Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases
Abstract
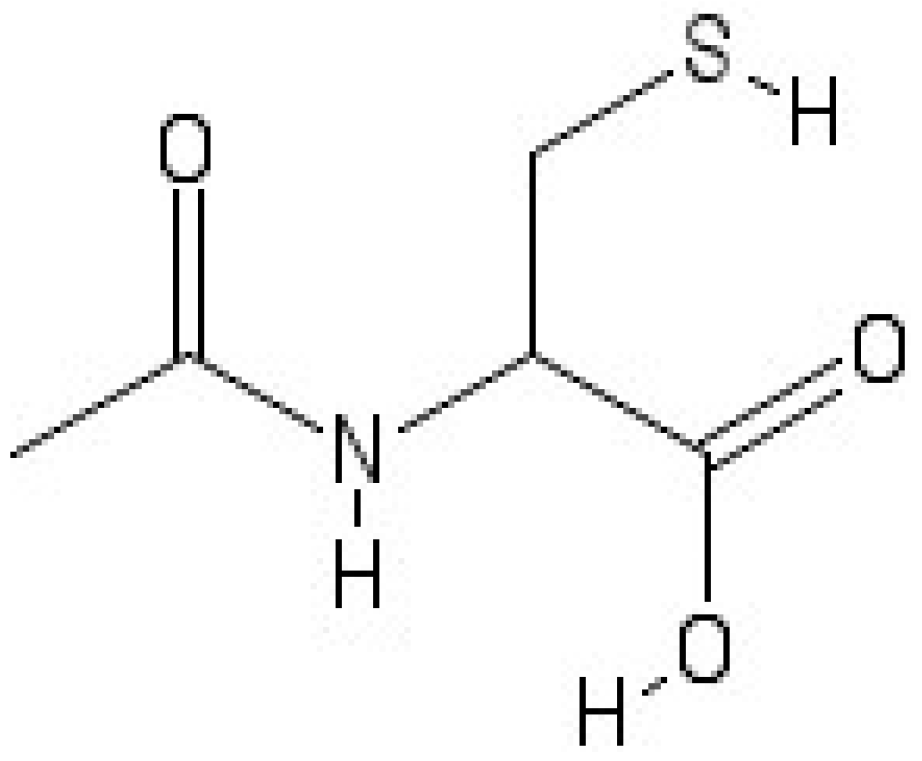
1. Introduction
2. NAC as Antioxidant and Anti-Inflammatory Compound
3. Effects of NAC on Neurotransmission
4. NAC in Parkinson’s Disease
5. NAC in Alzheimer’s Disease
6. NAC in Neuropathic Pain
7. NAC in Stroke
8. N-Acetylcysteine: Clinical Trials in Neurodegenerative Diseases
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Da Costa, M.; Bernardi, J.; Costa, L.; Fiuza, T.; Brandao, R.; Ribeiro, M.F.; Amaral, J.D.; Rodrigues, C.M.P.; Pereira, M.E. N-acetylcysteine treatment attenuates the cognitive impairment and synaptic plasticity loss induced by streptozotocin. Chem. Biol. Interact. 2017, 272, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bavarsad, S.R.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (nac) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.L.; Green, R.; Pak, S.C. N-acetylcysteine for the treatment of psychiatric disorders: A review of current evidence. Biomed. Res. Int. 2018, 2018, 2469486. [Google Scholar] [CrossRef] [PubMed]
- Witschi, A.; Reddy, S.; Stofer, B.; Lauterburg, B.H. The systemic availability of oral glutathione. Eur. J. Clin. Pharmacol. 1992, 43, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Hems, R.; Krebs, H.A. Maintenance of glutathione content is isolated hepatocyctes. Biochem. J. 1978, 170, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Sjodin, K.; Nilsson, E.; Hallberg, A.; Tunek, A. Metabolism of N-acetyl-l-cysteine. Some structural requirements for the deacetylation and consequences for the oral bioavailability. Biochem. Pharmacol. 1989, 38, 3981–3985. [Google Scholar] [CrossRef]
- Borgstrom, L.; Kagedal, B. Dose dependent pharmacokinetics of N-acetylcysteine after oral dosing to man. Biopharm. Drug Dispos. 1990, 11, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Holdiness, M.R. Clinical pharmacokinetics of N-acetylcysteine. Clin. Pharmacokinet. 1991, 20, 123–134. [Google Scholar] [CrossRef]
- Lavoie, S.; Murray, M.M.; Deppen, P.; Knyazeva, M.G.; Berk, M.; Boulat, O.; Bovet, P.; Bush, A.I.; Conus, P.; Copolov, D.; et al. Glutathione precursor, n-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 2008, 33, 2187–2199. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Pagel, M.A.; Hasler, B.P.; Deloughery, T.G.; Muldoon, L.L. Therapeutic efficacy of aortic administration of N-acetylcysteine as a chemoprotectant against bone marrow toxicity after intracarotid administration of alkylators, with or without glutathione depletion in a rat model. Cancer Res. 2001, 61, 7868–7874. [Google Scholar]
- Farr, S.A.; Poon, H.F.; Dogrukol-Ak, D.; Drake, J.; Banks, W.A.; Eyerman, E.; Butterfield, D.A.; Morley, J.E. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged samp8 mice. J. Neurochem. 2003, 84, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef] [PubMed]
- Omara, F.O.; Blakley, B.R.; Bernier, J.; Fournier, M. Immunomodulatory and protective effects of N-acetylcysteine in mitogen-activated murine splenocytes in vitro. Toxicology 1997, 116, 219–226. [Google Scholar] [CrossRef]
- Arakawa, M.; Ito, Y. N-acetylcysteine and neurodegenerative diseases: Basic and clinical pharmacology. Cerebellum 2007, 6, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Russo, A. The role of glutathione in radiation and drug induced cytotoxicity. Br. J. Cancer Suppl. 1987, 8, 96–104. [Google Scholar] [PubMed]
- Mitchell, J.B.; Biaglow, J.E.; Russo, A. Role of glutathione and other endogenous thiols in radiation protection. Pharmacol. Ther. 1988, 39, 269–274. [Google Scholar] [CrossRef]
- Bannai, S.; Tateishi, N. Role of membrane transport in metabolism and function of glutathione in mammals. J. Membrane. Biol. 1986, 89, 1–8. [Google Scholar] [CrossRef]
- Ishige, K.; Tanaka, M.; Arakawa, M.; Saito, H.; Ito, Y. Distinct nuclear factor-kappab/rel proteins have opposing modulatory effects in glutamate-induced cell death in ht22 cells. Neurochem. Int. 2005, 47, 545–555. [Google Scholar] [CrossRef]
- Sen, C.K. Nutritional biochemistry of cellular glutathione. J. Nutr. Biochem. 1997, 8, 660–672. [Google Scholar] [CrossRef]
- Meister, A. Glutathione metabolism. Methods Enzymol. 1995, 251, 3–7. [Google Scholar]
- Richman, P.G.; Meister, A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J. Biol. Chem. 1975, 250, 1422–1426. [Google Scholar] [PubMed]
- Emet, S.; Memis, D.; Pamukcu, Z. The influence of n-acetyl-l-cystein infusion on cytokine levels and gastric intramucosal ph during severe sepsis. Crit. Care 2004, 8, R172–179. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M.; Suliman, M.E.; Silva, M.; Chinaglia, T.; Marchioro, J.; Hayashi, S.Y.; Riella, M.C.; Lindholm, B.; Anderstam, B. Effect of oral N-acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: A placebo-controlled study. Periton. Dialysis Int. 2010, 30, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Paintlia, M.K.; Paintlia, A.S.; Contreras, M.A.; Singh, I.; Singh, A.K. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: Attenuation by n-acetyl cysteine. Exp. Neurol. 2008, 210, 560–576. [Google Scholar] [CrossRef] [PubMed]
- Pajonk, F.; Riess, K.; Sommer, A.; McBride, W.H. N-acetyl-l-cysteine inhibits 26s proteasome function: Implications for effects on NF-κB activation. Free Radical Bio. Med. 2002, 32, 536–543. [Google Scholar] [CrossRef]
- Oka, S.; Kamata, H.; Kamata, K.; Yagisawa, H.; Hirata, H. N-Acetylcysteine suppresses TNF-induced NF-κB activation through inhibition of IκB kinases. FEBS Lett. 2000, 472, 196–202. [Google Scholar] [CrossRef]
- Olive, M.F.; Cleva, R.M.; Kalivas, P.W.; Malcolm, R.J. Glutamatergic medications for the treatment of drug and behavioral addictions. Pharmacol. Biochem. Be. 2012, 100, 801–810. [Google Scholar] [CrossRef]
- Minarini, A.; Ferrari, S.; Galletti, M.; Giambalvo, N.; Perrone, D.; Rioli, G.; Galeazzi, G.M. N-acetylcysteine in the treatment of psychiatric disorders: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2017, 13, 279–292. [Google Scholar] [CrossRef]
- Kantrowitz, J.T.; Javitt, D.C. N-methyl-d-aspartate (nmda) receptor dysfunction or dysregulation: The final common pathway on the road to schizophrenia? Brain Res. Bull. 2010, 83, 108–121. [Google Scholar] [CrossRef]
- Moran, M.M.; McFarland, K.; Melendez, R.I.; Kalivas, P.W.; Seamans, J.K. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J. Neurosci. 2005, 25, 6389–6393. [Google Scholar] [CrossRef]
- Smith, L.; Tracy, D.K.; Giaroli, G. What future role might n-acetyl-cysteine have in the treatment of obsessive compulsive and grooming disorders?: A systematic review. J. Clin. Psychopharm. 2016, 36, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sattler, R.; Tymianski, M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol. 2001, 24, 107–129. [Google Scholar] [CrossRef]
- Paoletti, P.; Neyton, J. Nmda receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P. Molecular basis of nmda receptor functional diversity. Eur. J. Neurosci. 2011, 33, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.W.; Shyu, W.C.; Wang, Y.T. Stroke intervention pathways: Nmda receptors and beyond. Trends Mol. Med. 2011, 17, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, B.; Javitt, D. From revolution to evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012, 37, 4–15. [Google Scholar] [CrossRef]
- Cull-Candy, S.G.; Leszkiewicz, D.N. Role of distinct nmda receptor subtypes at central synapses. Sci. STKE 2004, 2004, re16. [Google Scholar] [CrossRef]
- Conn, P.J.; Pin, J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. 1997, 37, 205–237. [Google Scholar] [CrossRef]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar]
- D’Angelo, E.; Rossi, P. Integrated regulation of signal coding and plasticity by nmda receptors at a central synapse. Neural Plast. 1998, 6, 8–16. [Google Scholar] [CrossRef]
- Baker, D.A.; Xi, Z.X.; Shen, H.; Swanson, C.J.; Kalivas, P.W. The origin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci. 2002, 22, 9134–9141. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.A.; Madayag, A.; Kristiansen, L.V.; Meador-Woodruff, J.H.; Haroutunian, V.; Raju, I. Contribution of cystine-glutamate antiporters to the psychotomimetic effects of phencyclidine. Neuropsychopharmacology 2008, 33, 1760–1772. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.A.; McFarland, K.; Lake, R.W.; Shen, H.; Tang, X.C.; Toda, S.; Kalivas, P.W. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat. Neurosci. 2003, 6, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, K.R.; Aizenman, E.; Reynolds, I.J. Oxidized glutathione modulates n-methyl-d-aspartate- and depolarization-induced increases in intracellular Ca2+ in cultured rat forebrain neurons. Neurosci. Lett. 1991, 133, 11–14. [Google Scholar] [CrossRef]
- Varga, V.; Jenei, Z.; Janaky, R.; Saransaari, P.; Oja, S.S. Glutathione is an endogenous ligand of rat brain n-methyl-d-aspartate (nmda) and 2-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (ampa) receptors. Neurochem. Res. 1997, 22, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Steullet, P.; Neijt, H.C.; Cuenod, M.; Do, K.Q. Synaptic plasticity impairment and hypofunction of nmda receptors induced by glutathione deficit: Relevance to schizophrenia. Neuroscience 2006, 137, 807–819. [Google Scholar] [CrossRef]
- Berk, M.; Malhi, G.S.; Gray, L.J.; Dean, O.M. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol. Sci. 2013, 34, 167–177. [Google Scholar] [CrossRef]
- Deepmala; Slattery, J.; Kumar, N.; Delhey, L.; Berk, M.; Dean, O.; Spielholz, C.; Frye, R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rew. 2015, 55, 294–321. [Google Scholar] [CrossRef]
- Agid, Y.; Ruberg, M.; Javoy-Agid, F.; Hirsch, E.; Raisman-Vozari, R.; Vyas, S.; Faucheux, B.; Michel, P.; Kastner, A.; Blanchard, V.; et al. Are dopaminergic neurons selectively vulnerable to parkinson’s disease? Adv. Neurol. 1993, 60, 148–164. [Google Scholar]
- Fisone, G.; Bezard, E. Molecular mechanisms of l-dopa-induced dyskinesia. Int. Rev. Neurobiol. 2011, 98, 95–122. [Google Scholar]
- Jenner, P. Molecular mechanisms of l-dopa-induced dyskinesia. Nat. Rev. Neurosci. 2008, 9, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Guttman, M.; Slaughter, P.M.; Theriault, M.E.; DeBoer, D.P.; Naylor, C.D. Parkinsonism in ontario: Increased mortality compared with controls in a large cohort study. Neurology 2001, 57, 2278–2282. [Google Scholar] [CrossRef] [PubMed]
- Veldman, B.A.; Wijn, A.M.; Knoers, N.; Praamstra, P.; Horstink, M.W. Genetic and environmental risk factors in parkinson’s disease. Clin. Neurol. Neurosur. 1998, 100, 15–26. [Google Scholar] [CrossRef]
- Martinez, M.; Hernandez, A.I.; Martinez, N. N-acetylcysteine delays age-associated memory impairment in mice: Role in synaptic mitochondria. Brain Res. 2000, 855, 100–106. [Google Scholar] [CrossRef]
- Martinez, M.; Hernandez, A.I.; Martinez, N.; Ferrandiz, M.L. Age-related increase in oxidized proteins in mouse synaptic mitochondria. Brain Res. 1996, 731, 246–248. [Google Scholar] [CrossRef]
- Fitzmaurice, P.S.; Ang, L.; Guttman, M.; Rajput, A.H.; Furukawa, Y.; Kish, S.J. Nigral glutathione deficiency is not specific for idiopathic parkinson’s disease. Movement Disord. 2003, 18, 969–976. [Google Scholar] [CrossRef]
- Jha, N.; Jurma, O.; Lalli, G.; Liu, Y.; Pettus, E.H.; Greenamyre, J.T.; Liu, R.M.; Forman, H.J.; Andersen, J.K. Glutathione depletion in pc12 results in selective inhibition of mitochondrial complex i activity. Implications for parkinson’s disease. J. Biol. Chem. 2000, 275, 26096–26101. [Google Scholar] [CrossRef]
- Conway, K.A.; Rochet, J.C.; Bieganski, R.M.; Lansbury, P.T., Jr. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 2001, 294, 1346–1349. [Google Scholar] [CrossRef]
- Giasson, B.I.; Duda, J.E.; Murray, I.V.; Chen, Q.; Souza, J.M.; Hurtig, H.I.; Ischiropoulos, H.; Trojanowski, J.Q.; Lee, V.M. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 2000, 290, 985–989. [Google Scholar] [CrossRef]
- Hsu, M.; Srinivas, B.; Kumar, J.; Subramanian, R.; Andersen, J. Glutathione depletion resulting in selective mitochondrial complex i inhibition in dopaminergic cells is via an no-mediated pathway not involving peroxynitrite: Implications for parkinson’s disease. J. Neurochem. 2005, 92, 1091–1103. [Google Scholar] [CrossRef]
- Etminan, M.; Gill, S.S.; Samii, A. Intake of vitamin e, vitamin c, and carotenoids and the risk of parkinson’s disease: A meta-analysis. Lancet Neurol. 2005, 4, 362–365. [Google Scholar] [CrossRef]
- Sechi, G.; Deledda, M.G.; Bua, G.; Satta, W.M.; Deiana, G.A.; Pes, G.M.; Rosati, G. Reduced intravenous glutathione in the treatment of early parkinson’s disease. Prog. Neuro-Psychoph. 1996, 20, 1159–1170. [Google Scholar] [CrossRef]
- Wang, X.F.; Cynader, M.S. Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J. Neurosci. 2001, 21, 3322–3331. [Google Scholar] [CrossRef] [PubMed]
- Banaclocha, M.M. Therapeutic potential of N-acetylcysteine in age-related mitochondrial neurodegenerative diseases. Med. Hypotheses 2001, 56, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Martinez Banaclocha, M. N-acetylcysteine elicited increase in complex i activity in synaptic mitochondria from aged mice: Implications for treatment of parkinson’s disease. Brain Res. 2000, 859, 173–175. [Google Scholar] [CrossRef]
- Martinez, M.; Martinez, N.; Hernandez, A.I.; Ferrandiz, M.L. Hypothesis: Can N-acetylcysteine be beneficial in parkinson’s disease? Life Sci. 1999, 64, 1253–1257. [Google Scholar] [CrossRef]
- Medina, S.; Martinez, M.; Hernanz, A. Antioxidants inhibit the human cortical neuron apoptosis induced by hydrogen peroxide, tumor necrosis factor alpha, dopamine and beta-amyloid peptide 1-42. Free Radic. Res. 2002, 36, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Banaclocha, M.M.; Hernandez, A.I.; Martinez, N.; Ferrandiz, M.L. N-acetylcysteine protects against age-related increase in oxidized proteins in mouse synaptic mitochondria. Brain Res. 1997, 762, 256–258. [Google Scholar] [CrossRef]
- Pocernich, C.B.; La Fontaine, M.; Butterfield, D.A. In-vivo glutathione elevation protects against hydroxyl free radical-induced protein oxidation in rat brain. Neurochem. Int. 2000, 36, 185–191. [Google Scholar] [CrossRef]
- Martinez Banaclocha, M.; Martinez, N. N-acetylcysteine elicited increase in cytochrome c oxidase activity in mice synaptic mitochondria. Brain Res. 1999, 842, 249–251. [Google Scholar] [CrossRef]
- Offen, D.; Ziv, I.; Sternin, H.; Melamed, E.; Hochman, A. Prevention of dopamine-induced cell death by thiol antioxidants: Possible implications for treatment of parkinson’s disease. Exp. Neurol. 1996, 141, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Cocco, T.; Sgobbo, P.; Clemente, M.; Lopriore, B.; Grattagliano, I.; Di Paola, M.; Villani, G. Tissue-specific changes of mitochondrial functions in aged rats: Effect of a long-term dietary treatment with N-acetylcysteine. Free Radical Biol. Med. 2005, 38, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Clore, E.L.; Zheng, K.; Adame, A.; Masliah, E.; Simon, D.K. Oral n-acetyl-cysteine attenuates loss of dopaminergic terminals in alpha-synuclein overexpressing mice. PLoS ONE 2010, 5, e12333. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Aoki, E.; Yano, R.; Yokoyama, H.; Kato, H.; Araki, T. Role of nuclear transcription factor kappa b (nf-kappab) for mptp (1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine)-induced apoptosis in nigral neurons of mice. Exp. Mol. Pathol. 2009, 86, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Chin, L.S.; Li, L. Phosphorylation of parkin by Parkinson disease-linked kinase PINK1 activates parkin E3 ligase function and NF-κB signaling. Hum. Mol. Genet. 2010, 19, 352–363. [Google Scholar] [CrossRef]
- Talley, A.K.; Dewhurst, S.; Perry, S.W.; Dollard, S.C.; Gummuluru, S.; Fine, S.M.; New, D.; Epstein, L.G.; Gendelman, H.E.; Gelbard, H.A. Tumor necrosis factor alpha-induced apoptosis in human neuronal cells: protection by the antioxidant N-acetylcysteine and the genes bcl-2 and crmA. Mol. Cell. Biol. 1995, 15, 2359–2366. [Google Scholar] [CrossRef]
- Bagh, M.B.; Maiti, A.K.; Jana, S.; Banerjee, K.; Roy, A.; Chakrabarti, S. Quinone and oxyradical scavenging properties of N-acetylcysteine prevent dopamine mediated inhibition of Na+, K+-ATPase and mitochondrial electron transport chain activity in rat brain: Implications in the neuroprotective therapy of parkinson’s disease. Free Radic. Res. 2008, 42, 574–581. [Google Scholar] [CrossRef]
- Alzheimer’s, A. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016, 12, 459–509. [Google Scholar]
- Lahiri, D.K.; Rogers, J.T.; Greig, N.H.; Sambamurti, K. Rationale for the development of cholinesterase inhibitors as anti-alzheimer agents. Curr. Pharm. Design 2004, 10, 3111–3119. [Google Scholar] [CrossRef]
- Lee, H.P.; Zhu, X.; Casadesus, G.; Castellani, R.J.; Nunomura, A.; Smith, M.A.; Lee, H.G.; Perry, G. Antioxidant approaches for the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2010, 10, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. The new cholinesterase inhibitors for Alzheimer’s disease, part 2: Illustrating their mechanisms of action. J. Clin. Psychiat. 2000, 61, 813–814. [Google Scholar] [CrossRef]
- Herholz, K. Acetylcholine esterase activity in mild cognitive impairment and Alzheimer’s disease. Eur. J. Nucl. Med. 2008, 35, S25–S29. [Google Scholar] [CrossRef] [PubMed]
- Randall, A.D.; Witton, J.; Booth, C.; Hynes-Allen, A.; Brown, J.T. The functional neurophysiology of the amyloid precursor protein (app) processing pathway. Neuropharmacology 2010, 59, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Arima, K. Ultrastructural characteristics of tau filaments in tauopathies: Immuno-electron microscopic demonstration of tau filaments in tauopathies. Neuropathology 2006, 26, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Schwab, C.; McGeer, P.L. Inflammatory aspects of alzheimer disease and other neurodegenerative disorders. JAD 2008, 13, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Tiraboschi, P.; Hansen, L.A.; Thal, L.J.; Corey-Bloom, J. The importance of neuritic plaques and tangles to the development and evolution of ad. Neurology 2004, 62, 1984–1989. [Google Scholar] [CrossRef]
- Reddy, P.H. Mitochondrial dysfunction in aging and Alzheimer’s disease: Strategies to protect neurons. Antioxid. Redox. Sign. 2007, 9, 1647–1658. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. JAD 2001, 3, 75–80. [Google Scholar] [CrossRef]
- Dahlgren, K.N.; Manelli, A.M.; Stine, W.B.J.; Baker, L.K.; Krafft, G.A.; LaDu, M.J. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J. Biol. Chem. 2002, 277, 32046–32053. [Google Scholar] [CrossRef]
- Caughey, B.; Lansbury, P.T. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci. 2003, 26, 267–298. [Google Scholar] [CrossRef] [PubMed]
- Fawzi, N.L.; Okabe, Y.; Yap, E.H.; Head-Gordon, T. Determining the critical nucleus and mechanism of fibril elongation of the Alzheimer’s abeta(1-40) peptide. J. Mol. Biol. 2007, 365, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Hartley, D.M.; Condron, M.M.; Selkoe, D.J.; Teplow, D.B. In vitro studies of amyloid beta-protein fibril assembly and toxicity provide clues to the aetiology of flemish variant (ala692-->gly) Alzheimer’s disease. Biochem. J. 2001, 355, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.; Link, C.D.; Butterfield, D.A. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1-42) in a transgenic caenorhabditis elegans model. Neurobiol. Aging 2003, 24, 415–420. [Google Scholar] [CrossRef]
- Lauderback, C.M.; Hackett, J.M.; Huang, F.F.; Keller, J.N.; Szweda, L.I.; Markesbery, W.R.; Butterfield, D.A. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: The role of abeta1-42. J. Neurochem. 2001, 78, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Lauderback, C.M. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radical Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Poon, H.F.; St Clair, D.; Keller, J.N.; Pierce, W.M.; Klein, J.B.; Markesbery, W.R. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: Insights into the development of Alzheimer’s disease. Neurobiol. Dis. 2006, 22, 223–232. [Google Scholar] [CrossRef]
- Pocernich, C.B.; Lange, M.L.; Sultana, R.; Butterfield, D.A. Nutritional approaches to modulate oxidative stress in Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 452–469. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Bader Lange, M.L.; Sultana, R. Involvements of the lipid peroxidation product, hne, in the pathogenesis and progression of Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801, 924–929. [Google Scholar] [CrossRef]
- Lovell, M.A.; Markesbery, W.R. Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J. Neurosci. Res. 2007, 85, 3036–3040. [Google Scholar] [CrossRef]
- Hara, Y.; McKeehan, N.; Dacks, P.A.; Fillit, H.M. Evaluation of the neuroprotective potential of N-acetylcysteine for prevention and treatment of cognitive aging and dementia. JPAD 2017, 4, 201–206. [Google Scholar] [PubMed]
- Koppal, T.; Drake, J.; Butterfield, D.A. In vivo modulation of rodent glutathione and its role in peroxynitrite-induced neocortical synaptosomal membrane protein damage. Biochim. Biophys. Acta 1999, 1453, 407–411. [Google Scholar] [CrossRef]
- Pocernich, C.B.; Cardin, A.L.; Racine, C.L.; Lauderback, C.M.; Butterfield, D.A. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: Relevance to brain lipid peroxidation in neurodegenerative disease. Neurochem. Int. 2001, 39, 141–149. [Google Scholar] [CrossRef]
- LaFontaine, M.A.; Geddes, J.W.; Butterfield, D.A. 3-nitropropionic acid-induced changes in bilayer fluidity in synaptosomal membranes: Implications for huntington’s disease. Neurochem. Res. 2002, 27, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.L.; Dong, Z.H.; Sun, M.J. Protective effect of N-acetyl-l-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain Res. 2006, 1109, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Aluise, C.D.; Joshi, G.; Sultana, R.; St Clair, D.K.; Markesbery, W.R.; Butterfield, D.A. Potential in vivo amelioration by N-acetyl-l-cysteine of oxidative stress in brain in human double mutant app/ps-1 knock-in mice: Toward therapeutic modulation of mild cognitive impairment. J. Neurosci. Res. 2010, 88, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Estus, S.; Tucker, H.M.; van Rooyen, C.; Wright, S.; Brigham, E.F.; Wogulis, M.; Rydel, R.E. Aggregated amyloid-beta protein induces cortical neuronal apoptosis and concomitant “apoptotic” pattern of gene induction. J. Neurosci. 1997, 17, 7736–7745. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.H.; Chen, P.S.; Yeh, S.H.; Lin, C.H.; Gean, P.W. N-acetylcysteine prevents beta-amyloid toxicity by a stimulatory effect on p35/cyclin-dependent kinase 5 activity in cultured cortical neurons. J. Neurosci. Res. 2008, 86, 2685–2695. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, X.Y.; Liu, Y.; Zong, Y.Y. Different protection of k252a and N-acetyl-l-cysteine against amyloid-beta peptide-induced cortical neuron apoptosis involving inhibition of mlk3-mkk7-jnk3 signal cascades. J. Neurosci. Res. 2009, 87, 918–927. [Google Scholar] [CrossRef]
- Yan, C.Y.; Greene, L.A. Prevention of pc12 cell death by N-acetylcysteine requires activation of the ras pathway. J. Neurosci. 1998, 18, 4042–4049. [Google Scholar] [CrossRef]
- Studer, R.; Baysang, G.; Brack, C. N-acetyl-l-cystein downregulates beta-amyloid precursor protein gene transcription in human neuroblastoma cells. Biogerontology 2001, 2, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Steele, M.; Munch, G. Activated astroglia during chronic inflammation in Alzheimer’s disease--do they neglect their neurosupportive roles? Mutat. Res. 2010, 690, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Cho, T.; Jantaratnotai, N.; Wang, Y.T.; McGeer, E.; McGeer, P.L. Depletion of gsh in glial cells induces neurotoxicity: Relevance to aging and degenerative neurological diseases. FASEB J. 2010, 24, 2533–2545. [Google Scholar] [CrossRef] [PubMed]
- Pahan, K.; Sheikh, F.G.; Namboodiri, A.M.; Singh, I. N-acetyl cysteine inhibits induction of no production by endotoxin or cytokine stimulated rat peritoneal macrophages, c6 glial cells and astrocytes. Free Radical Biol. Med. 1998, 24, 39–48. [Google Scholar] [CrossRef]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; O’Connor, A.B.; Backonja, M.; Farrar, J.T.; Finnerup, N.B.; Jensen, T.S.; Kalso, E.A.; Loeser, J.D.; Miaskowski, C.; Nurmikko, T.J.; et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 2007, 132, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Wiffen, P.J.; Derry, S.; Toelle, T.; Rice, A.S. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane. DB Syst. Rev. 2014, 4, CD007938. [Google Scholar]
- Dworkin, R.H.; O’Connor, A.B.; Audette, J.; Baron, R.; Gourlay, G.K.; Haanpaa, M.L.; Kent, J.L.; Krane, E.J.; Lebel, A.A.; Levy, R.M.; et al. Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clin. Proc. 2010, 85, S3–S14. [Google Scholar] [CrossRef]
- Horst, A.; Kolberg, C.; Moraes, M.S.; Riffel, A.P.; Finamor, I.A.; Bello-Klein, A.; Pavanato, M.A.; Partata, W.A. Effect of N-acetylcysteine on the spinal-cord glutathione system and nitric-oxide metabolites in rats with neuropathic pain. Neurosci. Lett. 2014, 569, 163–168. [Google Scholar] [CrossRef]
- Horst, A.; de Souza, J.A.; Santos, M.C.Q.; Riffel, A.P.K.; Kolberg, C.; Partata, W.A. Effects of N-acetylcysteine on spinal cord oxidative stress biomarkers in rats with neuropathic pain. Braz. J. Med. Biol. Res. 2017, 50, e6533. [Google Scholar] [CrossRef]
- Horst, A.; de Souza, J.A.; Santos, M.C.; Riffel, A.P.; Kolberg, C.; Ribeiro, M.F.; de Fraga, L.S.; Partata, W.A. N-acetylcysteine downregulates phosphorylated p-38 expression but does not reverse the increased superoxide anion levels in the spinal cord of rats with neuropathic pain. Braz. J. Med. Biol. Res. 2017, 50, e5801. [Google Scholar] [CrossRef] [PubMed]
- Chiechio, S.; Copani, A.; Zammataro, M.; Battaglia, G.; Gereau, R.W.t.; Nicoletti, F. Transcriptional regulation of type-2 metabotropic glutamate receptors: An epigenetic path to novel treatments for chronic pain. Trends Pharmacol. Sci. 2010, 31, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Varney, M.A.; Gereau, R.W.t. Metabotropic glutamate receptor involvement in models of acute and persistent pain: Prospects for the development of novel analgesics. Curr. Drug Targets: CNS Neurol. Disord. 2002, 1, 283–296. [Google Scholar] [CrossRef]
- Goudet, C.; Magnaghi, V.; Landry, M.; Nagy, F.; Gereau, R.W.t.; Pin, J.P. Metabotropic receptors for glutamate and gaba in pain. Brain Res. 2009, 60, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009, 10, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Bridges, R.J.; Natale, N.R.; Patel, S.A. System xc− cystine/glutamate antiporter: An update on molecular pharmacology and roles within the cns. Br. J. Pharmacol. 2012, 165, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, M.; Notartomaso, S.; Zappulla, C.; Fazio, F.; Cannella, M.; Motolese, M.; Battaglia, G.; Bruno, V.; Gradini, R.; Nicoletti, F. N-acetyl-cysteine causes analgesia by reinforcing the endogenous activation of type-2 metabotropic glutamate receptors. Mol. Pain 2012, 8, 77. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Deng, X.; Jiang, C.; Pan, C.; Chen, L.; Han, Y.; Dai, W.; Hu, L.; Zhang, G.; et al. N-acetyl-cysteine attenuates neuropathic pain by suppressing matrix metalloproteinases. Pain 2016, 157, 1711–1723. [Google Scholar] [CrossRef]
- Sozbir, E.; Naziroglu, M. Diabetes enhances oxidative stress-induced TRPM2 channel activity and its control by N-acetylcysteine in rat dorsal root ganglion and brain. Metab. Brain Dis. 2016, 31, 385–393. [Google Scholar] [CrossRef]
- Tsai, W.Y.; Tsai, R.Y.; Liu, C.C.; Wu, J.L.; Wong, C.S. Sulfasalazine attenuates acl transection and medial menisectomy-induced cartilage destruction by inhibition of cystine/glutamate antiporter. J. Orthop. Res. 2016, 34, 650–657. [Google Scholar] [CrossRef]
- Nishio, N.; Taniguchi, W.; Sugimura, Y.K.; Takiguchi, N.; Yamanaka, M.; Kiyoyuki, Y.; Yamada, H.; Miyazaki, N.; Yoshida, M.; Nakatsuka, T. Reactive oxygen species enhance excitatory synaptic transmission in rat spinal dorsal horn neurons by activating trpa1 and trpv1 channels. Neuroscience 2013, 247, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Hacimuftuoglu, A.; Handy, C.R.; Goettl, V.M.; Lin, C.G.; Dane, S.; Stephens, R.L.J. Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Behav. Brain Res. 2006, 173, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.K.; Tandan, S.K.; Dudhgaonkar, S.P.; Jadhav, S.H.; Kataria, M.; Prakash, V.R.; Kumar, D. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-l-cysteine in rats. Eur. J. Pain 2006, 10, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, S.; Cekic Gonenc, O.; Karaca, Y.; Mentese, A.; Demir, S.; Beyhun, E.; Sahin, A.; Gunduz, A.; Yulug, E.; Turedi, S. The effect of ethyl pyruvate and N-acetylcysteine on ischemia-reperfusion injury in an experimental model of ischemic stroke. Am. J. Emerg. Med. 2016, 34, 1804–1807. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.; Sekhon, C.; Khan, M.; Patel, S.J.; Singh, I.; Singh, A.K. N-acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res. 2003, 971, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.C.; Sun, Y.; Shen, X.; Wang, X.; Shu, H.; Pan, R.; Liu, C.F.; Liu, W.; Liu, K.J.; et al. Normobaric hyperoxia extends neuro- and vaso-protection of N-acetylcysteine in transient focal ischemia. Mol. Neurobiol. 2017, 54, 3418–3427. [Google Scholar] [CrossRef]
- Wang, B.; Aw, T.Y.; Stokes, K.Y. The protection conferred against ischemia-reperfusion injury in the diabetic brain by N-acetylcysteine is associated with decreased dicarbonyl stress. Free Radical Biol. Med. 2016, 96, 89–98. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Taheri, S.; Liu, K.J.; Shi, H. Hypoxia-inducible factor 1 contributes to N-acetylcysteine’s protection in stroke. Free Radical Biol. Med. 2014, 68, 8–21. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Ding, Q.; Hu, B.; Xie, Y.; Li, X.; Yang, Q.; Xiong, L. Limb remote postconditioning alleviates cerebral reperfusion injury through reactive oxygen species-mediated inhibition of delta protein kinase c in rats. Anesth. Analg. 2011, 113, 1180–1187. [Google Scholar] [CrossRef]
- Khan, M.; Sekhon, B.; Jatana, M.; Giri, S.; Gilg, A.G.; Sekhon, C.; Singh, I.; Singh, A.K. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J. Neurosci. Res. 2004, 76, 519–527. [Google Scholar] [CrossRef]
- Knuckey, N.W.; Palm, D.; Primiano, M.; Epstein, M.H.; Johanson, C.E. N-acetylcysteine enhances hippocampal neuronal survival after transient forebrain ischemia in rats. Stroke 1995, 26, 305–310, discussion 311. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.D.; Tuite, P.J.; Oz, G.; Mishra, U.R.; Kartha, R.V.; Sullivan, K.M.; Cloyd, J.C.; Terpstra, M. Repeated-dose oral N-acetylcysteine in parkinson’s disease: Pharmacokinetics and effect on brain glutathione and oxidative stress. J. Clin. Pharmacol. 2018, 58, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Holmay, M.J.; Terpstra, M.; Coles, L.D.; Mishra, U.; Ahlskog, M.; Oz, G.; Cloyd, J.C.; Tuite, P.J. N-acetylcysteine boosts brain and blood glutathione in gaucher and parkinson diseases. Clin. Neuropharmacol. 2013, 36, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.W.; Wintering, N.A.; Cai, J.; Wei, X.; Bazzan, A.J.; Zhong, L.; Bowen, B.; et al. N-acetyl cysteine may support dopamine neurons in parkinson’s disease: Preliminary clinical and cell line data. PLoS ONE 2016, 11, e0157602. [Google Scholar] [CrossRef]
- Remington, R.; Bechtel, C.; Larsen, D.; Samar, A.; Page, R.; Morrell, C.; Shea, T.B. Maintenance of cognitive performance and mood for individuals with Alzheimer’s disease following consumption of a nutraceutical formulation: A one-year, open-label study. JAD 2016, 51, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Remington, R.; Bechtel, C.; Larsen, D.; Samar, A.; Doshanjh, L.; Fishman, P.; Luo, Y.; Smyers, K.; Page, R.; Morrell, C.; et al. A phase ii randomized clinical trial of a nutritional formulation for cognition and mood in Alzheimer’s disease. JAD 2015, 45, 395–405. [Google Scholar] [CrossRef] [PubMed]
| Study Title | Conditions | Status | Treatments | Identifier |
|---|---|---|---|---|
| Repeated-Dose Oral N-acetylcysteine for the Treatment of Parkinson’s Disease | Parkinson’s Disease | Phase 2 completed | Orally (6000 mg/day) | NCT02212678 |
| Intravenous N-acetylcysteine for the Treatment of Gaucher’s Disease and Parkinson’s Disease | Parkinson’s Disease | Phase 1 completed | Intravenously (150 mg/kg) | NCT01427517 |
| Does N-acetylcysteine Decrease Spontaneous Oxidation of Central Neural Dopamine in Parkinson’s Disease? | Parkinson’s Disease | Recruiting | Orally (4000 mg/day) | NCT03104725 |
| N-acetylcysteine for Neuroprotection in Parkinson’s Disease | Parkinson’s Disease | Phase 1/2 completed | Orally (1800 and 3600 mg/day) | NCT01470027 |
| Physiological Effects of Nutritional Support in Patients With Parkinson’s Disease | Parkinson’s Disease | Active, not recruiting | Intravenously/orally (50 mg in 200 mL of D5W and 1200 mg/day) | NCT02445651 |
| Study of the Efficacy of N-acetylcysteine (NAC) on Impulse Control Disorders | Parkinson’s Disease | Phase 3 Recruiting | Doses no reported | NCT03146130 |
| Comparative Effectiveness of MCI and Dementia Treatments in a Community-Based Dementia Practice | Parkinson’s and Alzheimer’s Disease | Completed | Doses no reported | NCT02860338 |
| A Clinical Trial of a Vitamin/Nutriceutical Formulation for Alzheimer’s Disease | Alzheimer’s Disease | Phase 2 completed | Orally (600 mg/day) | NCT01320527 |
| NAC-003 P.L.U.S. Program (Progress Through Learning Understanding & Support) | Alzheimer’s Disease | Completed | Orally (600 mg/day) | NCT01370954 |
| The Role of N-acetyl-l-cysteine (NAC) as an Adjuvant to Opioid Treatment in Patients With Chronic Neuropathic Pain | Neuropathic Pain | Phase 2 completed | Orally (2400 mg/day) | NCT01840345 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules 2018, 23, 3305. https://doi.org/10.3390/molecules23123305
Tardiolo G, Bramanti P, Mazzon E. Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules. 2018; 23(12):3305. https://doi.org/10.3390/molecules23123305
Chicago/Turabian StyleTardiolo, Giuseppe, Placido Bramanti, and Emanuela Mazzon. 2018. "Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases" Molecules 23, no. 12: 3305. https://doi.org/10.3390/molecules23123305
APA StyleTardiolo, G., Bramanti, P., & Mazzon, E. (2018). Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules, 23(12), 3305. https://doi.org/10.3390/molecules23123305






