Ionic Liquid–Ultrasound-Based Extraction of Biflavonoids from Selaginella helvetica and Investigation of Their Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening Different ILs
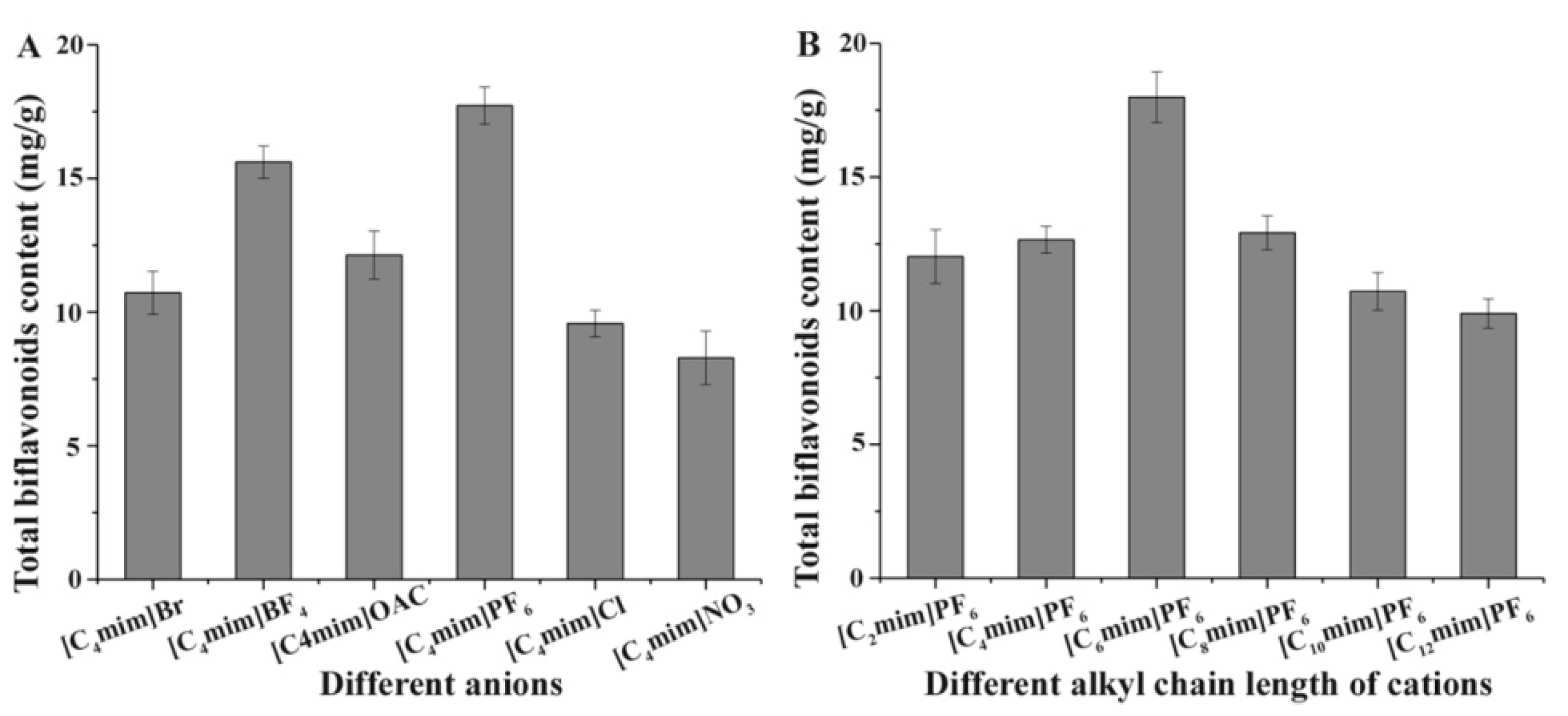
2.1.1. Effect of Anion
2.1.2. Effect of Cation
2.2. Single-Factor Experiment
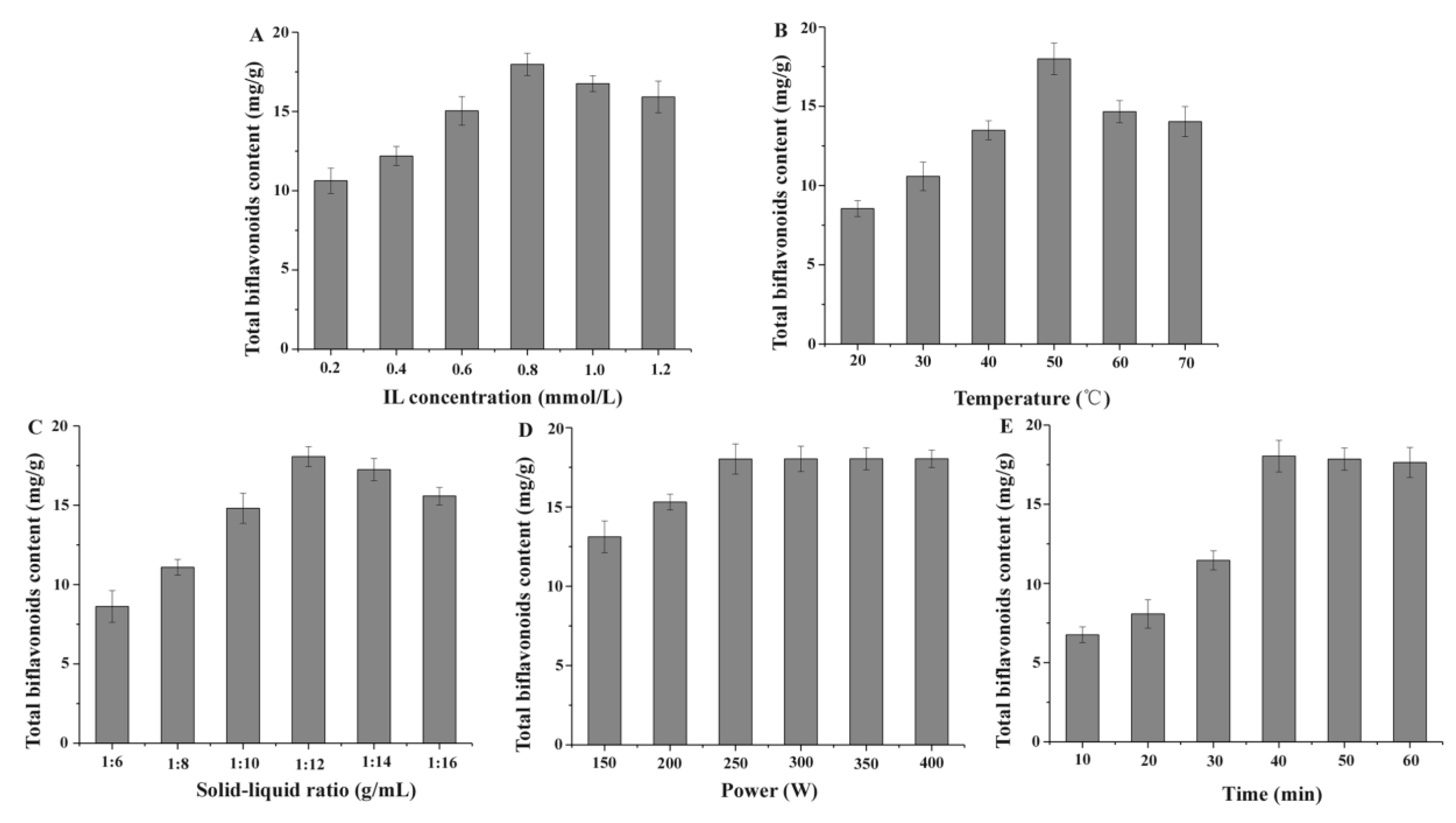
2.2.1. Effect of IL Concentration
2.2.2. Effect of Extracting Temperature
2.2.3. Effect of Solid–Liquid Ratio
2.2.4. Effect of Ultrasonic Power
2.2.5. Effect of Extraction Time
2.3. Analysis of Response Surfaces
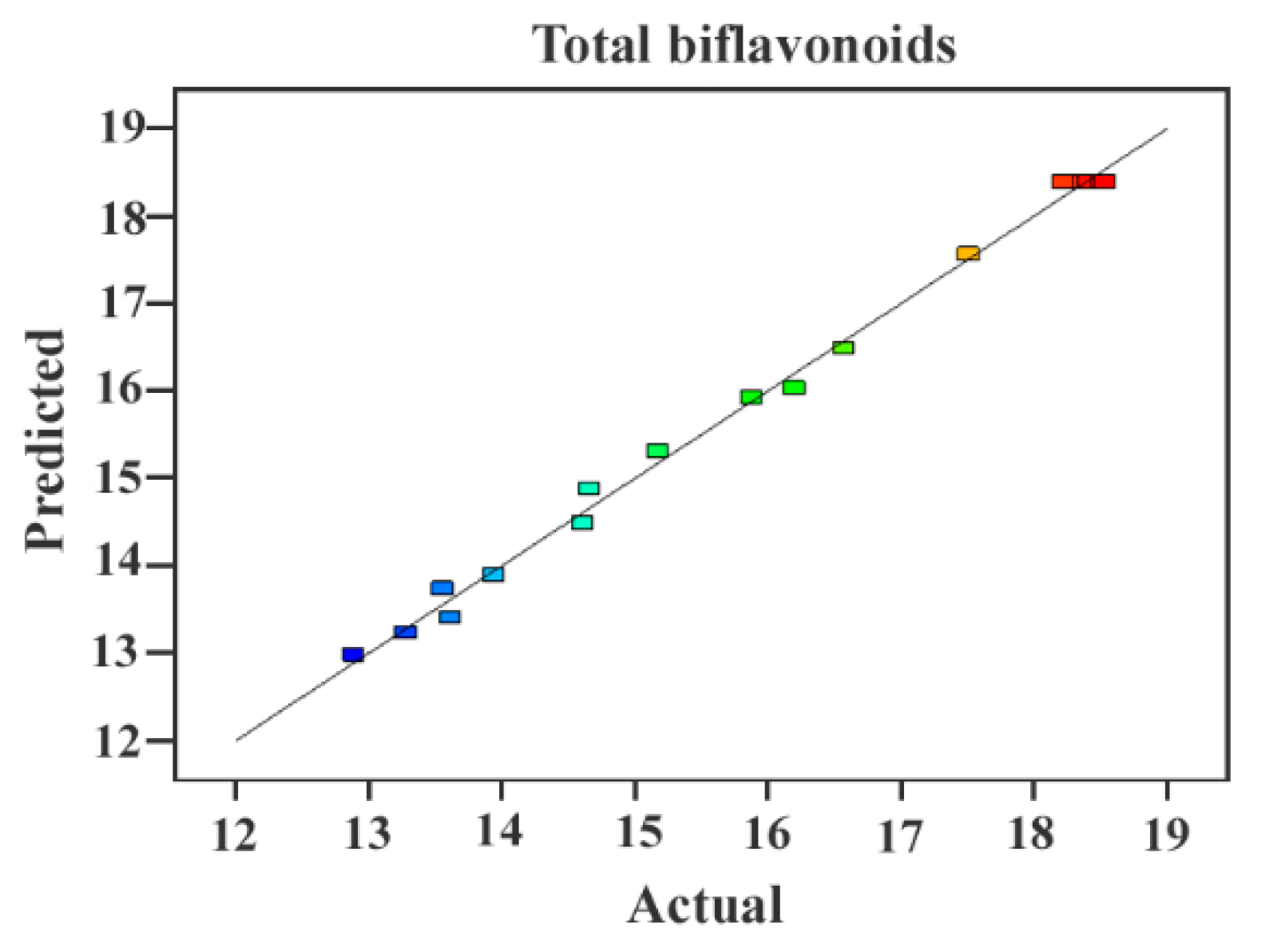
2.3.1. Fitting the Model
2.3.2. Effect of Extraction Parameters
2.4. Recovery of TBF and IL
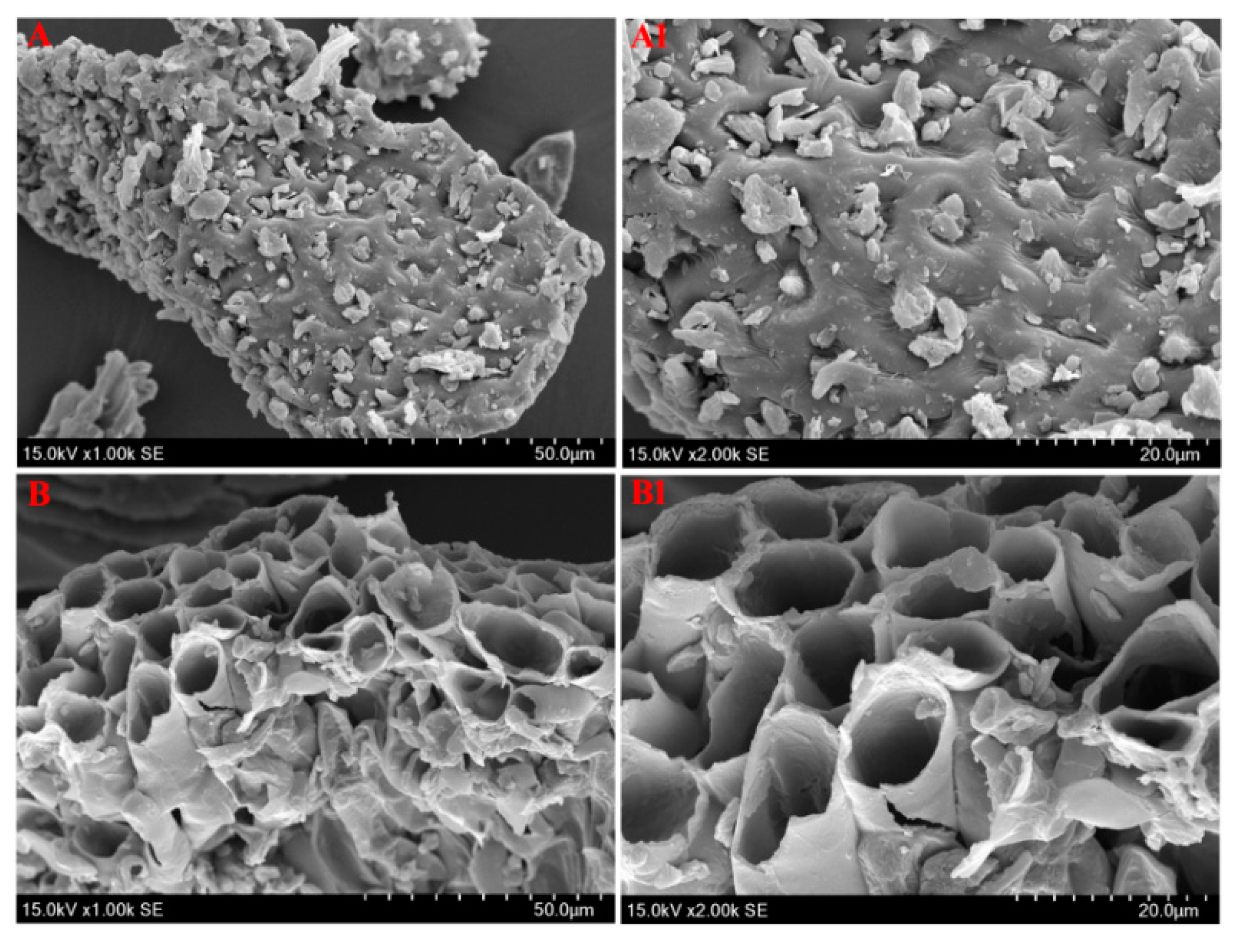
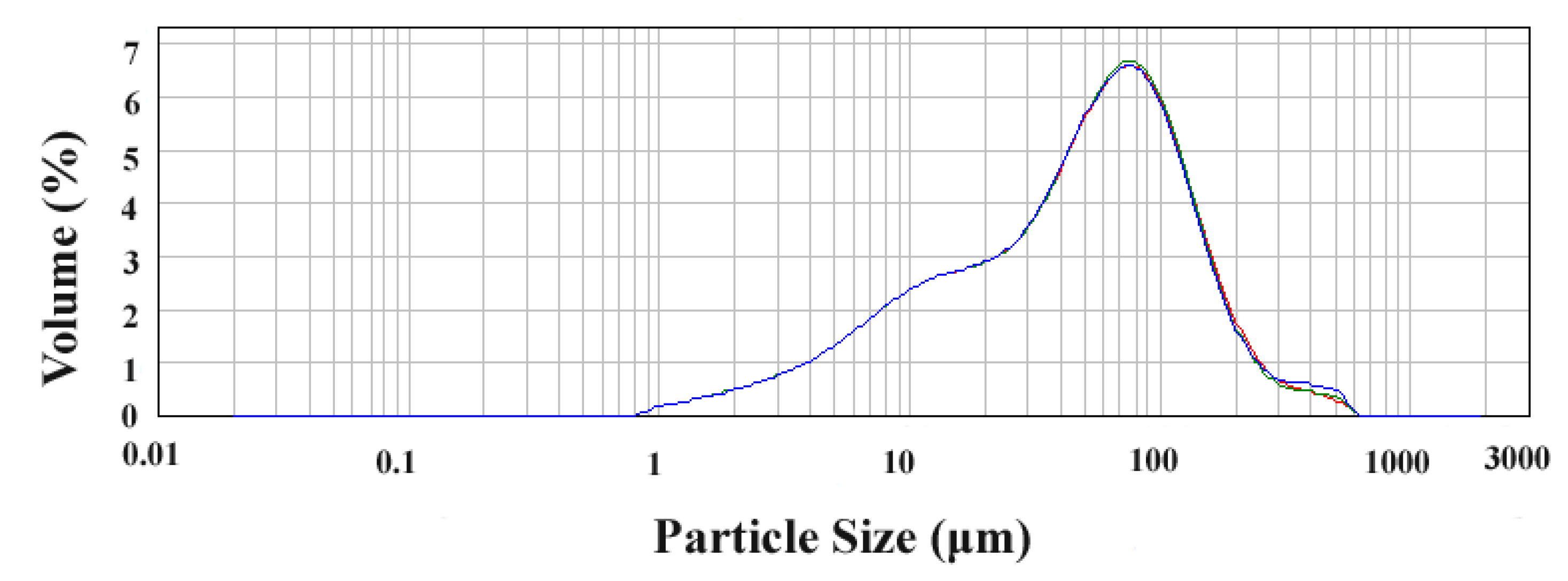
2.5. Comparison of Five Extraction Methods
2.6. Antioxidant of Total Biflavonoid Extract
3. Material and Methods
3.1. Materials
3.2. Samples Preparation
3.3. IL-Ultrasonic-Assisted Extraction
3.4. Determination of Total Biflavonoid Content
3.5. Experimental Design
3.5.1. Single-Factor Experiments
3.5.2. RSM Experiments
3.6. Recovery of TBF and IL
3.7. Conventional Reference Extraction Methods
3.7.1. Ethanol-Based UAE
3.7.2. Heat-Reflux Extraction
3.7.3. Soxhelt Extraction
3.7.4. Percolation Extraction
3.8. Evaluation of Antioxidant Activity
3.8.1. DPPH Radical Scavenging Assay
3.8.2. ABTS Radical Scavenging Assay
3.8.3. Ferric Ion Reducing Assay
3.8.4. Chelation of Ferrous Ions Assay
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Okigawa, M.; Hwa, C.W.; Kawano, N.; Rahman, W. Biflavones in Selaginella species. Phytochemistry 1971, 10, 3286–3287. [Google Scholar] [CrossRef]
- Alston, A.H.G. The Brazilian species of Selaginella. Feddes. Repert. 2010, 40, 303–319. [Google Scholar] [CrossRef]
- Gayathri, V.; Asha, V.V.; Subramoniam, A. Preliminary studies on the immunomodulatory and antioxidant properties of Selaginella species. Indian J. Pharmacol. 2005, 37, 381–385. [Google Scholar]
- Yan, Y.U.; Cui, G.H.; Zhong, L.I.; Wei, X. Preliminary studies of RAPD markers for ten Selaginella species from Guangdong Province. Guihaia 2007, 27, 48–52. [Google Scholar] [CrossRef]
- Wan, D.R.; Chen, K.L.; Wang, B.E. Anatomical study of the stems of the 10 Selaginella species and its taxonomic significance. J. Wuhan Bot. Res. 2008, 26, 343–349. [Google Scholar]
- Wang, G.; Yao, S.; Zhang, X.X.; Song, H. Rapid screening and structural characterization of antioxidants from the extract of Selaginella doederleinii Hieron with DPPH-UPLC-Q-TOF/MS method. Int. J. Anal. Chem. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.M.; Liang, L.Z.; Gan, T.Q.; Li, D.; Wang, G. Phytochemistry and Bioactivities of Biflavonoids: A Review. In Proceedings of the 6th International Conference on Bioinformatics and Biomedical Science, Jurong West, Singapore, 22–24 June 2017; pp. 105–108. [Google Scholar] [CrossRef]
- Huang, J.Y.; Shao, L.I.; Zhao, M.F.; Yu, L.I.; Lin, X.H.; Yao, H.; Pharmacy, D.O. Simultaneous determination of four biflavonoids in Selaginella doederleinii Hieron by HPLC. Chin. J. Hosp. Pharm. 2013, 23, 1945–1948. [Google Scholar]
- Lin, R.C.; Skaltsounis, A.L.; Seguin, E.; Tillequin, F.; Koch, M. Phenolic constttuents of Selaginella doederleinii. Planta Med. 1994, 60, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Hatware, K.V.; Sharma, S.; Patil, K.; Shete, M.; Karri, S.; Gupta, G. Evidence for gastroprotective, anti-inflammatory and antioxidant potential of methanolic extract of Cordia dichotoma leaves on indomethacin and stress induced gastric lesions in Wistar rats. Biomed. Pharmacother. 2018, 103, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Kim, H.S.; Yu, K.A.; Choe, Y.K.; Lim, J.S.; Yoon, D.Y. Identification of p7F, a bioflavonoid from natural product and analysis of its anti-inflammatory effects. FASEB J. 2002, 16, 1054–1058. [Google Scholar]
- Li, A.N.; Li, S.; Li, Y.; Xu, D.P.; Li, H.B. Optimization of ultrasound-assisted extraction of natural antioxidants from the Osmanthus fragrans flower. Molecules 2016, 21. [Google Scholar] [CrossRef]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Wang, G.; Yao, S.; Cheng, L.; Luo, Y.; Song, H. Antioxidant and anticancer effection of the volatile oil from various habitats of Selaginella doederleinii Hieron. Technol. Health Care 2015, 23, 21–27. [Google Scholar] [CrossRef]
- Wang, G.; Song, H.; Yao, S.; Zhang, Z.R. Optimization of the microwave-assisted extraction, GC-MS analysis and antimicrobial activity of the volatile oil constituents from Selaginella doederleinii Hieron. J. Invest. Med. 2013, 61, 13–16. [Google Scholar]
- Gonzalez, E.J.; Diaz, I.; Gonzalez-Miquel, M.; Rodriguez, M.; Sueiras, A. On the behavior of imidazolium versus pyrrolidinium ionic liquids as extractants of phenolic compounds from water: Experimental and computational analysis. Sep. Purif. Technol. 2018, 201, 214–222. [Google Scholar] [CrossRef]
- Dong, B.; Tang, J.; Yonannes, A.; Yao, S. Hexafluorophosphate salts with tropine-type cations in the extraction of alkaloids with the same nucleus from radix physochlainae. RSC Adv. 2018, 8, 262–277. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Domanska, U.; Schroeder, B.; Coutinho, J.A.P.; Pinho, S.P. Selection of ionic liquids to be used as separation agents for terpenes and terpenoids. ACS Sustain. Chem. Eng. 2016, 4, 548–556. [Google Scholar] [CrossRef]
- Santamarta, F.; Vilas, M.; Tojo, E.; Fall, Y. Synthesis and properties of novel chiral imidazolium-based ionic liquids derived from carvone. RSC Adv. 2016, 6, 31177–31180. [Google Scholar] [CrossRef]
- Lopez, C.J.; Caleja, C.; Prieto, M.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization and comparison of heat and ultrasound assisted extraction techniques to obtain anthocyanin compounds from Arbutus unedo L. fruits. Food Chem. 2018, 264, 81–91. [Google Scholar] [CrossRef]
- Pereira, M.G.; Hamerski, F.; Andrade, E.F.; Scheer, A.D.P.; Corazza, M.L. Assessment of subcritical propane, ultrasound-assisted and Soxhlet extraction of oil from sweet passion fruit (Passiflora alata Curtis) seeds. J. Supercrit. Fluid 2017, 128, 338–348. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, L.; Liu, F.; Li, T.; Yu, Z.; Xu, Y.; Yang, Y. Optimization of ultrasound-assisted extraction and structural characterization of the Polysaccharide from Pumpkin (Cucurbita moschata) Seeds. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Wang, T.; Guo, N.; Wang, S.X.; Kou, P.; Zhao, C.; Fu, Y.J. Ultrasound-negative pressure cavitation extraction of phenolic compounds from blueberry leaves and evaluation of its DPPH radical scavenging activity. Food. Biopro. Process 2018, 108, 69–80. [Google Scholar] [CrossRef]
- Ji, S.; Wang, Y.J.; Su, Z.Y.; He, D.D.; Du, Y.; Guo, M.Z.; Yang, D.Z.; Tang, D.Q. Ionic liquids-ultrasound based efficient extraction of flavonoid glycosides and triterpenoid saponins from licorice. RSC Adv. 2018, 8, 13989–13996. [Google Scholar] [CrossRef]
- Tan, Z.J.; Wang, C.Y.; Yang, Z.Z.; Yi, Y.J.; Wang, H.Y.; Zhou, W.L.; Li, F.F. Ionic liquid-based ultrasonic-assisted extraction of Secoisolariciresinol diglucoside from flaxseed (Linum usitatissimum L.) with further purification by an aqueous two-phase system. Molecules 2015, 20. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Lee Kee, H.A. Ionic liquid-based ultrasound-assisted dispersive liquid–liquid microextraction followed high-performance liquid chromatography for the determination of ultraviolet filters in environmental water samples. Anal. Chim. Acta 2012, 750, 120–126. [Google Scholar] [CrossRef]
- He, Z.J.; Wang, X.M.; Yao, T.; Song, H.; Yao, S. Determination and correlation of solubilities of four novel benzothiazolium ionic liquids with 6 PF- in six alcohols. Chin. J. Chem. Eng. 2014, 22, 435–446. [Google Scholar] [CrossRef]
- Li, D.; Qian, Y.; Tian, Y.J.; Yuan, S.M.; Wei, W.; Wang, G. Optimization of ionic liquid-assisted extraction of biflavonoids from Selaginella doederleinii and evaluation of its antioxidant and antitumor activity. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wen, X.; Chen, X.; He, Z.; Ni, Y. Optimization of ultrasound-assisted extraction of okra (Abelmoschus esculentus (L.) Moench) polysaccharides based on response surface methodology and antioxidant activity. Int. J. Biol. Macromol. 2018, 114, 1056–1063. [Google Scholar] [CrossRef]
- Al-Othman, A.; Tawalbeh, M.; Assad, M.E.; Alkayyali, T.; Eisa, A. Novel multi-stage flash (MSF) desalination plant driven by parabolic trough collectors and a solar pond: A simulation study in UAE. Desalination 2018, 443, 237–244. [Google Scholar] [CrossRef]
- Giacometti, J.; Zauhar, G.; Zuvic, M. Optimization of ultrasonic-assisted extraction of major phenolic compounds from Olive leaves (Olea europaea L.) using response surface methodology. Foods 2018, 7, 149. [Google Scholar] [CrossRef]
- Liu, S.; Dong, Z.B.; Xiang, D.; Jiang, Y.; Tao, Q.B.; Cao, Y. Crossing-link of experimental reducibility tests, XPS characterizations and DFT estimates on ferrite oxygen carriers in CLC. Appl. Catal. B Environ. 2018, 238, 647–655. [Google Scholar] [CrossRef]
- Wang, G.; Li, S.H.; Zhou, H.L. Comparison of antioxidant and anticancer of the extracts from various habitats of Selageinella doederleinii. Adv. Eng. Res. 2016, 12, 231–235. [Google Scholar]
- Espada-Bellido, E.; Ferreiro-Gonzalez, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Mei, X.; Shen, B.X.; Lu, F.J.; Zhou, W.J.; Si, M.; Chakraborty, S. Oxidation of NO by in situ Fenton reaction system with dual ions as reagents. Chem. Eng. J. 2018, 351, 660–667. [Google Scholar] [CrossRef]
- Peng, L.Q.; Yu, W.Y.; Xu, J.J.; Cao, J. Pyridinium ionic liquid-based liquid-solid extraction of inorganic and organic iodine from Laminaria. Food Chem. 2018, 239, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Zhang, P.; Yan, S.; He, X.; Chen, F. Ionic liquid-based ultrasound-assisted extraction of chlorogenic acid from Lonicera japonica thunb. Chromatographia 2011, 73, 129–133. [Google Scholar] [CrossRef]
- Ali, N.; Shah, I.; Shah, S.W.A.; Ahmed, G.; Shoaib, M.; Junaid, M.; Ali, W.; Ahmed, Z. Antioxidant and relaxant activity of fractions of crude methanol extract and essential oil of Artemisia macrocephala jacquem. BMC Complement. Altern. Med. 2013, 13, 96–100. [Google Scholar] [CrossRef]
- Rontani, J.F.; Amiraux, R.; Lalande, C.; Babin, M.; Kim, H.R.; Belt, S.T. Use of palmitoleic acid and its oxidation products for monitoring the degradation of ice algae in Arctic waters and bottom sediments. Org. Geochem. 2018, 124, 88–102. [Google Scholar] [CrossRef]
- Wang, G.; Li, S.H.; Zhou, H.L.; Jiang, Y.M. Phytochemical screening, antioxidant, antibacterial and cytotoxic activities of different extracts of Selaginella doederleinii Bangladesh. J. Bot. 2017, 46, 1193–1201. [Google Scholar] [CrossRef]
- Arti, A.; Muraleedharan, G.N.; Gale, M.S. Structure-activity relationships for antioxidant activities of a series flavonoids in a liposomal system. Free Radic. Biol. Med. 1998, 24, 1355–1363. [Google Scholar] [CrossRef]
- Paola, M.; Alessandra, B.; Cosimo, P.; Nunziatina, D.T. Structure–antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as ‘green’ solvents for extractions. J. Chem. Technol. Biot. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Wang, W.C.; Li, Q.Y.; Liu, Y.H.; Chen, B.B. Ionic liquid-aqueous solution ultrasonic-assisted extraction of three kinds of alkaloids from Phellodendron amurense Rupr and optimize conditions use response surface. Ultrason. Sonochem. 2015, 24, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Bai, M.G.; Qin, Y.C.; Liu, B.T. Application of ionic liquid-based ultrasonic-assisted extraction of flavonoids from bamboo leaves. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Zhang, J.J.; Li, Y.; Meng, X.; Li, H.B. Microwave-assisted extraction of phenolic compounds from Melastoma sanguineum fruit: Optimization and identification. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S. Percolation as new method of preparation of modified biosorbents for pollutants remova. Chem. Eng. J. 2016, 283, 1305–1314. [Google Scholar] [CrossRef]
- Ullah, F.; Iqbal, N.; Ayaz, M.; Sadiq, A.; Ullah, I.; Ahmad, S.; Imran, M. DPPH, ABTS free radical scavenging, antibacterial and phytochemical evaluation of crude methanolic extract and subsequent fractions of Chenopodium botrys aerial parts. Pak. J. Pharm. Sci. 2017, 30, 761–766. [Google Scholar] [PubMed]
- Chung, J.H.; Kong, J.N.; Choi, H.E.; Kong, K.H. Antioxidant, anti-inflammatory, and anti-allergic activities of the sweet-tasting protein brazzein. Food Chem. 2018, 267, 163–169. [Google Scholar] [CrossRef]
- Oztaskin, N.; Taslimi, P.; Maras, A.; Gulcin, I.; Goksu, S. Novel antioxidant bromophenols with acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase inhibitory actions. Bioorg. Chem. 2017, 74, 104–114. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrieres, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef] [PubMed]
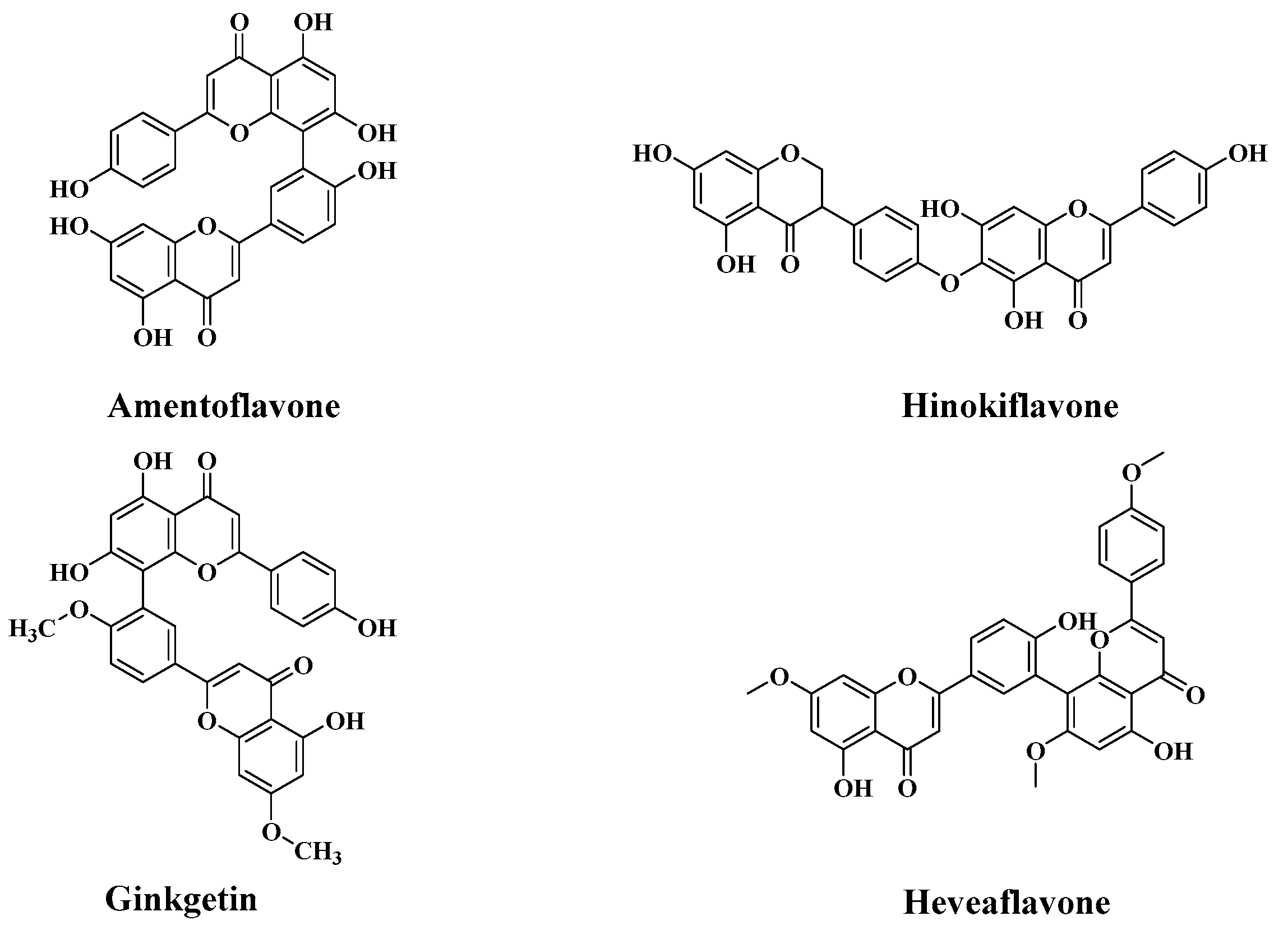
Sample Availability: Samples of the compounds amentoflavone, ginkgetin, hinokiflavone and heveaflavone are available from the authors. |





| Source | Sum of Squares | df | F Value | p Value | R2 | R2 (Adj) | Significant |
|---|---|---|---|---|---|---|---|
| Model | 67.92 | 9 | 180.95 | <0.0001 | 0.9957 | 0.9902 | significant |
| X1 | 4.06 | 1 | 97.38 | 0.0015 | |||
| X2 | 0.73 | 1 | 17.41 | 0.0042 | |||
| X3 | 16.79 | 1 | 402.60 | <0.0001 | |||
| X1X2 | 2.250 × 10−4 | 1 | 5.395 × 10−3 | 0.9435 | |||
| X1X3 | 1.58 | 1 | 37.76 | 0.0005 | |||
| X2X3 | 0.023 | 1 | 0.54 | 0.4865 | |||
| X12 | 13.04 | 1 | 312.54 | <0.0001 | |||
| X22 | 12.49 | 1 | 299.36 | <0.0001 | |||
| X32 | 14.52 | 1 | 348.14 | <0.0001 | |||
| Residual | 0.29 | 7 | |||||
| Lack of Fit | 0.21 | 3 | 3.37 | 0.1355 | not significant | ||
| Pure Error | 0.083 | 4 | |||||
| Cor Total | 68.21 | 16 |
| Ethyl Acetate | Chloroform | n-Butanol | Dichloromethane | Ether | n-Hexane | |
|---|---|---|---|---|---|---|
| TBFs | 96.23 ± 0.53 | 38.22 ± 0.31 | 87.56 ± 0.28 | 50.75 ± 0.37 | 73.69 ± 0.34 | 15.76 ± 0.49 |
| IL | 97.67 ± 0.44 | 94.59 ± 0.45 | 97.21 ± 0.39 | 98.46 ± 0.25 | 95.17 ± 0.52 | 96.29 ± 0.38 |
| Ionic Liquids | Cations | Anions |
|---|---|---|
| [C4mim]Cl (1-butyl-3-methylimidazolium-chloride) | 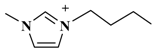 | Cl− |
| [C4mim]NO3 (1-butyl-3-methylimidazolium-nitrate) | 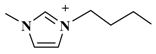 | NO3− |
| [C4mim]PF6 (1-butyl-3-methylimidazolium-hexafluorophosphate) |  | PF6− |
| [C4mim]Br (1-butyl-3-methylimidazolium-bromine) |  | Br− |
| [C4mim]BF4 (1-butyl-3-methylimidazolium-tetrafluoroborate) | 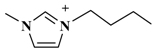 | BF4− |
| [C4mim]CH3COO (1-butyl-3-methyli-midazolium-acetate) | 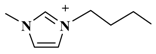 | CH3COO− |
| [C2mim]PF6 (1-ethyl-3-methylimidazolium-hexafluorophosphate) | 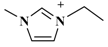 | PF6− |
| [C6mim]PF6 (1-hexyl-3-methylimidazolium-hexafluorophosphate) | 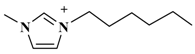 | PF6− |
| [C8mim]PF6 (1-octyl-3-methylimida-zolium-hexafluorophosphate) | 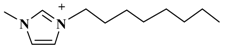 | PF6− |
| [C10mim]PF6 (1-decyl-3-methylimidazolium-hexafluorophosphate) | 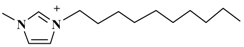 | PF6− |
| [C12mim]PF6 (1-dodecyl-3-methylimidazolium-hexafluorophosphate) |  | PF6− |
| Level | Factors | ||
|---|---|---|---|
| Solid–Liquid Ratio (g/mL) | IL Concentration (mmol/L) | Extract Temperature (°C) | |
| −1 | 1:10 | 0.6 | 40 |
| 0 | 1:12 | 0.8 | 50 |
| 1 | 1:14 | 1.0 | 60 |
| Run | X1 | X2 | X3 | Y |
|---|---|---|---|---|
| Extract Temperature (°C) | Solid–Liquid Ratio (g/mL) | IL Concentration (mmol/L) | Yield of TBFs (mg/g) | |
| 1 | 60 | 12 | 0.6 | 14.66 |
| 2 | 60 | 10 | 0.8 | 13.94 |
| 3 | 60 | 14 | 0.8 | 14.61 |
| 4 | 50 | 12 | 0.8 | 18.22 |
| 5 | 50 | 12 | 0.8 | 18.51 |
| 6 | 50 | 14 | 0.6 | 16.57 |
| 7 | 40 | 12 | 1.0 | 13.62 |
| 8 | 40 | 10 | 0.8 | 15.18 |
| 9 | 50 | 12 | 0.8 | 18.25 |
| 10 | 50 | 14 | 1.0 | 13.56 |
| 11 | 50 | 12 | 0.8 | 18.41 |
| 12 | 50 | 10 | 1.0 | 12.89 |
| 13 | 50 | 10 | 0.6 | 16.20 |
| 14 | 40 | 12 | 0.6 | 17.51 |
| 15 | 60 | 12 | 1.0 | 13.28 |
| 16 | 50 | 12 | 0.8 | 18.53 |
| 17 | 40 | 14 | 0.8 | 15.88 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Li, D.; Ma, X.; Jiang, F.; He, Q.; Qiu, S.; Li, Y.; Wang, G. Ionic Liquid–Ultrasound-Based Extraction of Biflavonoids from Selaginella helvetica and Investigation of Their Antioxidant Activity. Molecules 2018, 23, 3284. https://doi.org/10.3390/molecules23123284
Jiang Y, Li D, Ma X, Jiang F, He Q, Qiu S, Li Y, Wang G. Ionic Liquid–Ultrasound-Based Extraction of Biflavonoids from Selaginella helvetica and Investigation of Their Antioxidant Activity. Molecules. 2018; 23(12):3284. https://doi.org/10.3390/molecules23123284
Chicago/Turabian StyleJiang, Yongmei, Dan Li, Xiankui Ma, Fengqin Jiang, Qun He, Shaoliang Qiu, Yan Li, and Gang Wang. 2018. "Ionic Liquid–Ultrasound-Based Extraction of Biflavonoids from Selaginella helvetica and Investigation of Their Antioxidant Activity" Molecules 23, no. 12: 3284. https://doi.org/10.3390/molecules23123284
APA StyleJiang, Y., Li, D., Ma, X., Jiang, F., He, Q., Qiu, S., Li, Y., & Wang, G. (2018). Ionic Liquid–Ultrasound-Based Extraction of Biflavonoids from Selaginella helvetica and Investigation of Their Antioxidant Activity. Molecules, 23(12), 3284. https://doi.org/10.3390/molecules23123284




