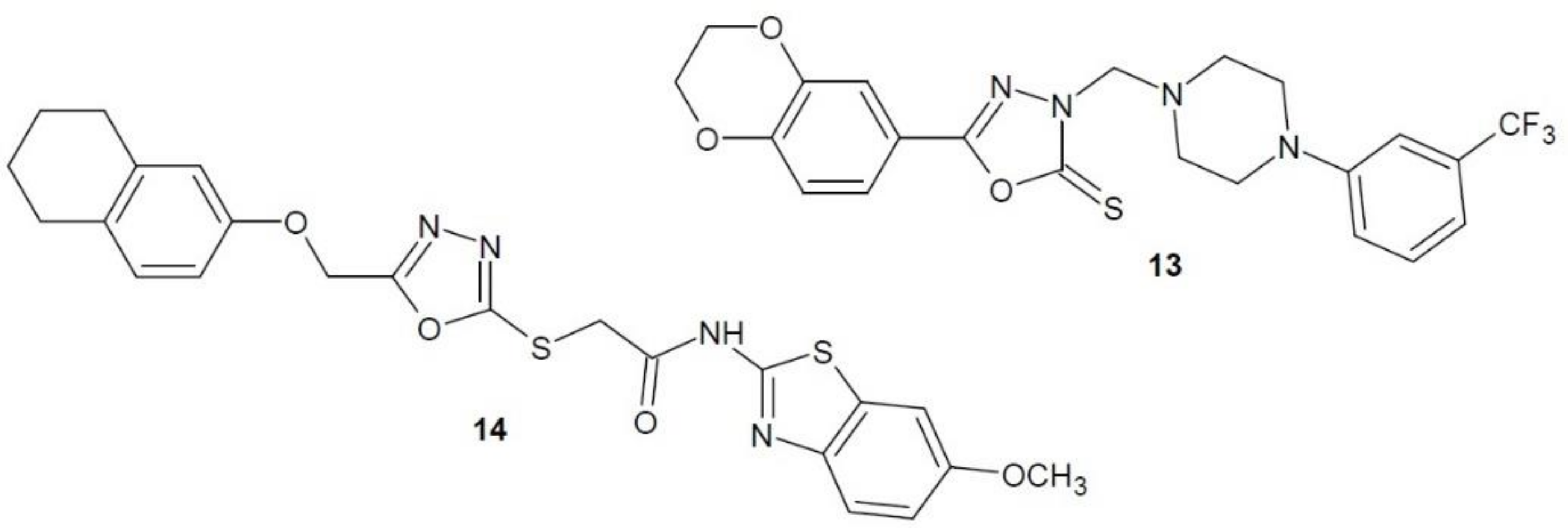
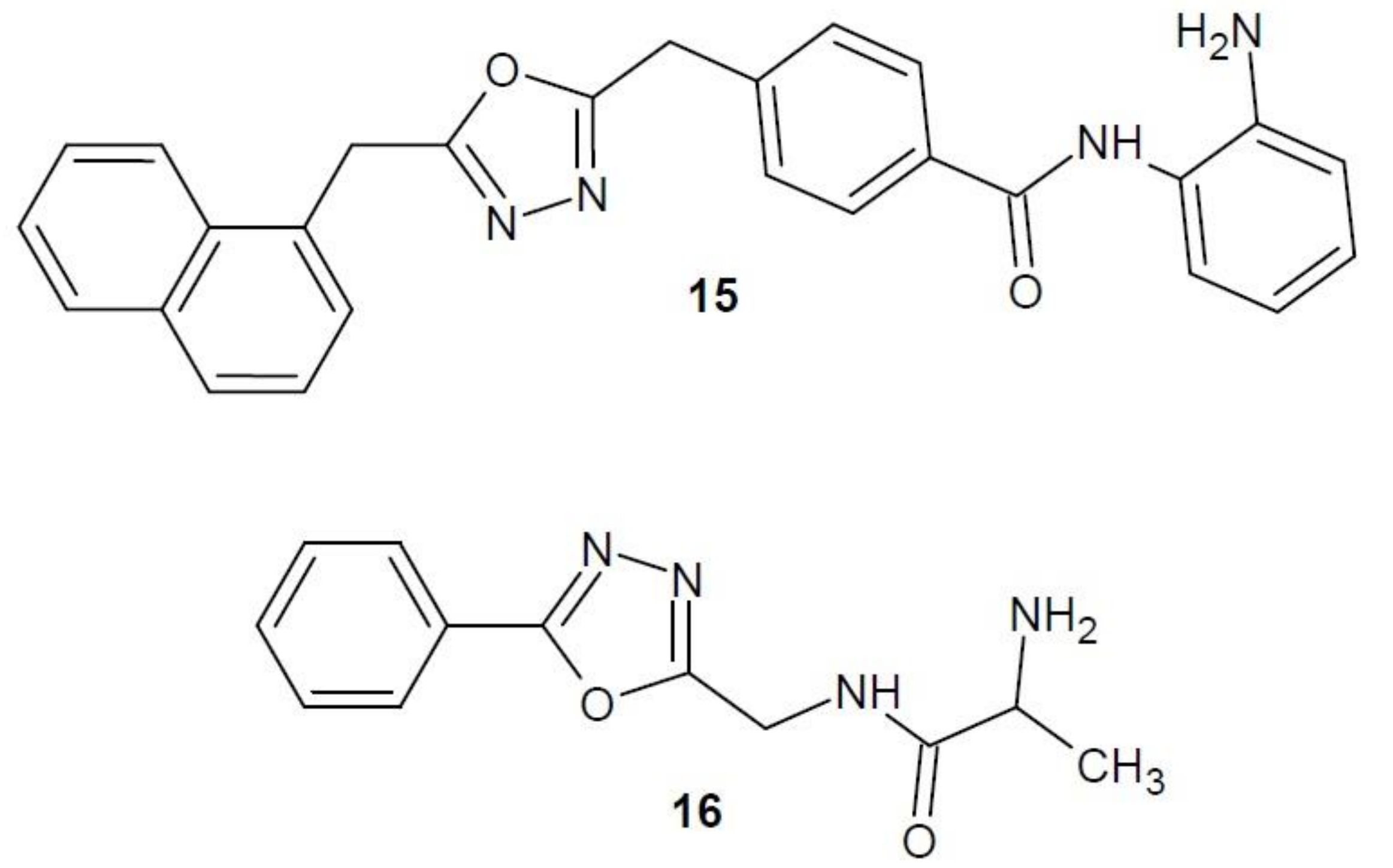
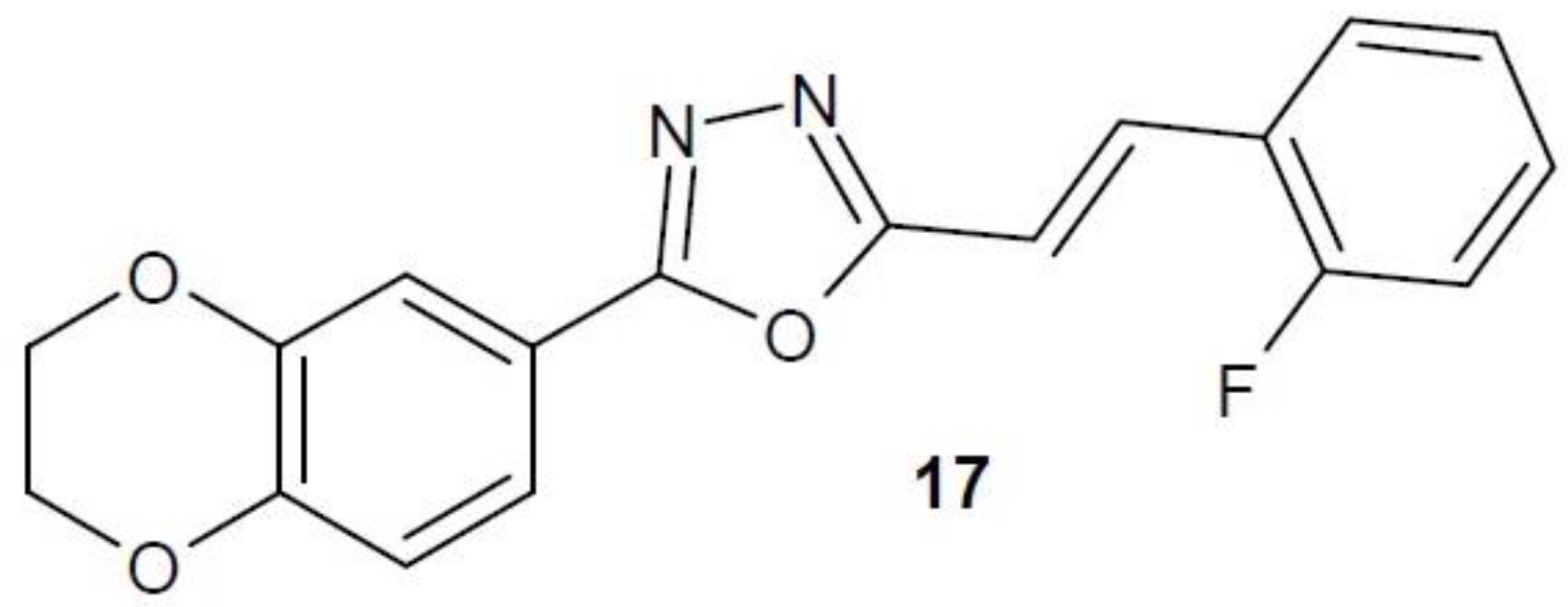
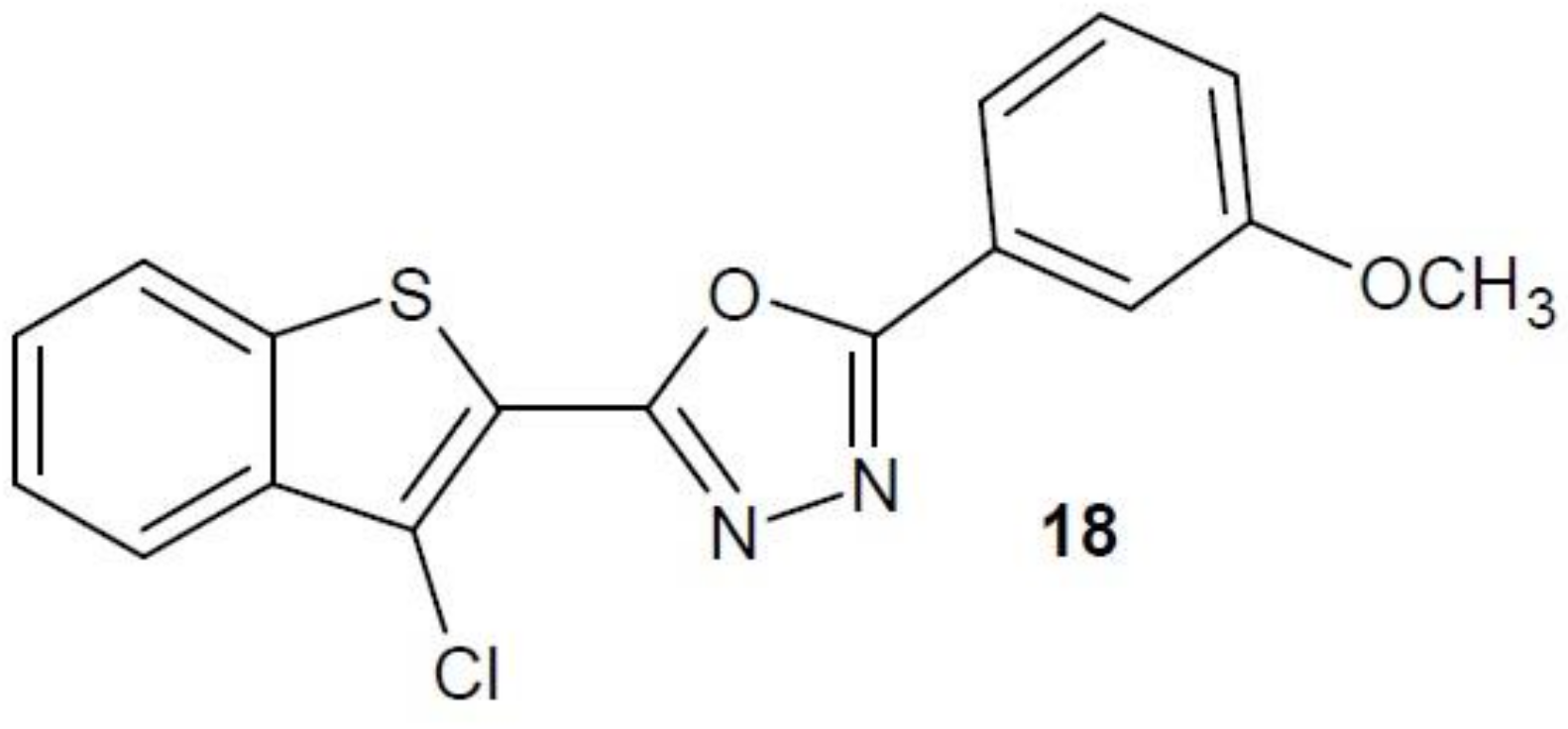
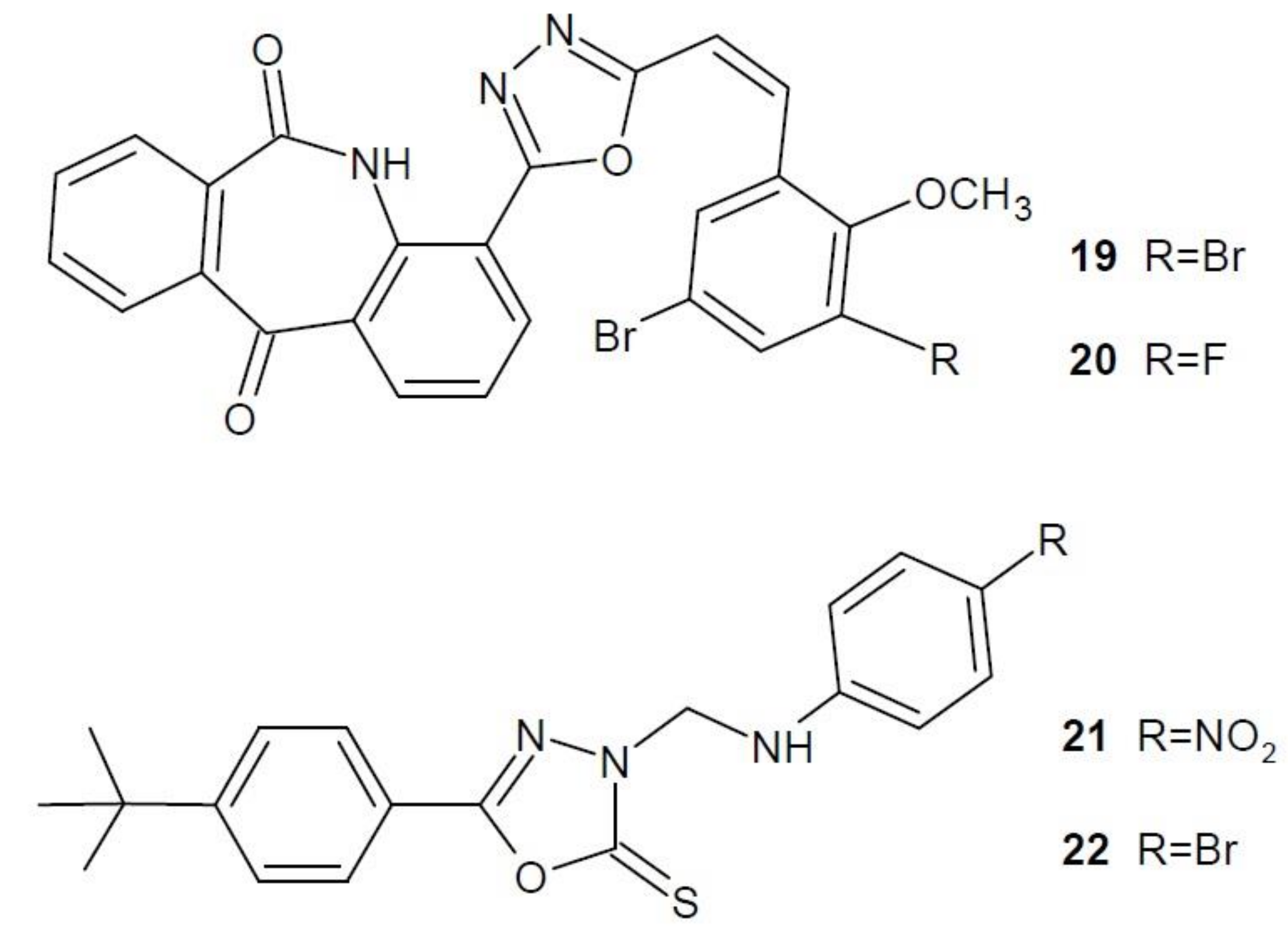
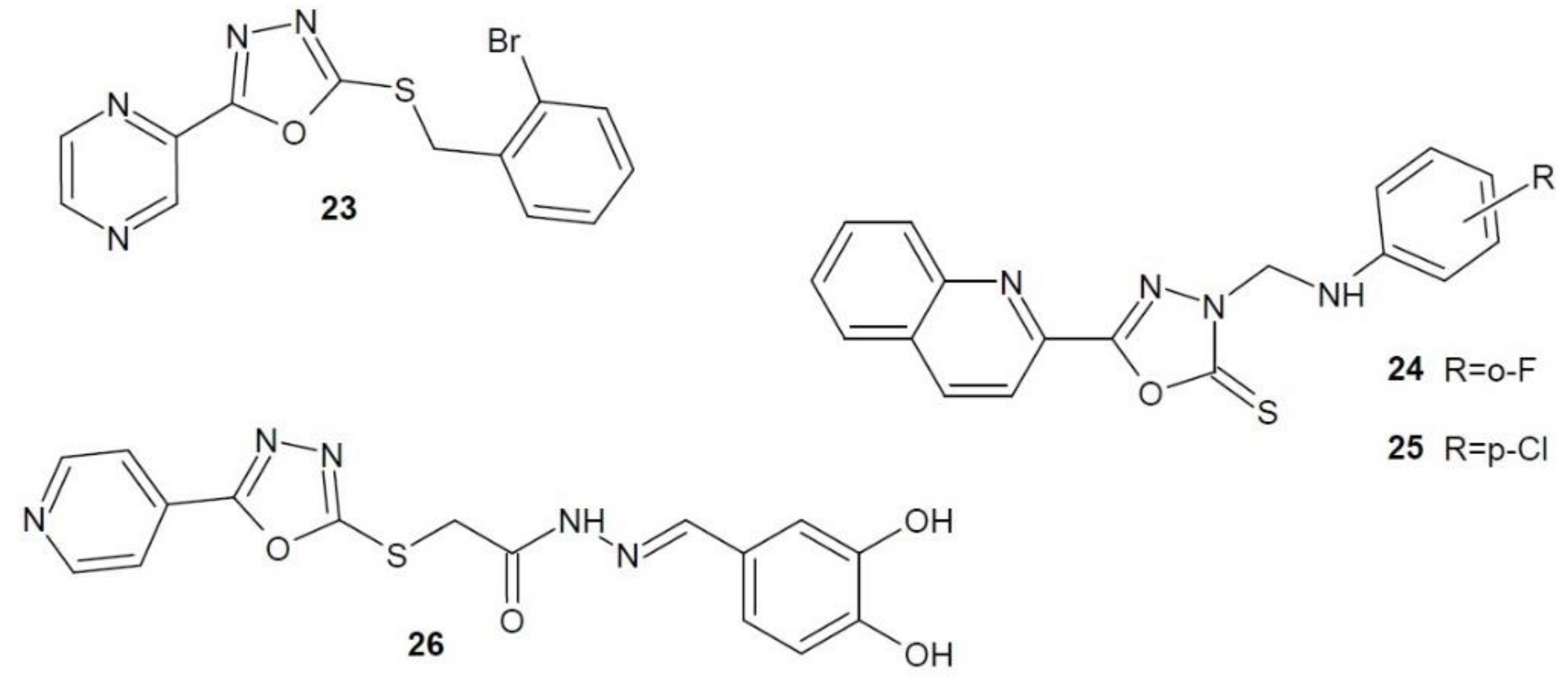
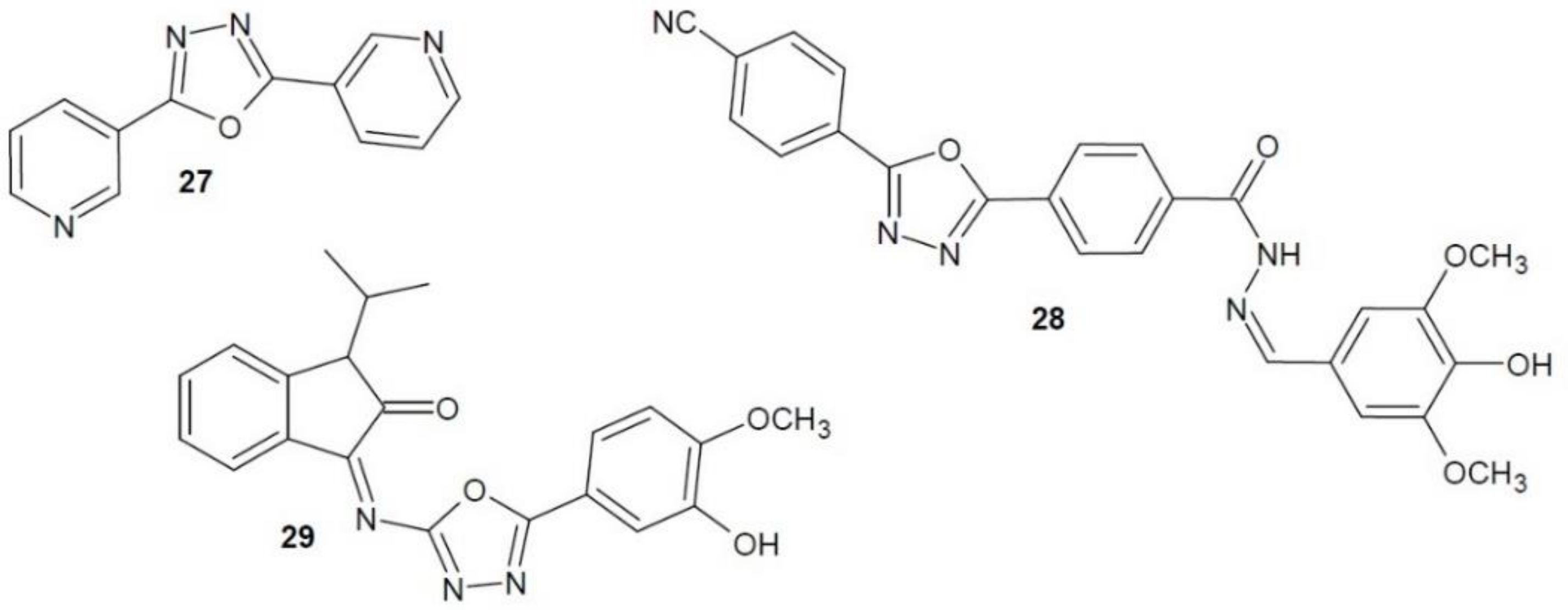
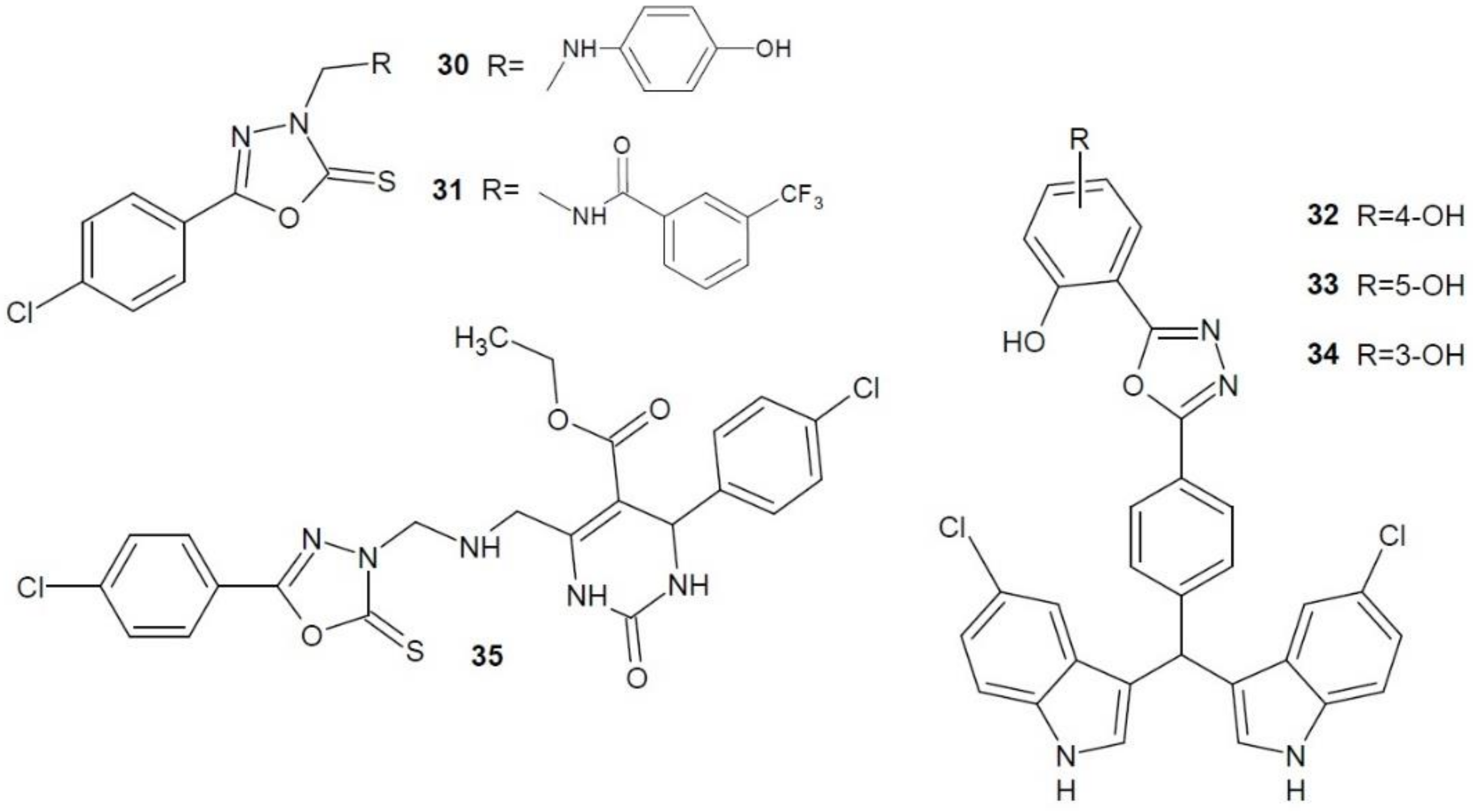
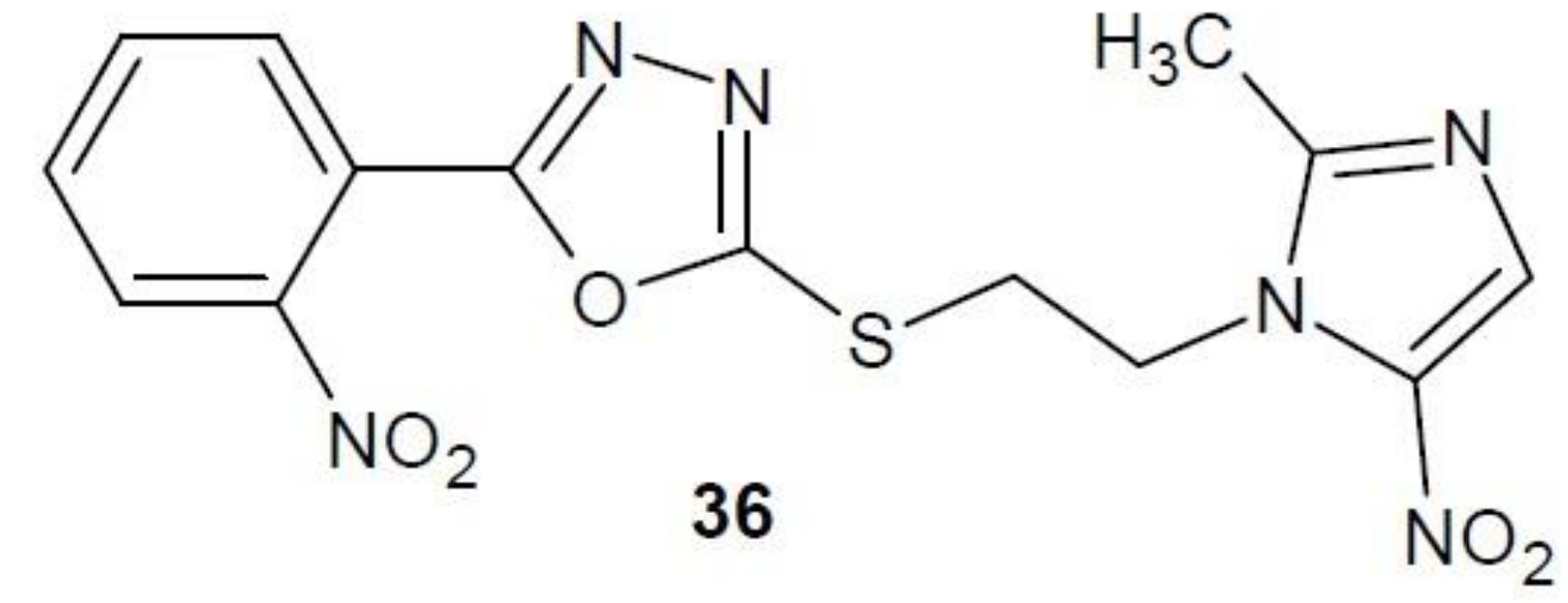
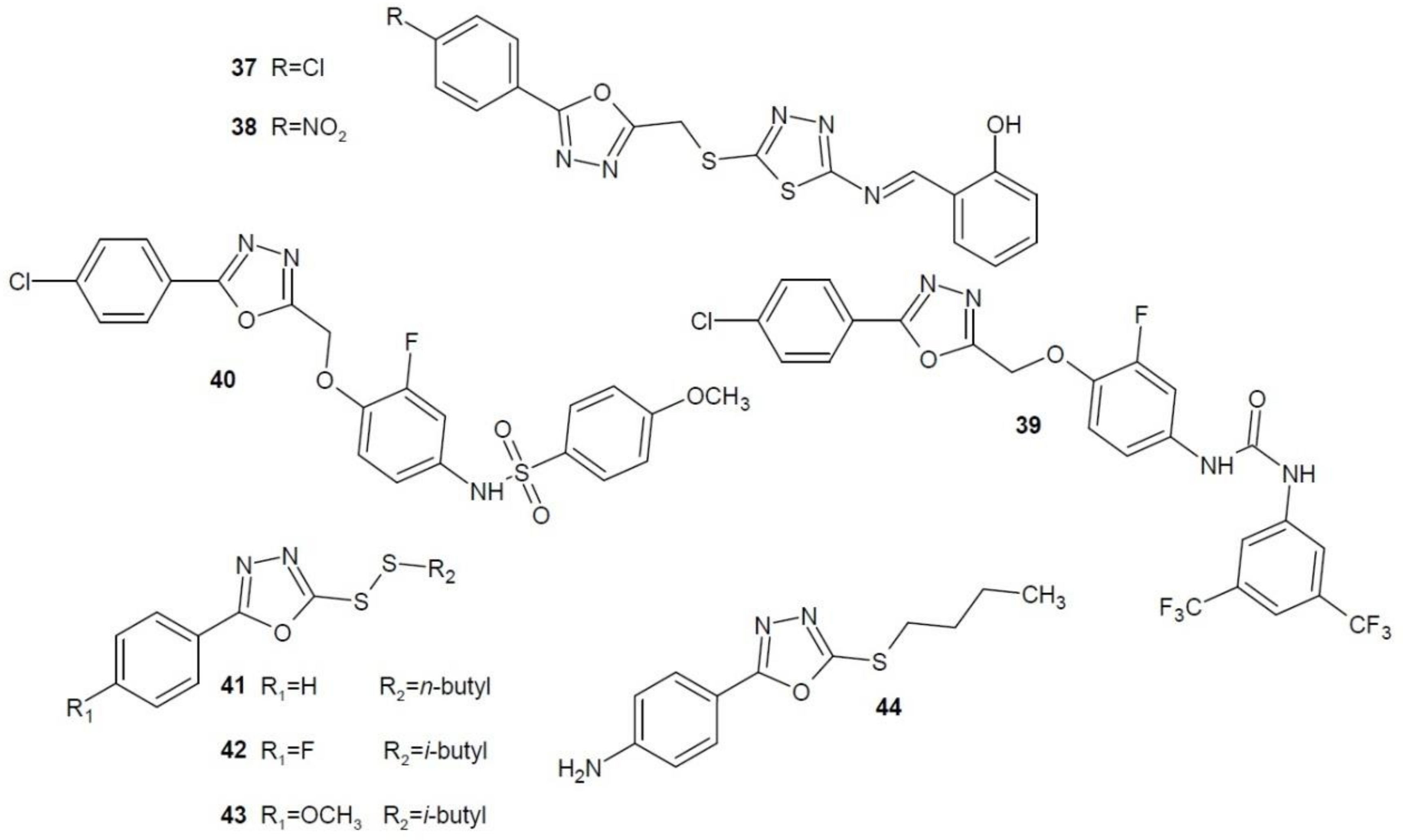
2.12. 1,3,4-Oxadiazole Derivatives with Unknown Anti-Cancer Mechanism of Action
3. Summary
Funding
Conflicts of Interest
References
- World Health Organization. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 15 November 2018).
- Mutschler, E.; Geisslinger, G.; Kroemer, H.K.; Ruth, P.; Schaefer-Korting, M. Mutschler Farmakologia I Toksykologia, Wydanie II; MedPharm Polska: Wrocław, Poland, 2010; pp. 945–987. ISBN 978-83-60466-81-0. [Google Scholar]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Joule, J.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons: Hoboken, New Jersey, USA, 2010; pp. 569–574. ISBN 978-1-405-19365-8. [Google Scholar]
- Boström, J.; Hogner, A.; Llinàs, A.; Wellner, E.; Plowright, A.T. Oxadiazoles in Medicinal Chemistry. J. Med. Chem. 2012, 55, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.N.; Sadiq, B.; Al-Masoudi, N.A.; Yasin, K.A.; Hameed, S.; Mahmood, T.; Ayub, K.; Tahir, M.N. Synthesis, Crystal Structures, Computational Studies and Antimicrobial Activity of New Designed Bis((5-Aryl-1,3,4-Oxadiazol-2-Yl)Thio)Alkanes. J. Mol. Struct. 2018, 1155, 403–413. [Google Scholar] [CrossRef]
- Verma, G.; Chashoo, G.; Ali, A.; Khan, M.F.; Akhtar, W.; Ali, I.; Akhtar, M.; Alam, M.M.; Shaquiquzzaman, M. Synthesis of Pyrazole Acrylic Acid Based Oxadiazole and Amide Derivatives as Antimalarial and Anticancer Agents. Bioorg. Chem. 2018, 77, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ellah, H.S.; Abdel-Aziz, M.; Shoman, M.E.; Beshr, E.A.M.; Kaoud, T.S.; Ahmed, A.S.F.F. New 1,3,4-Oxadiazole/Oxime Hybrids: Design, Synthesis, Anti-Inflammatory, COX Inhibitory Activities and Ulcerogenic Liability. Bioorg. Chem. 2017, 74, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Tantray, M.A.; Khan, I.; Hamid, H.; Alam, M.S.; Dhulap, A.; Kalam, A. Synthesis of Benzimidazole-Linked-1,3,4-Oxadiazole Carboxamides as GSK-3β Inhibitors with in Vivo Antidepressant Activity. Bioorg. Chem. 2018, 77, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Yadagiri, B.; Gurrala, S.; Bantu, R.; Nagarapu, L.; Polepalli, S.; Srujana, G.; Jain, N. Synthesis and Evaluation of Benzosuberone Embedded with 1,3,4-Oxadiazole, 1,3,4-Thiadiazole and 1,2,4-Triazole Moieties as New Potential Anti Proliferative Agents. Bioorganic Med. Chem. Lett. 2015, 25, 2220–2224. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, K.; Poojary, B.; Lobo, P.L.; Fernandes, J.; Kumari, N.S. Synthesis and Biological Evaluation of Some 1,3,4-Oxadiazole Derivatives. Eur. J. Med. Chem. 2010, 45, 5225–5233. [Google Scholar] [CrossRef] [PubMed]
- Hajimahdi, Z.; Zarghi, A.; Zabihollahi, R.; Aghasadeghi, M.R. Synthesis, Biological Evaluation, and Molecular Modeling Studies of New 1,3,4-Oxadiazole- and 1,3,4-Thiadiazole-Substituted 4-Oxo-4H-Pyrido[1-a] Pyrimidines as Anti-HIV-1 Agents. Med. Chem. Res. 2013, 22, 2467–2475. [Google Scholar] [CrossRef]
- Xu, W.-M.; Li, S.-Z.; He, M.; Yang, S.; Li, X.-Y.; Li, P. Synthesis and Bioactivities of Novel Thioether/Sulfone Derivatives Containing 1,2,3-Thiadiazole and 1,3,4-Oxadiazole/Thiadiazole Moiety. Bioorg. Med. Chem. Lett. 2013, 23, 5821–5824. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz, H.; Gavai, A.V.; Fink, B.; Struzynski, C.; Tarrant, J.; Vite, G.D.; Wong, T.W.; Zhang, G.; Vyas, D.M. Pyrrolotriazine-5-Carboxylate Ester Inhibitors of EGFR and HER2 Protein Tyrosine Kinases and a Novel One-Pot Synthesis of C-4 Subsitituted Pyrrole-2,3-Dicarboxylate Diesters. Can. J. Chem. 2006, 84, 528–533. [Google Scholar] [CrossRef]
- Bakr, R.B.; Abdelall, E.K.A.; Abdel-Hamid, M.K.; Kandeel, M.M. Design and Synthesis of New EGFR-Tyrosine Kinase Inhibitors Containing Pyrazolo[3,4-d]Pyrimidine Cores as Anticancer Agents. Bull. Pharm. Sci. 2012, 35, 27–42. [Google Scholar]
- Yarden, Y. The EGFR Family and Its Ligands in Human Cancer. Eur. J. Cancer 2001, 37, 3–8. [Google Scholar] [CrossRef]
- Abou-Seri, S.M. Synthesis and Biological Evaluation of Novel 2,4′-Bis Substituted Diphenylamines as Anticancer Agents and Potential Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. Eur. J. Med. Chem. 2010, 45, 4113–4121. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Siddiqui, A.A.; Khan, A.A.; Ali, Z.; Dewangan, R.P.; Pasha, S.; Yar, M.S. Design, Synthesis, Docking and QSAR Study of Substituted Benzimidazole Linked Oxadiazole as Cytotoxic Agents, EGFR and ErbB2 Receptor Inhibitors. Eur. J. Med. Chem. 2017, 126, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, F.; Radi, M.; Brullo, C.; Schenone, S. Vascular Endothelial Growth Factor (VEGF) Receptors: Drugs and New Inhibitors. J. Med. Chem. 2012, 55, 10797–10822. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.W.; Wei, D.; Borzilleri, R.M.; Qian, L.; Kamath, A.; Mortillo, S.; Wautlet, B.; Henley, B.J.; Jeyaseelan, R.; Tokarski, J.; et al. Synthesis, SAR, and Evaluation of 4-[2,4-Difluoro-5-(Cyclopropylcarbamoyl)Phenylamino]Pyrrolo[2,1-f][1,2,4]Triazine-Based VEGFR-2 Kinase Inhibitors. Bioorganic Med. Chem. Lett. 2008, 18, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Ruel, R.; Thibeault, C.; L’Heureux, A.; Martel, A.; Cai, Z.W.; Wei, D.; Qian, L.; Barrish, J.C.; Mathur, A.; D’Arienzo, C.; et al. Discovery and Preclinical Studies of 5-Isopropyl-6-(5-Methyl-1,3,4-Oxadiazol-2-Yl)-N-(2-Methyl-1H-Pyrrolo[2,3-b]Pyridin-5-Yl)Pyrrolo[2,1-f][1,2,4]Triazin-4-Amine (BMS-645737), an in Vivo Active Potent VEGFR-2 Inhibitor. Bioorganic Med. Chem. Lett. 2008, 18, 2985–2989. [Google Scholar] [CrossRef] [PubMed]
- Bhanushali, U.; Kalekar-Joshi, S.; Kulkarni-Munshi, R.; Yellanki, S.; Medishetty, R.; Kulkarni, P.; Chelakara, R.S. Design, Synthesis and Evaluation of 5-Pyridin-4-Yl-2-Thioxo-[1,3,4]Oxadiazol-3-Yl Derivatives as Anti-Angiogenic Agents Targeting VEGFR-2. Anticancer Agents Med. Chem. 2017, 17, 67–74. [Google Scholar] [PubMed]
- Warren, R.; Liu, G. ZD4054: A Specific Endothelin A Receptor Antagonist with Promising Activity in Metastatic Castration-Resistant Prostate Cancer. Expert Opin. Investig. Drugs 2008, 17, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.-U.; Dashwood, M.R.; Heetun, M.; Shiwen, X.; Farooqui, N.; Ramesh, B.; Welch, H.; Savage, F.J.; Ogunbiyi, O.; Abraham, D.J.; et al. Efficacy of the Specific Endothelin A Receptor Antagonist Zibotentan (ZD4054) in Colorectal Cancer: A Preclinical Study. Mol. Cancer Ther. 2013, 12, 1556–1567. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shao, N.; Shen, Z.X.; Li, Q.; Wang, Y.; Li, C.; Ma, G.; Dong, J.; Lu, X.J.; Feng, N.H. The Efficacy and Safety of Zibotentan in the Treatment of Castration-Resistant Prostate Cancer: A Meta-Analysis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3291–3296. [Google Scholar] [PubMed]
- Pharmafile. Available online: http://www.pharmafile.com/news/147640/phase-iii-failure-astrazeneca-prostate-cancer-zibotentan (accessed on 22 November 2018).
- Lee, B.Y.; Timpson, P.; Horvath, L.G.; Daly, R.J. FAK Signaling in Human Cancer as a Target for Therapeutics. Pharmacol. Ther. 2015, 146, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ren, S.Z.; Lu, X.Y.; Li, J.J.; Shen, F.Q.; Xu, C.; Zhu, H.L. Discovery of a Series of 1,3,4-Oxadiazole-2(3H)-Thione Derivatives Containing Piperazine Skeleton as Potential FAK Inhibitors. Bioorganic Med. Chem. 2017, 25, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Sever, B.; Akalın Çiftçi, G.; Turan-Zitouni, G.; Kaplancıklı, Z.A.; Özdemir, A. Design, Synthesis, in Vitro and in Silico Evaluation of a New Series of Oxadiazole-Based Anticancer Agents as Potential Akt and FAK Inhibitors. Eur. J. Med. Chem. 2018, 155, 905–924. [Google Scholar] [CrossRef] [PubMed]
- Valente, S.; Trisciuoglio, D.; De Luca, T.; Nebbioso, A.; Labella, D.; Lenoci, A.; Bigogno, C.; Dondio, G.; Miceli, M.; Brosch, G.; et al. 1,3,4-Oxadiazole-Containing Histone Deacetylase Inhibitors: Anticancer Activities in Cancer Cells. J. Med. Chem. 2014, 57, 6259–6265. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, S.; Chini, M.G.; Terracciano, S.; Bruno, I.; Riccio, R.; Bifulco, G. Structural Basis for the Design and Synthesis of Selective HDAC Inhibitors. Bioorganic Med. Chem. 2013, 21, 3795–3807. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.L.; Hazzalin, C.A.; Mahadevan, L.C. Enhanced Histone Acetylation and Transcription: A Dynamic Perspective. Mol. Cell. 2006, 23, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Pidugu, V.R.; Yarla, N.S.; Pedada, S.R.; Kalle, A.M.; Satya, A.K. Design and Synthesis of Novel HDAC8 Inhibitory 2,5-Disubstituted-1,3,4-Oxadiazoles Containing Glycine and Alanine Hybrids with Anti Cancer Activity. Bioorganic Med. Chem. 2016, 24, 5611–5617. [Google Scholar] [CrossRef] [PubMed]
- Pidugu, V.R.; Yarla, N.S.; Bishayee, A.; Kalle, A.M.; Satya, A.K. Novel Histone Deacetylase 8-Selective Inhibitor 1,3,4-Oxadiazole-Alanine Hybrid Induces Apoptosis in Breast Cancer Cells. Apoptosis 2017, 22, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, P.; Lakshmikuttyamma, A.; Dimmock, J.R.; Sharma, R.K. Methionine Aminopeptidase 2 and Cancer. Biochim. Biophys. Acta Rev. Cancer 2006, 1765, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, M.H.; Qian, S.S.; Guo, F.J.; Dang, X.F.; Wang, X.M.; Xue, Y.R.; Zhu, H.L. Synthesis and Antitumor Activity of 1,3,4-Oxadiazole Possessing 1,4-Benzodioxan Moiety as a Novel Class of Potent Methionine Aminopeptidase Type II Inhibitors. Bioorganic Med. Chem. Lett. 2013, 23, 2876–2879. [Google Scholar] [CrossRef] [PubMed]
- Spiros, A.V. Aberrant Control of NF-ΚB in Cancer Permits Transcriptional and Phenotypic Plasticity, to Curtail Dependence on Host Tissue: Molecular Mode. Cancer Biol. Med. 2017, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Chai, E.Z.P.; Siveen, K.S.; Shanmugam, M.K.; Arfuso, F.; Sethi, G. Analysis of the Intricate Relationship between Chronic Inflammation and Cancer. Biochem. J. 2015, 468, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Puar, Y.; Shanmugam, M.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the Involvement of the Master Transcription Factor NF-ΚB in Cancer Initiation and Progression. Biomedicines 2018, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sethi, G. Targeting Transcription Factor NF-ΚB to Overcome Chemoresistance and Radioresistance in Cancer Therapy. Biochim. Biophys. Acta Rev. Cancer 2010, 1805, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. NF-ΚB in Cancer Therapy. Arch. Toxicol. 2015, 89, 711–731. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Anilkumar, N.C.; Rangappa, S.; Shanmugam, M.K.; Mishra, S.; Chinnathambi, A.; Alharbi, S.A.; Bhattacharjee, A.; Sethi, G.; Kumar, A.P.; et al. Novel 1,3,4-Oxadiazole Induces Anticancer Activity by Targeting NF-ΚB in Hepatocellular Carcinoma Cells. Front. Oncol. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, C.; Li, F.; Guan, D.; Wu, X.; Fu, X.; Lu, A.; Zhang, G. PARP1 in Carcinomas and PARP1 Inhibitors as Antineoplastic Drugs. Int. J. Mol. Sci. 2017, 18, 2111. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Kumar, P.; Chhikara, A.; Chopra, M. Development of 1,3,4-Oxadiazole Thione Based Novel Anticancer Agents: Design, Synthesis and in-Vitro Studies. Biomed. Pharmacother. 2017, 95, 721–730. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, X.Y.; Liang, J.W.; Cao, C.; Li, S.; Zhang, T.J.; Meng, F.H. Design, Synthesis and Anticancer Activities Evaluation of Novel 5H-Dibenzo[b,e]Azepine-6,11-Dione Derivatives Containing 1,3,4-Oxadiazole Units. Bioorganic Med. Chem. Lett. 2018, 28, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Cohn, E.P.M.T.; Wu, K.L.; Pettus, T.R.R.; Reich, N.O. A New Strategy for Detection and Development of Tractable Telomerase Inhibitors. J. Med. Chem. 2012, 55, 3678–3686. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific Association of Human Telomerase Activity with Immortal Cells and Cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Wang, X.L.; Liu, W.; Yang, Y.S.; Tang, J.F.; Zhu, H.L. Design, Synthesis and Biological Evaluation of Heterocyclic Azoles Derivatives Containing Pyrazine Moiety as Potential Telomerase Inhibitors. Bioorganic Med. Chem. 2012, 20, 6356–6365. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhu, H.; Yang, Z.M.; Zhu, H.L. Synthesis, Molecular Modeling and Biological Evaluation of 2-Aminomethyl-5-(Quinolin-2-Yl)-1,3,4-Oxadiazole-2(3H)-Thione Quinolone Derivatives as Novel Anticancer Agent. Eur. J. Med. Chem. 2013, 60, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, X.L.; Shi, J.; Wang, S.F.; Yin, Y.; Yang, Y.S.; Zhang, W.M.; Zhu, H.L. Synthesis, Molecular Modeling and Biological Evaluation of N-Benzylidene-2-((5-(Pyridin-4-Yl)-1,3,4-Oxadiazol-2-Yl)Thio)Acetohydrazide Derivatives as Potential Anticancer Agents. Bioorganic Med. Chem. 2014, 22, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, S.; Nitanda, T.; Furukawa, T.; Sumizawa, T.; Tani, A.; Nishimoto, K.; Akiba, S.; Miyadera, K.; Fukushima, M.; Yamada, Y.; et al. The Effect of a Thymidine Phosphorylase Inhibitor on Angiogenesis and Apoptosis in Tumors. Cancer Res. 1999, 59, 1911–1916. [Google Scholar] [PubMed]
- Khan, K.M.; Rani, M.; Ambreen, N.; Ali, M.; Hussain, S.; Perveen, S.; Choudhary, M.I. 2,5-Disubstituted-1,3,4-Oxadiazoles: Thymidine Phosphorylase Inhibitors. Med. Chem. Res. 2013, 22, 6022–6028. [Google Scholar] [CrossRef]
- Ullah, H.; Rahim, F.; Taha, M.; Uddin, I.; Wadood, A.; Shah, S.A.A.; Farooq, R.K.; Nawaz, M.; Wahab, Z.; Khan, K.M. Synthesis, Molecular Docking Study and in Vitro Thymidine Phosphorylase Inhibitory Potential of Oxadiazole Derivatives. Bioorg. Chem. 2018, 78, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Javid, M.; Rahim, F.; Taha, M.; Nawaz, M.; Wadood, A.; Ali, M.; Mosaddik, A.; Shah, S.A.A.; Farooq, R.K. Sythesis, SAR Elucidations and Molecular Docking Study of Newly Designed Isatin Based Oxadiazol Analogs as Potent Inhibitors of Thymidine Phosphorylase. Bioorg. Chem. 2018, 79, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Roy, P.P.; Singh, J. Synthesis, Thymidine Phosphorylase Inhibitory and Computational Study of Novel 1,3,4-Oxadiazole-2-Thione Derivatives as Potential Anticancer Agents. Comput. Biol. Chem. 2018, 76, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Rashid, U.; Imran, S.; Ali, M. Rational Design of Bis-Indolylmethane-Oxadiazole Hybrids as Inhibitors of Thymidine Phosphorylase. Bioorganic Med. Chem. 2018, 26, 3654–3663. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, F.; Yaqoob, F.; Tabassum, N.; Jan, M.S.; Sadiq, A.; Tahir, S.; Batool, T.; Niaz, B.; Ansari, F.L.; Choudhary, M.I.; et al. Design, Synthesis, in-Vitro Thymidine Phosphorylase Inhibition, in-Vivo Antiangiogenic and in-Silico Studies of C-6 Substituted Dihydropyrimidines. Bioorg. Chem. 2018, 80, 99–111. [Google Scholar] [CrossRef] [PubMed]
- El-Mesallamy, H.O.; El Magdoub, H.M.; Chapman, J.M.; Hamdy, N.M.; Schaalan, M.F.; Hammad, L.N.; Berger, S.H. Biomolecular Study of Human Thymidylate Synthase Conformer-Selective Inhibitors: New Chemotherapeutic Approach. PLoS ONE 2018, 13, e0193810. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Liang, J.W.; Mohamed, O.K.; Zhang, T.J.; Lu, G.Q.; Meng, F.H. Design, Synthesis and Biological Evaluation of N-Phenyl-(2,4-Dihydroxypyrimidine-5-Sulfonamido)Benzoyl Hydrazide Derivatives as Thymidylate Synthase (TS) Inhibitors and as Potential Antitumor Drugs. Eur. J. Med. Chem. 2018, 154, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.R.; Li, D.D.; Pi, Y.Z.; Li, J.R.; Sun, J.; Fang, F.; Zhong, W.Q.; Gong, H.B.; Zhu, H.L. Novel 1,3,4-Oxadiazole Thioether Derivatives Targeting Thymidylate Synthase as Dual Anticancer/Antimicrobial Agents. Bioorganic Med. Chem. 2013, 21, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, P.; Xuan, L.N.; Fu, X.Y.; Jing, F.; Li, S.; Liu, Y.M.; Chen, B.Q. Synthesis and Antitumor Activities of Novel Hybrid Molecules Containing 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Bearing Schiff Base Moiety. Bioorganic Med. Chem. Lett. 2014, 24, 5154–5156. [Google Scholar] [CrossRef] [PubMed]
- Gamal El-Din, M.M.; El-Gamal, M.I.; Abdel-Maksoud, M.S.; Yoo, K.H.; Oh, C.H. Synthesis and Broad-Spectrum Antiproliferative Activity of Diarylamides and Diarylureas Possessing 1,3,4-Oxadiazole Derivatives. Bioorganic Med. Chem. Lett. 2015, 25, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Gamal El-Din, M.M.; El-Gamal, M.I.; Abdel-Maksoud, M.S.; Yoo, K.H.; Oh, C.-H. Synthesis and in Vitro Antiproliferative Activity of New 1,3,4-Oxadiazole Derivatives Possessing Sulfonamide Moiety. Eur. J. Med. Chem. 2015, 90, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-J.; Wang, X.-F.; Li, B.-L.; Zhang, R.-L.; Li, B.; Liu, Y.-M.; Li, C.-W.; Liu, J.-B.; Chen, B.-Q. Synthesis and in Vitro Antiproliferative Evaluation of Novel Nonsymmetrical Disulfides Bearing 1,3,4-Oxadiazole Moiety. Bioorg. Med. Chem. Lett. 2016, 26, 4414–4416. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ghosh, R.; Gilda, J.E.; Gomes, A.V. The Necessity of and Strategies for Improving Confidence in the Accuracy of Western Blots. Expert Rev. Proteomics 2014, 11, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Khanam, R.; Ahmad, K.; Hejazi, I.I.; Siddique, I.A.; Kumar, V.; Bhat, A.R.; Azam, A.; Athar, F. Inhibitory Growth Evaluation and Apoptosis Induction in MCF-7 Cancer Cells by New 5-Aryl-2-Butylthio-1,3,4-Oxadiazole Derivatives. Cancer Chemother. Pharmacol. 2017, 80, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glomb, T.; Szymankiewicz, K.; Świątek, P. Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole. Molecules 2018, 23, 3361. https://doi.org/10.3390/molecules23123361
Glomb T, Szymankiewicz K, Świątek P. Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole. Molecules. 2018; 23(12):3361. https://doi.org/10.3390/molecules23123361
Chicago/Turabian StyleGlomb, Teresa, Karolina Szymankiewicz, and Piotr Świątek. 2018. "Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole" Molecules 23, no. 12: 3361. https://doi.org/10.3390/molecules23123361
APA StyleGlomb, T., Szymankiewicz, K., & Świątek, P. (2018). Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole. Molecules, 23(12), 3361. https://doi.org/10.3390/molecules23123361




