Purification and Functional Characterization of the C-Terminal Domain of the β-Actin-Binding Protein AIM1 In Vitro
Abstract
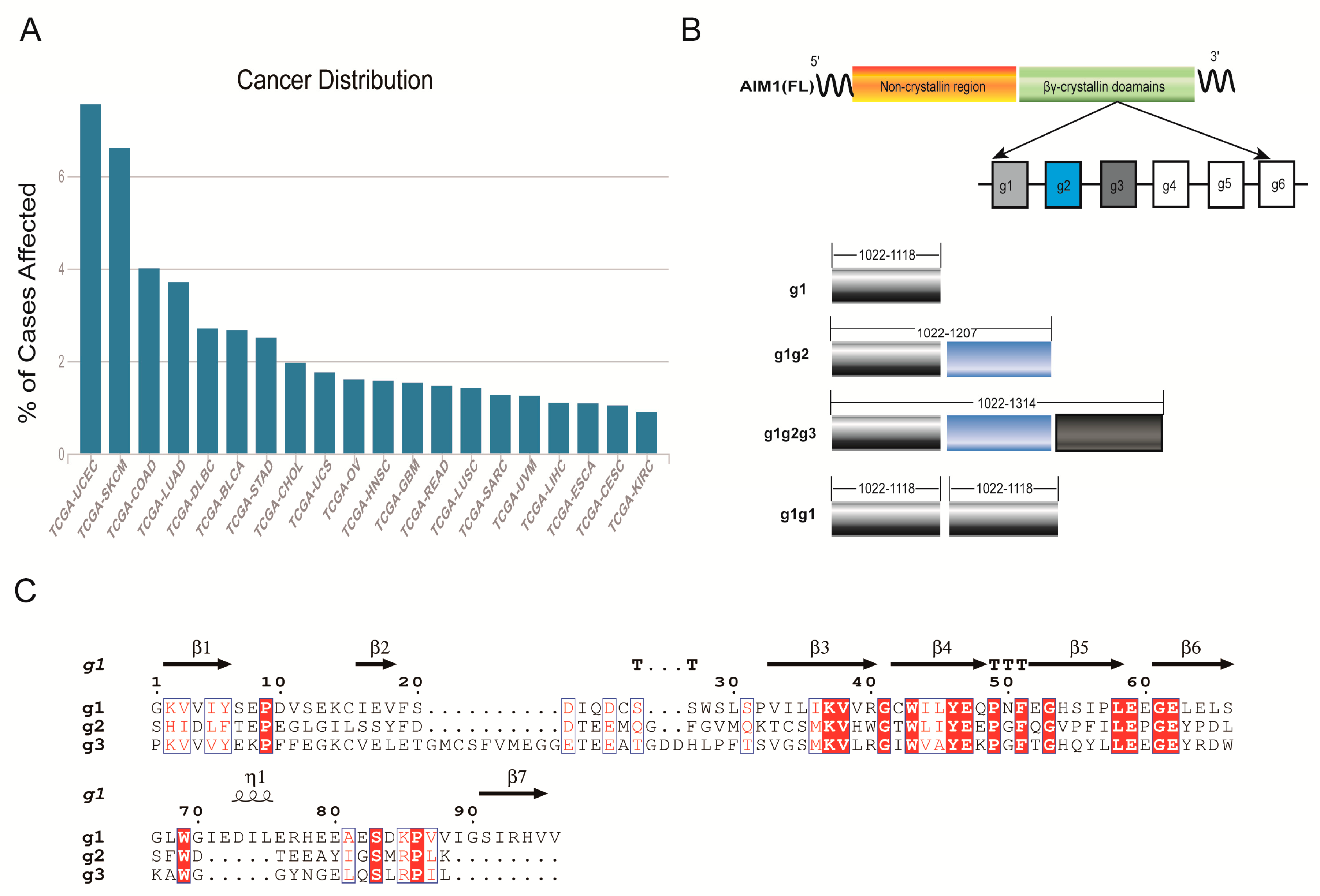
1. Introduction
2. Results
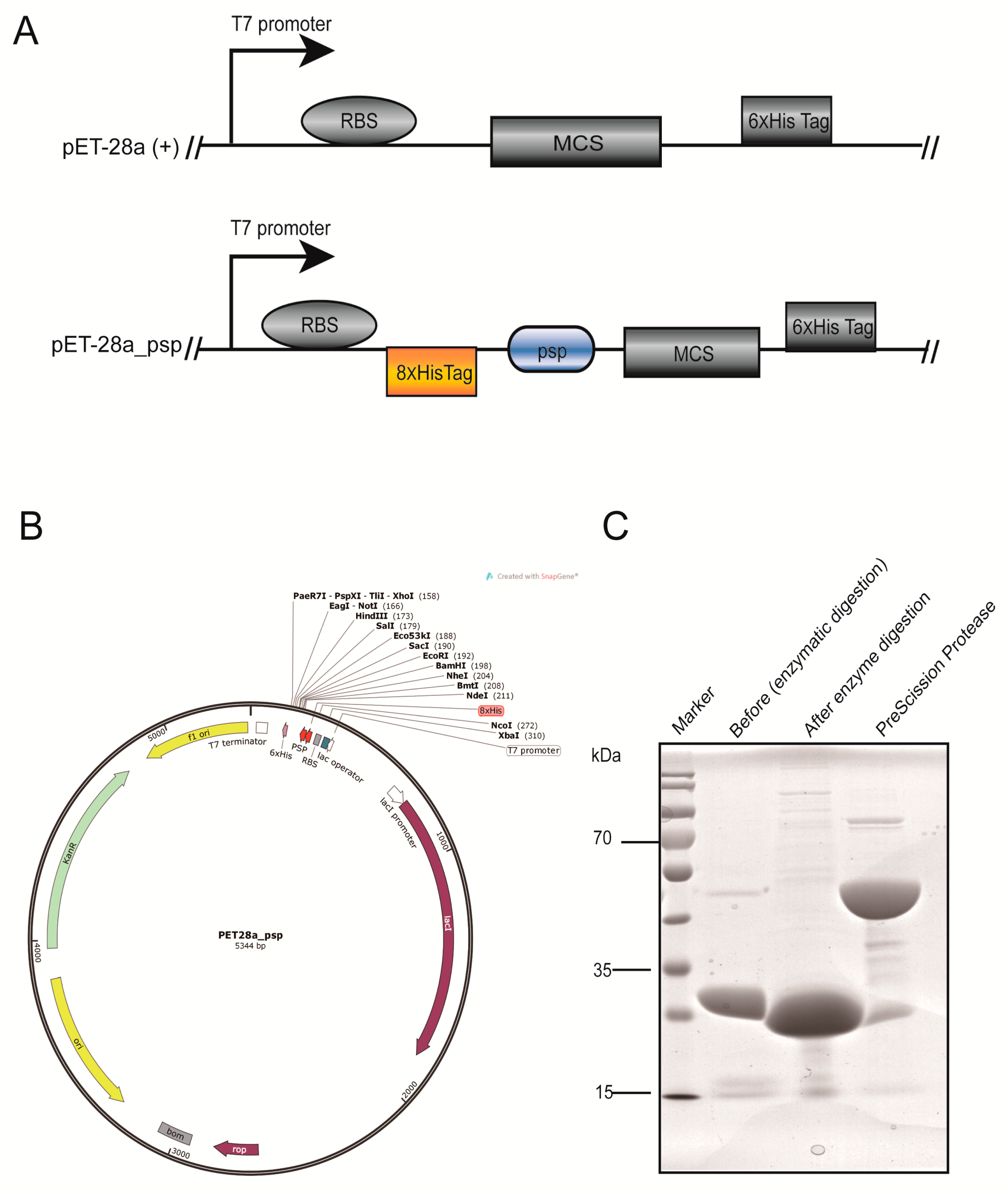
2.1. Modification of the Vector and Preparation of Prescission Protease
2.2. The Cloning and Overexpression of g1, g1g2, g1g2g3, and g1g1
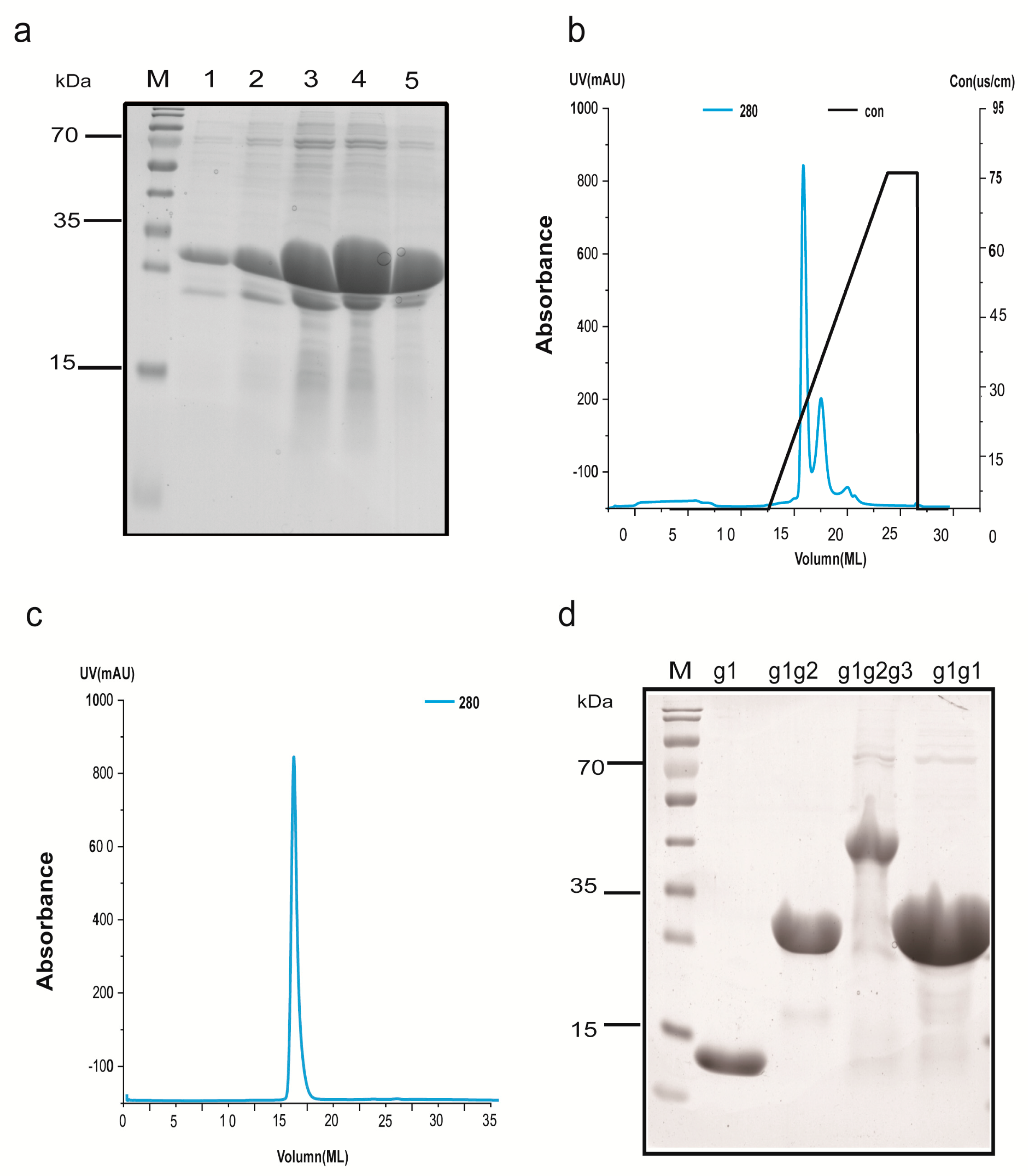
2.3. Protein Extraction and Purification
2.4. Characterization of AIM1 (g1, g1g2, g1g2g3, and g1g1) Formation in Solution
2.5. Actin-Binding Assay
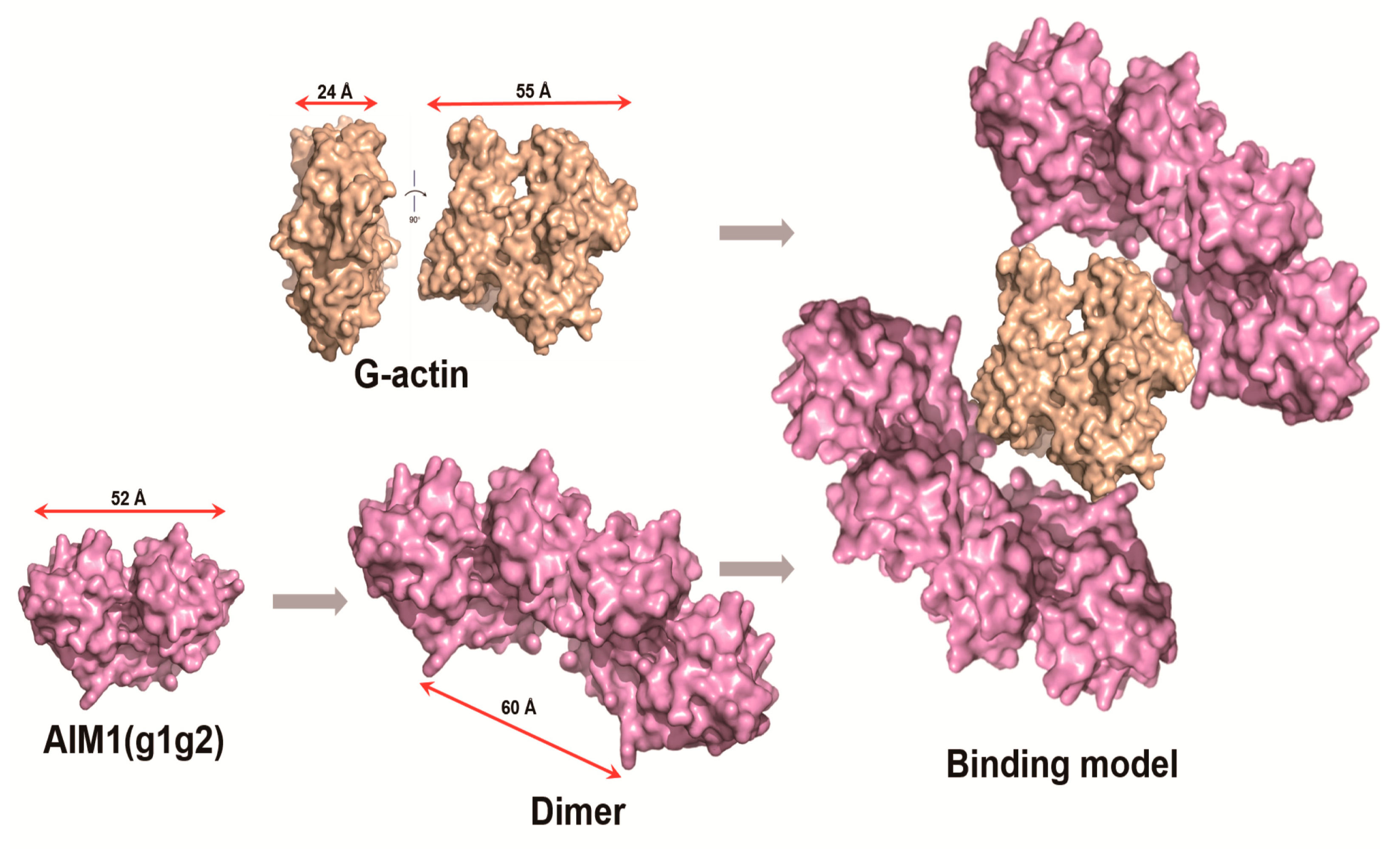
2.6. Structural Model of g1g2 Interacting with G-Actin
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Construction of the pET-28a_psp Vector
4.3. Cloning of AIM1 (g1, g1g2, g1g2g3, g1g1) into the pET-28a_psp Expression Vector
4.4. Expression of AIM1 (g1, g1g2, g1g2g3, and g1g1) in Escherichia coli
4.5. Protein Extraction and Purification
4.6. Dot Blot Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Gorlov, I.P.; Byun, J.; Gorlova, O.Y.; Aparicio, A.M.; Efstathiou, E.; Logothetis, C.J. Candidate pathways and genes for prostate cancer: A meta-analysis of gene expression data. BMC Med. Genom. 2009, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Nurnberg, A.; Kitzing, T.; Grosse, R. Nucleating actin for invasion. Nat. Rev. Cancer 2011, 11, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Purnell, D.M.; Heatfield, B.M.; Anthony, R.L.; Trump, B.F. Immunohistochemistry of the cytoskeleton of human prostatic epithelium. Evidence for disturbed organization in neoplasia. Am. J. Pathol. 1987, 126, 384–395. [Google Scholar] [PubMed]
- Sanz-Moreno, V.; Marshall, C.J. The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr. Opin. Cell Biol. 2010, 22, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ali, M.R.K. Gold Nanorod Photothermal Therapy Alters Cell Junctions and Actin Network in Inhibiting Cancer Cell Collective Migration. ACS Nano 2018, 12, 9279–9290. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Esopi, D.M.; Chaux, A.; Gurel, M.; Ghosh, S.; Vaghasia, A.M.; Tsai, H.; Kim, K.; Castagna, N.; Lam, H.; et al. AIM1 is an actin-binding protein that suppresses cell migration and micrometastatic dissemination. Nat. Commun. 2017, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Winder, S.J.; Ayscough, K.R. Actin-binding proteins. J. Cell Sci. 2005, 118, 651–654. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ling, L.; Liao, Y.; Li, S.; Han, W.; Zhao, B.; Sun, Y.; Qiu, H.J. Beta-actin interacts with the E2 protein and is involved in the early replication of classical swine fever virus. Virus Res. 2014, 179, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Pantaloni, D.; Le Clainche, C.; Carlier, M.F. Mechanism of actin-based motility. Science 2001, 292, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Trent, J.M.; Stanbridge, E.J.; McBride, H.L.; Meese, E.U.; Casey, G.; Araujo, D.E.; Witkowski, C.M.; Nagle, R.B. Tumorigenicity in human melanoma cell lines controlled by introduction of human chromosome 6. Science 1990, 247, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.E.; Su, Y.A.; Meltzer, P.S.; Trent, J.M. Isolation and characterization of genes associated with chromosome-6 mediated tumor suppression in human malignant melanoma. Oncogene 1996, 12, 2527–2533. [Google Scholar] [PubMed]
- Hoshimoto, S.; Kuo, C.T.; Chong, K.K.; Takeshima, T.L.; Takei, Y.; Li, M.W.; Huang, S.K.; Sim, M.S.; Morton, D.L.; Hoon, D.S. AIM1 and LINE-1 epigenetic aberrations in tumor and serum relate to melanoma progression and disease outcome. J. Investig. Dermatol. 2012, 132, 1689–1697. [Google Scholar] [CrossRef]
- Rosenbaum, E.; Begum, S.; Brait, M.; Zahurak, M.; Maldonado, L.; Mangold, L.A.; Eisenberger, M.A.; Epstein, J.I.; Partin, A.W.; Sidransky, D.; et al. AIM1 promoter hypermethylation as a predictor of decreased risk of recurrence following radical prostatectomy. Prostate 2012, 72, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, U.; Ray, M.E.; Ellison, J.; Graham, C.; Wistow, G.; Meltzer, P.S.; Trent, J.M.; Pavan, W.J. Cloning and tissue expression of the mouse ortholog of AIM1, a betagamma-crystallin superfamily member. Mamm. Genome 1998, 9, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.E.; Wistow, G.; Su, Y.A.; Meltzer, P.S.; Trent, J.M. AIM1, a novel non-lens member of the betagamma-crystallin superfamily, is associated with the control of tumorigenicity in human malignant melanoma. Proce. Natl. Acad. Sci. USA 1997, 94, 3229–3234. [Google Scholar] [CrossRef]
- Bhat, S.P. Crystallins, genes and cataract. Prog. Drug Res. 2003, 60, 205–262. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.A.; Cooper, J.A. Control of actin assembly at filament ends. Annu. Rev. Cell Dev. Biol. 1995, 11, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Aravind, P.; Rajini, B.; Sharma, Y.; Sankaranarayanan, R. Crystallization and preliminary X-ray crystallographic investigations on a βγ-crystallin domain of absent in melanoma 1 (AIM1), a protein from Homo sapiens. Acta Crystallogr. 2006, 62, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Krishnan, B.; Srivastava, S.S.; Sharma, Y. Microbial betagamma-crystallins. Prog. Biophys. Mol. Biol. 2014, 115, 42–51. [Google Scholar] [CrossRef]
- Wang, H.; Robinson, R.C.; Burtnick, L.D. The structure of native G-actin. Cytoskeleton 2010, 67, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Brait, M.; Begum, S.; Carvalho, A.L.; Dasgupta, S.; Vettore, A.L.; Czerniak, B.; Caballero, O.L.; Westra, W.H.; Sidransky, D.; Hoque, M.O. Aberrant promoter methylation of multiple genes during pathogenesis of bladder cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Loyo, M.; Brait, M.; Kim, M.S.; Ostrow, K.L.; Jie, C.C.; Chuang, A.Y.; Califano, J.A.; Liegeois, N.J.; Begum, S.; Westra, W.H.; et al. A survey of methylated candidate tumor suppressor genes in nasopharyngeal carcinoma. Int. J. Cancer 2011, 128, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Nakayama, Y.; Hori, A.; Yoshimura, K. Biomarkers for predicting the sensitivity of cancer cells to TRAIL-R1 agonistic monoclonal antibody. Cancer Lett. 2010, 292, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Iwasa, M.; Aihara, T.; Maeda, Y.; Narita, A. The nature of the globular- to fibrous-actin transition. Nature 2009, 457, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Rajini, B.; Graham, C.; Wistow, G.; Sharma, Y. Stability, homodimerization, and calcium-binding properties of a single, variant betagamma-crystallin domain of the protein absent in melanoma 1 (AIM1). Biochemistry 2003, 42, 4552–4559. [Google Scholar] [CrossRef] [PubMed]
- Aravind, P.; Wistow, G.; Sharma, Y.; Sankaranarayanan, R. Exploring the limits of sequence and structure in a variant betagamma-crystallin domain of the protein absent in melanoma-1 (AIM1). J. Mol. Biol. 2008, 381, 509–518. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Gene Name | Primers |
|---|---|
| g1 | F: GCGGCAGCGGATCCATGGGTAAAGTGGTTATCTACAGC R: TTGCACTTGTCGACTCAAACCACGTGACGAATGCTACC |
| g1g2 | F: GCGGCAGCGGATCCATGGGTAAAGTGGTTATCTACAGC R: TTGCACTTGTCGACTCACTTCAGCGGACGCATGCTGCC |
| g1g2g3 | F: GCGGCAGCGGATCCATGGGTAAAGTGGTTATCTACAGC R: TTGCACTTGTCGACTCACAGGATCGGGCGCAGGCTTTG |
| g1g1 | F1: GCGGCAGCGGATCCATGGGTAAAGTGGTTATCTACAG R1: TTGCACTTGAATTCAACACGGTAGTCCTGAACCAC F2: GCGGCAGCGAATTCATGGGTAAAGTGGTTATCTACAGC R2: TTGCACTTGTCGACTCAAACCACGTGACGAATGCTACC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Cheng, L.; Yu, Q.; Zhang, L.; Li, H.; Wang, C. Purification and Functional Characterization of the C-Terminal Domain of the β-Actin-Binding Protein AIM1 In Vitro. Molecules 2018, 23, 3281. https://doi.org/10.3390/molecules23123281
Wu F, Cheng L, Yu Q, Zhang L, Li H, Wang C. Purification and Functional Characterization of the C-Terminal Domain of the β-Actin-Binding Protein AIM1 In Vitro. Molecules. 2018; 23(12):3281. https://doi.org/10.3390/molecules23123281
Chicago/Turabian StyleWu, Fang, Liangkai Cheng, Qi Yu, Lin Zhang, Hong Li, and Caiyan Wang. 2018. "Purification and Functional Characterization of the C-Terminal Domain of the β-Actin-Binding Protein AIM1 In Vitro" Molecules 23, no. 12: 3281. https://doi.org/10.3390/molecules23123281
APA StyleWu, F., Cheng, L., Yu, Q., Zhang, L., Li, H., & Wang, C. (2018). Purification and Functional Characterization of the C-Terminal Domain of the β-Actin-Binding Protein AIM1 In Vitro. Molecules, 23(12), 3281. https://doi.org/10.3390/molecules23123281





