Metabolomics Research Reveals the Mechanism of Action of Astragalus Polysaccharide in Rats with Digestive System Disorders
Abstract
1. Introduction
2. Results
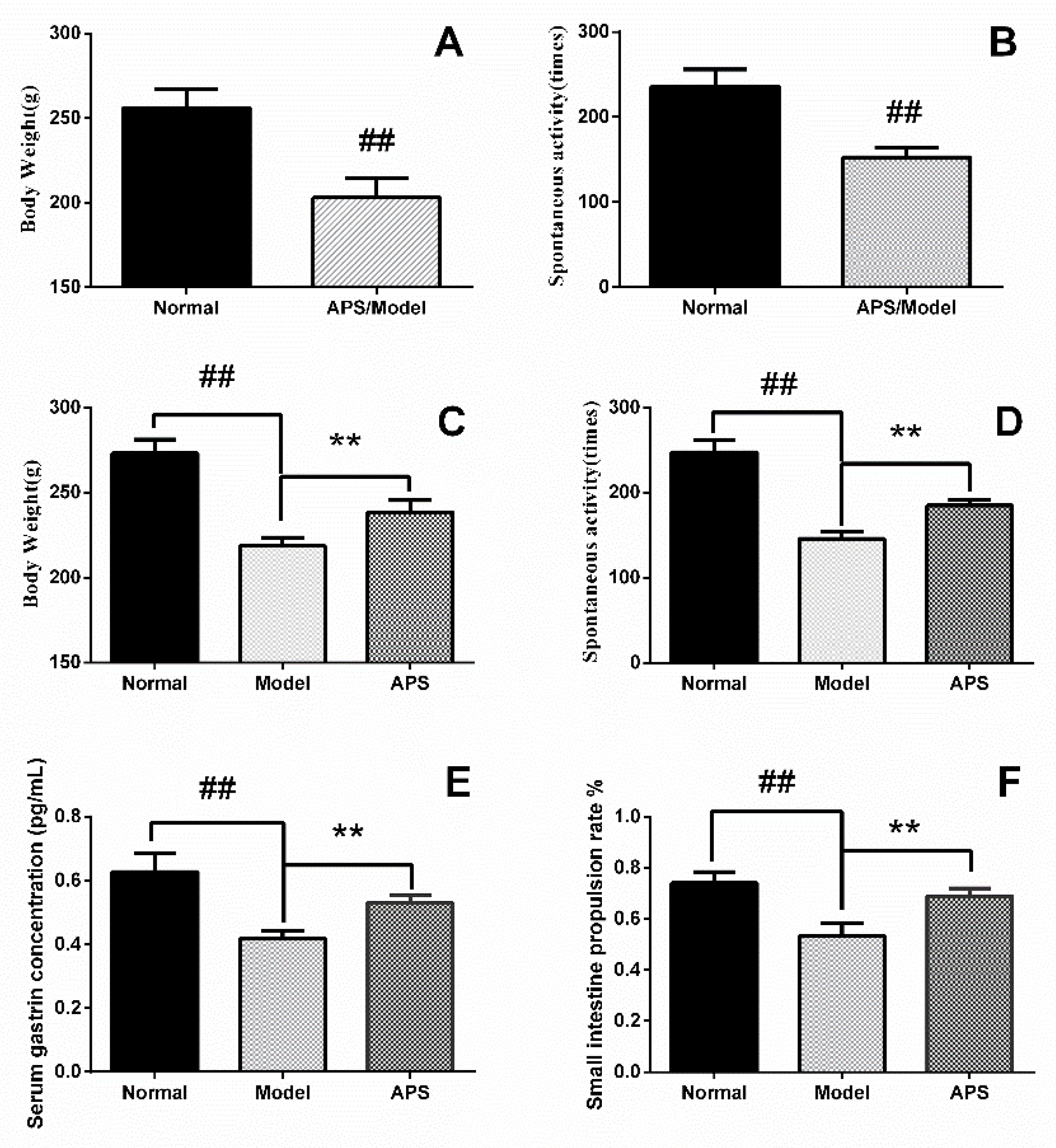
2.1. Animal Model of DSD
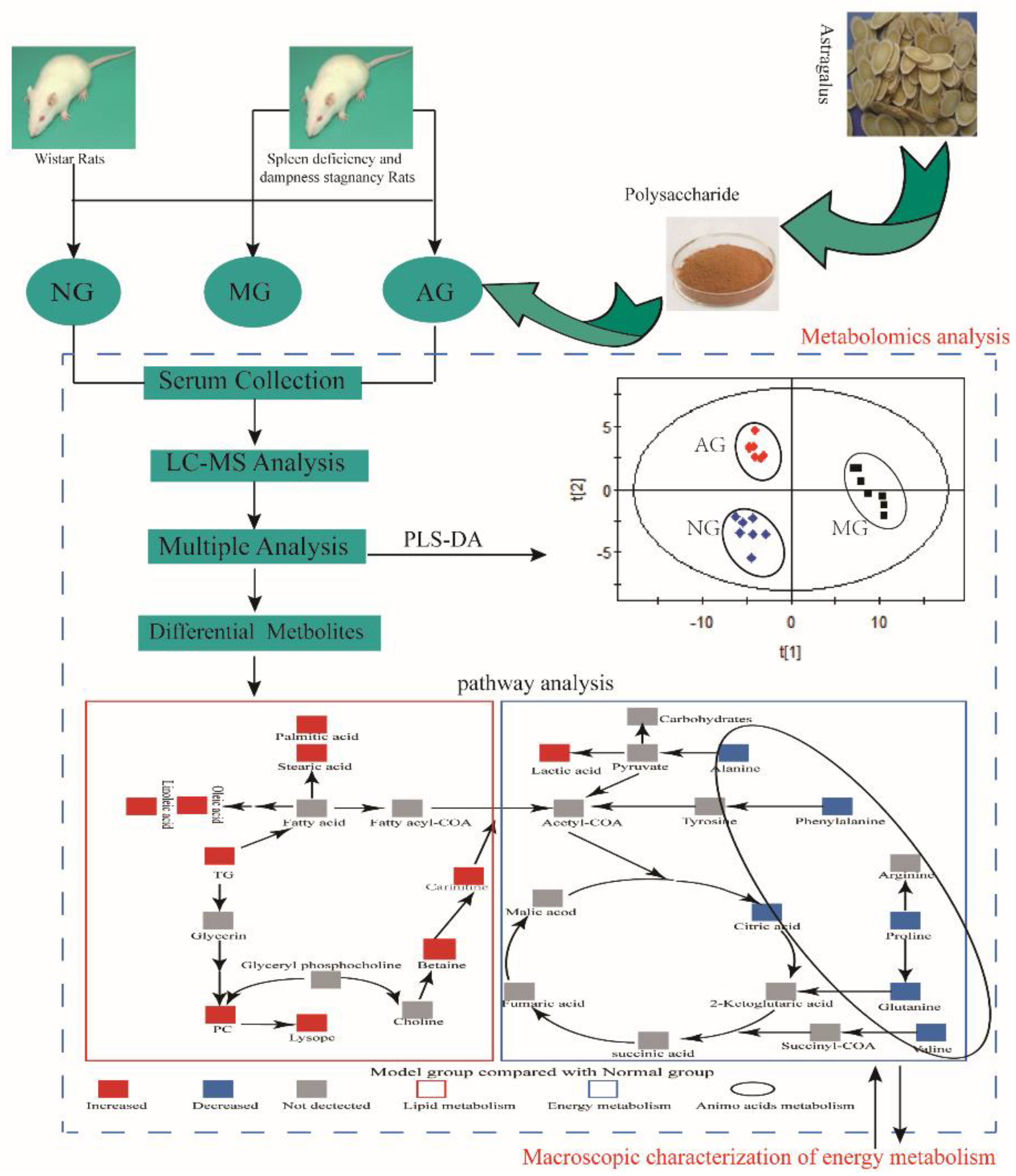
2.2. Multivariate Statistical Analysis of Metabolomics Data
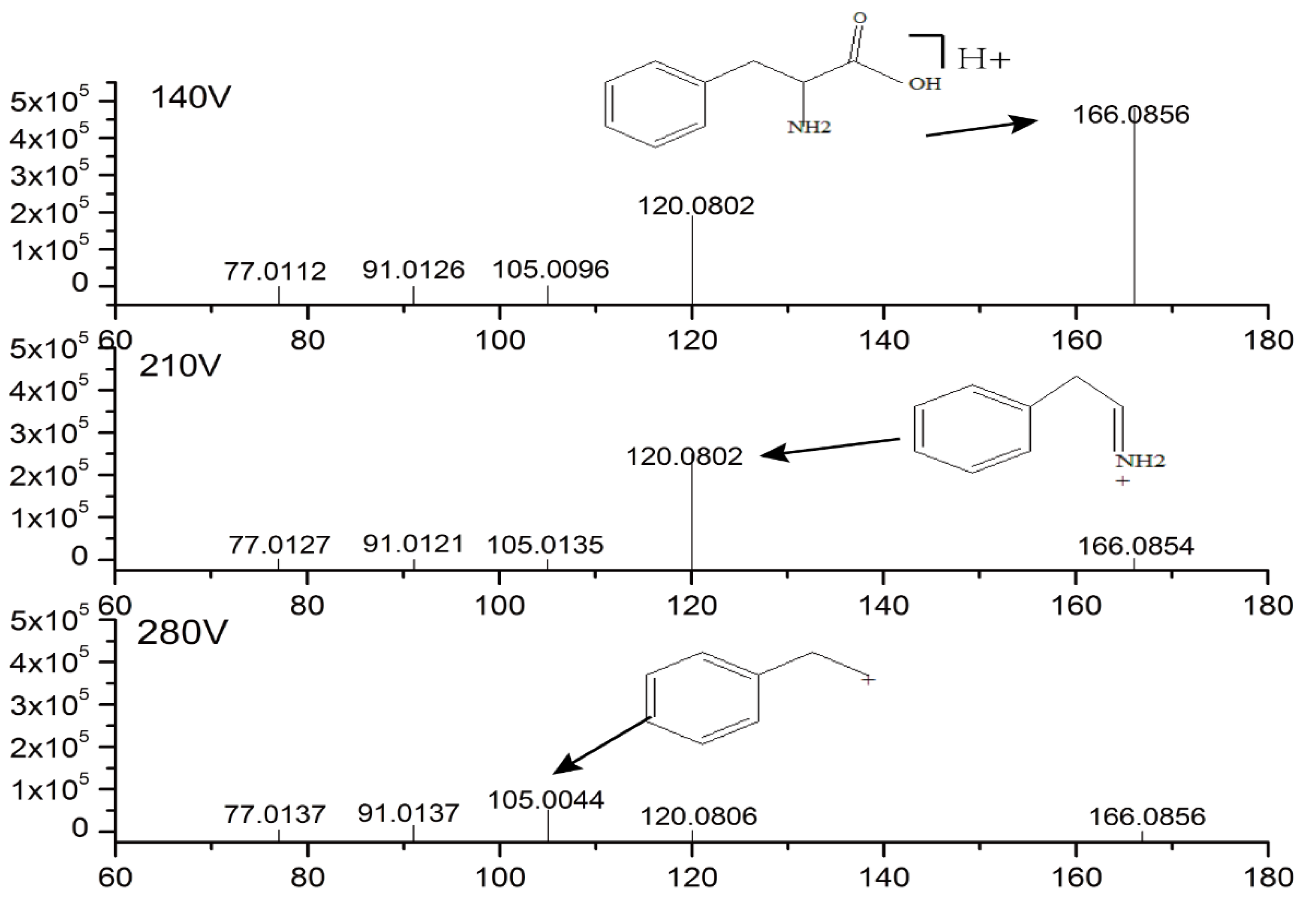
2.3. Identification of Potential Biomarkers
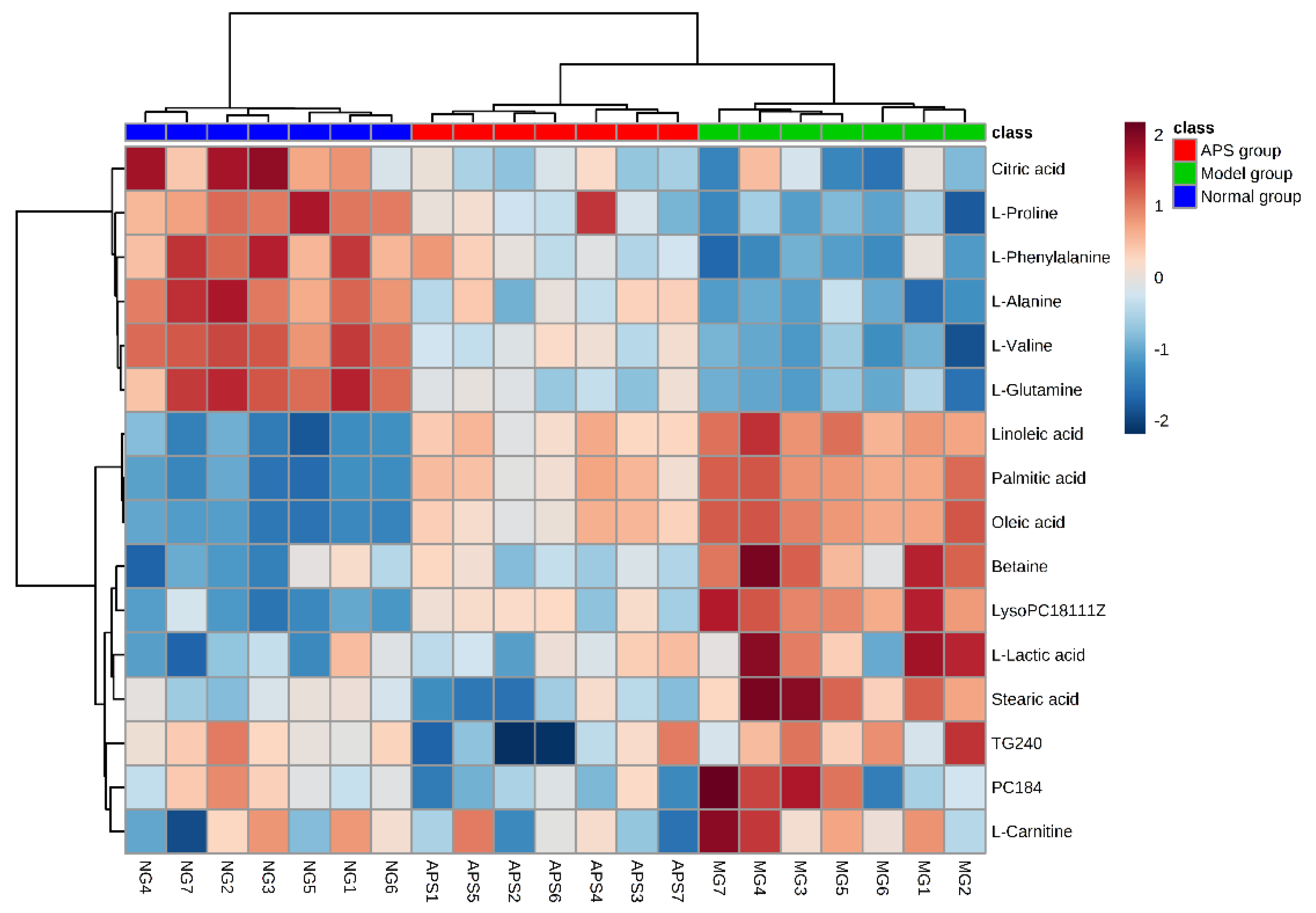
2.4. Heat Map Analysis
3. Discussion
3.1. Energy Metabolism
3.2. Amino Acid Metabolism
3.3. Lipid Metabolism
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of APS
4.3. Animal Experiment
4.4. Behavior Detection
4.5. Collection of Serum Samples
4.6. Serum Gastrin Concentration
4.7. Small Intestinal Propulsion Rate
4.8. Preparation of Serum Samples for Metabolomics
4.9. HPLC–TOF/MS Analysis
4.10. Data Handling
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chapman, M.J.; Nguyen, N.Q.; Deane, A.M. Gastrointestinal dysmotility: Evidence and clinical management. Curr. Opin. Clin. Nutr. Metab. Care. 2013, 16, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, E. Gastrointestinal dysfunction in liver cirrhosis. World J. Gastroenterol. 2014, 20, 14686–14695. [Google Scholar] [CrossRef] [PubMed]
- Lo Cascio, C.M.; Goetze, O.; Latshang, T.D.; Bluemel, S.; Frauenfelder, T.; Bloch, K.E. Gastrointestinal Dysfunction in Patients with Duchenne Muscular Dystrophy. PLoS ONE. 2016, 11, e0163779. [Google Scholar] [CrossRef] [PubMed]
- Kukla, U.; Łabuzek, K.; Chronowska, J.; Krzystanek, M.; Okopień, B. Mental disorders in digestive system diseases internist’s and psychiatrist’s insight. Pol. Merkur. Lekarski 2015, 38, 245–249. [Google Scholar] [PubMed]
- Peery, A.F.; Dellon, E.S.; Lund, J.; Crockett, S.D.; Mcgowan, C.E.; Bulsiewicz, W.J.; Gangarosa, L.M.; Thiny, M.T.; Stizenberg, K.; Morgan, D.R. Burden of gastrointestinal disease in the united states: 2012 update. Gastroenterology 2012, 143, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.R.; Choudhury, M.D.; Ningthoujam, S.S.; Mitra, A.; Nath, D.; Talukdar, A.D. Plant utilization against digestive system disorder in Southern Assam, India. J. Ethnopharmacol. 2015, 175, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Inês, P.; Joao Vasco, S.; Mário, D.R.; Alberto, F. Burden of digestive diseases in portugal: Trends in hospitalizations between 2000 and 2010. Eur. J. Gastroenterol. Hepatol. 2015, 27, 279–289. [Google Scholar]
- Cao, Y.; Ruan, Y.; Shen, T.; Huang, X.; Li, M.; Yu, W.; Zhu, Y.; Man, Y.; Wang, S.; Li, J. Astragalus polysaccharide suppresses doxorubicin-induced cardiotoxicity by regulating the PI3k/Akt and p38MAPK pathways. Oxid. Med. Cell. Longev. 2014, 2014, 674219. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Yang, Y.; Li, D.; Lv, J.; Ou, Y.; Sun, F.; Chen, J.; Shi, Y.; Xia, P. Astragalus polysaccharide ameliorates ionizing radiation-induced oxidative stress in mice. Int. J. Biol. Macromol. 2014, 68, 209–214. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Li, Y.; Wang, F.; Yang, X.; Yao, J. Sulfated Astragalus polysaccharide can regulate the inflammatory reaction induced by LPS in Caco2 cells. Int. J. Biol. Macromol. 2013, 60, 248–252. [Google Scholar] [CrossRef]
- Tu, S.; Shao, A.; Ren, L.; Chen, T.; Yao, D. Angiogenesis effect of Astragalus polysaccharide combined with endothelial progenitor cells therapy in diabetic male rat following experimental hind limb ischemia. Chin. Med. J. 2014, 127, 2121–2228. [Google Scholar] [PubMed]
- Yang, B.; Xiao, B.; Sun, T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, Y.; Tian, Z.; Liu, F.; Shi, Y.; Liu, Y.; Xia, P. Astragalus polysaccharides protect against dextran sulfate sodium-induced colitis by inhibiting NF-kappacapital VE. Int. J. Biol. Macromol. 2017, 98, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liu, Y.; Yang, B.; Zhang, J.; He, H.; Ge, H.; Wu, Y.; Shen, Z. Astagalus polysaccharide attenuates murine colitis through inhibiton of the NLRP3 inflammasome. Planta Med. 2017, 83, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, S.; Zhou, J.; Wang, H.; Fu, R.; Wu, X.; Wang, J.; Lu, F. Effect of Astragalus polysaccharides on chronic atrophic gastritis induced by N-methyl-N’-nitro-N-nitrosoguanidine in rats. Drug Res. 2013, 63, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.G.; Liu, Y.L.; Yin, Y.L.; Kong, X.F.; Huang, R.L.; Li, T.J.; Wu, G.Y.; Hou, Y. Dietary supplementation with Astragalus polysaccharide enhances ileal digestibilities and serum concentrations of amino acids in early weaned piglets. Amino Acids 2009, 37, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Raamsdonk, L.M.; Teusink, B.; Broadhurst, D.; Zhang, N.; Hayes, A.; Walsh, M.C.; Berden, J.A.; Brindle, K.M.; Kell, D.B.; Rowland, J.J. A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nat. Biotechnol. 2001, 19, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.K.; Joo Ern, A.; Revell, V.L.; Ben, H.; Anuska, M.; Robertson, F.P.; Nanyi, C.; Benita, M.; Katrin, A.; Manfred, K. Effect of sleep deprivation on the human metabolome. Proc. Nat. Acad. Sci. U S A 2014, 111, 10761–10766. [Google Scholar] [CrossRef]
- Zhao, W.X.; Cui, N.; Jiang, H.Q.; Ji, X.M.; Han, X.C.; Han, B.B.; Wang, T.; Wang, S.J. Effects of radix astragali and Its split components on gene expression profiles related to water metabolism in rats with the dampness stagnancy due to spleen deficiency syndrome. Evid. Based Complement. Alternat. Med. 2017, 2017, 4946031. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, N.; Han, B.; Xuming, J.I.; Gao, J.; Han, X.; Wang, S. Effect of Astragalus membranaceus and its components on the rats model of spleen deficiency and water-dampness stagnancy. J. Shandong Univ. TCM. 2015, 39, 268–270. [Google Scholar]
- Pesi, R.; Balestri, F.; Ipata, P.L. Metabolic interaction between urea cycle and citric acid cycle shunt: A guided approach. Biochem. Mol. Biol. Educ. 2018, 46, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Feng, G.; Zhao, B.; Zhao, J.; Pi, Z.; Liu, S.; Song, F.; Liu, Z. A non-target urinary and serum metabolomics strategy reveals therapeutical mechanism of Radix Astragali on adjuvant-induced arthritis rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1048, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, H.; Chen, J.; Qian, Q. Lactic Acid: No Longer an Inert and End-Product of Glycolysis. Physiology 2017, 32, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Tunaru, S.; Tang, C.; Müller, M.; Gille, A.; Sassmann, A.; Hanson, J.; Offermanns, S. An autocrine lactate loop mediates insulin–dependent inhibition of lipolysis through GPR81. Cell MeTab. 2010, 11, 311–319. [Google Scholar] [CrossRef]
- Brooks, G.A. Cell-cell and intracellular lactate shuttles. J. Physiol. 2010, 587, 5591–5600. [Google Scholar] [CrossRef]
- Changlu, L.; Jiejun, W.; Jessica, Z.; Chester, K.; Jingxue, Y.; Jonathan, S.; Sutton, S.W.; Xiaorong, L.; Su Jin, Y.; Taraneh, M. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 2009, 284, 2811–2822. [Google Scholar]
- Tao, Y.; Chen, X.; Li, W.; Cai, B.; Di, L.; Shi, L.; Hu, L. Global and untargeted metabolomics evidence of the protective effect of different extracts of Dipsacus asper Wall. ex C.B. Clarke on estrogen deficiency after ovariectomia in rats. J. Ethnopharmacol. 2017, 199, 20–29. [Google Scholar] [CrossRef]
- Manoli, I.; Venditti, C.P. Disorders of branched chain amino acid metabolism. Transl. Sci. Rare Dis. 2016, 1, 91–110. [Google Scholar] [CrossRef]
- Cha, X.L.; Yao, L.B. Biochemistry and Molecular Biology, 8th ed.; People’s Medical Publishing House: Beijing, China, 2013. [Google Scholar]
- Katagiri, R.; Goto, A.; Budhathoki, S.; Yamaji, T.; Yamamoto, H.; Kato, Y.; Iwasaki, M.; Tsugane, S. Association between plasma concentrations of branched-chain amino acids and adipokines in Japanese adults without diabetes. Sci. Rep. 2018, 8, 1043. [Google Scholar] [CrossRef]
- Nakamura, H.; Jinzu, H.; Nagao, K.; Noguchi, Y.; Shimba, N.; Miyano, H.; Watanabe, T.; Iseki, K. Plasma amino acid profiles are associated with insulin, C-peptide and adiponectin levels in type 2 diabetic patients. Nutr. Diabetes 2014, 4, e133. [Google Scholar] [CrossRef]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Stinkens, R.; Goossens, G.H.; Jocken, J.W.E.; Blaak, E.E. Targeting fatty acid metabolism to improve glucose metabolism. Obes. Rev. 2015, 16, 715–757. [Google Scholar] [CrossRef] [PubMed]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Róg, T.; Vattulainen, I. Cholesterol, sphingolipids, and glycolipids: What do we know about their role in raft-like membranes? Chem. Phys. Lipids 2014, 184, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Gerd, S.; Katharina, R. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis 2010, 208, 10–18. [Google Scholar]
- Smith, C.A.; Want, E.J.; Grace, O.M.; Ruben, A.; Gary, S. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds astragalus polysaccharide, d-glucose, l-phenylalanine are available from the authors. |

| No | RT (min) | Exact Mass | Biomarkers | VIP | p-Value | Change Trend | FC | Pathway | ||
|---|---|---|---|---|---|---|---|---|---|---|
| M/N | T/M | N/M | T/M | |||||||
| 1 | 1.126 | 89.047 | L-Alanine | 1.250 | 8.37 × 10−6 | ↓ | ↑ | 4.59 | 2.90 | Alanine aspartate and glutamate metabolism |
| 2 | 1.054 | 90.031 | L-Lactic acid | 1.306 | 9.87 × 10−5 | ↑ | ↓ | 287 | 2.24 | Glycolytic pathway |
| 3 | 1.000 | 117.079 | L-Valine | 1.285 | 2.85 × 10−10 | ↓ | ↑ | 3.96 | 2.39 | Glycine leucine isoleucine degradation |
| 4 | 1.001 | 117.146 | Betaine | 1.178 | 2.03 × 10−8 | ↑ | ↓ | 4.27 | 3.01 | Fatty metabolism |
| 5 | 1.089 | 146.069 | L-Glutamine | 1.757 | 8.74 × 10−7 | ↓ | ↑ | 7.13 | 2.68 | Arginine biosynthesis purine metabolism |
| 6 | 1.297 | 165.079 | L-Phenylalanine | 1.825 | 2.28 × 10−5 | ↓ | ↑ | 3.60 | 2.09 | Phenylalanine metabolism |
| 7 | 1.290 | 192.123 | Citric acid | 1.493 | 0.0058 | ↓ | ↑ | 3.23 | 2.11 | Three-carboxylic acid cycle |
| 8 | 1.002 | 115.063 | L-Proline | 1.629 | 4.35 × 10−7 | ↓ | ↑ | 6.09 | 2.61 | Arginine and proline metabolism |
| 9 | 16.521 | 776.034 | PC(18:4) | 3.905 | 7.01 × 10−4 | ↑ | ↓ | 2.58 | 2.20 | Phospholipid metabolism |
| 10 | 25.114 | 521.667 | LysoPC (18:1(11Z)) | 1.101 | 6.58 × 10−8 | ↑ | ↓ | 3.68 | 2.15 | Phospholipid metabolism |
| 11 | 42.535 | 256.240 | Palmitic acid | 1.856 | 2.09 × 10−21 | ↑ | ↓ | 2.37 | 2.03 | Biosynthesis of fatty acid |
| 12 | 24.900 | 284.271 | Stearic acid | 1.774 | 3.06 × 10−7 | ↑ | ↓ | 7.93 | 3.61 | Biosynthesis of fatty acid |
| 13 | 38.232 | 280.240 | Linoleic acid | 1.680 | 1.94 × 10−17 | ↑ | ↓ | 3.14 | 2.16 | Linoleic acid metabolism |
| 14 | 43.266 | 282.255 | Oleic acid | 1.819 | 6.29 × 10−19 | ↑ | ↓ | 8.1 | 2.45 | Biosynthesis of fatty acid |
| 15 | 14.505 | 161.105 | L-Carnitine | 1.291 | 4.39 × 10−4 | ↑ | ↓ | 4.42 | 3.78 | Bile secretion |
| 16 | 19.094 | 1017.76 | TG(24:0) | 1.626 | 0.0029 | ↑ | ↓ | 2.60 | 2.07 | Fatty metabolism |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, A.; Zhao, W.; Zhao, H.; Gong, L.; Chen, E.; Cui, N.; Ji, X.; Wang, S.; Jiang, H. Metabolomics Research Reveals the Mechanism of Action of Astragalus Polysaccharide in Rats with Digestive System Disorders. Molecules 2018, 23, 3333. https://doi.org/10.3390/molecules23123333
Wang H, Liu A, Zhao W, Zhao H, Gong L, Chen E, Cui N, Ji X, Wang S, Jiang H. Metabolomics Research Reveals the Mechanism of Action of Astragalus Polysaccharide in Rats with Digestive System Disorders. Molecules. 2018; 23(12):3333. https://doi.org/10.3390/molecules23123333
Chicago/Turabian StyleWang, Huanjun, Ana Liu, Wenxiao Zhao, Haijun Zhao, Lili Gong, Erdong Chen, Ning Cui, Xuming Ji, Shijun Wang, and Haiqiang Jiang. 2018. "Metabolomics Research Reveals the Mechanism of Action of Astragalus Polysaccharide in Rats with Digestive System Disorders" Molecules 23, no. 12: 3333. https://doi.org/10.3390/molecules23123333
APA StyleWang, H., Liu, A., Zhao, W., Zhao, H., Gong, L., Chen, E., Cui, N., Ji, X., Wang, S., & Jiang, H. (2018). Metabolomics Research Reveals the Mechanism of Action of Astragalus Polysaccharide in Rats with Digestive System Disorders. Molecules, 23(12), 3333. https://doi.org/10.3390/molecules23123333





