HPLC-ESI-MS/MS Profiling of Polyphenolics of a Leaf Extract from Alpinia zerumbet (Zingiberaceae) and Its Anti-Inflammatory, Anti-Nociceptive, and Antipyretic Activities In Vivo
Abstract
1. Introduction
2. Results
2.1. Identification of the Polyphenolics in a Methanol Extract from A. Zerumbet Leaves
2.2. Antioxidant Activities
2.3. Inhibition of Cyclooxygenase (COX1/2) and Lipoxygenase (LOX)
2.4. Effects of the Extract on Carrageenan-Induced Paw Edema in Rats
2.5. Effects of the Extract on Carrageenan-Induced Leukocyte Migration into The Peritoneal Cavity in Mice
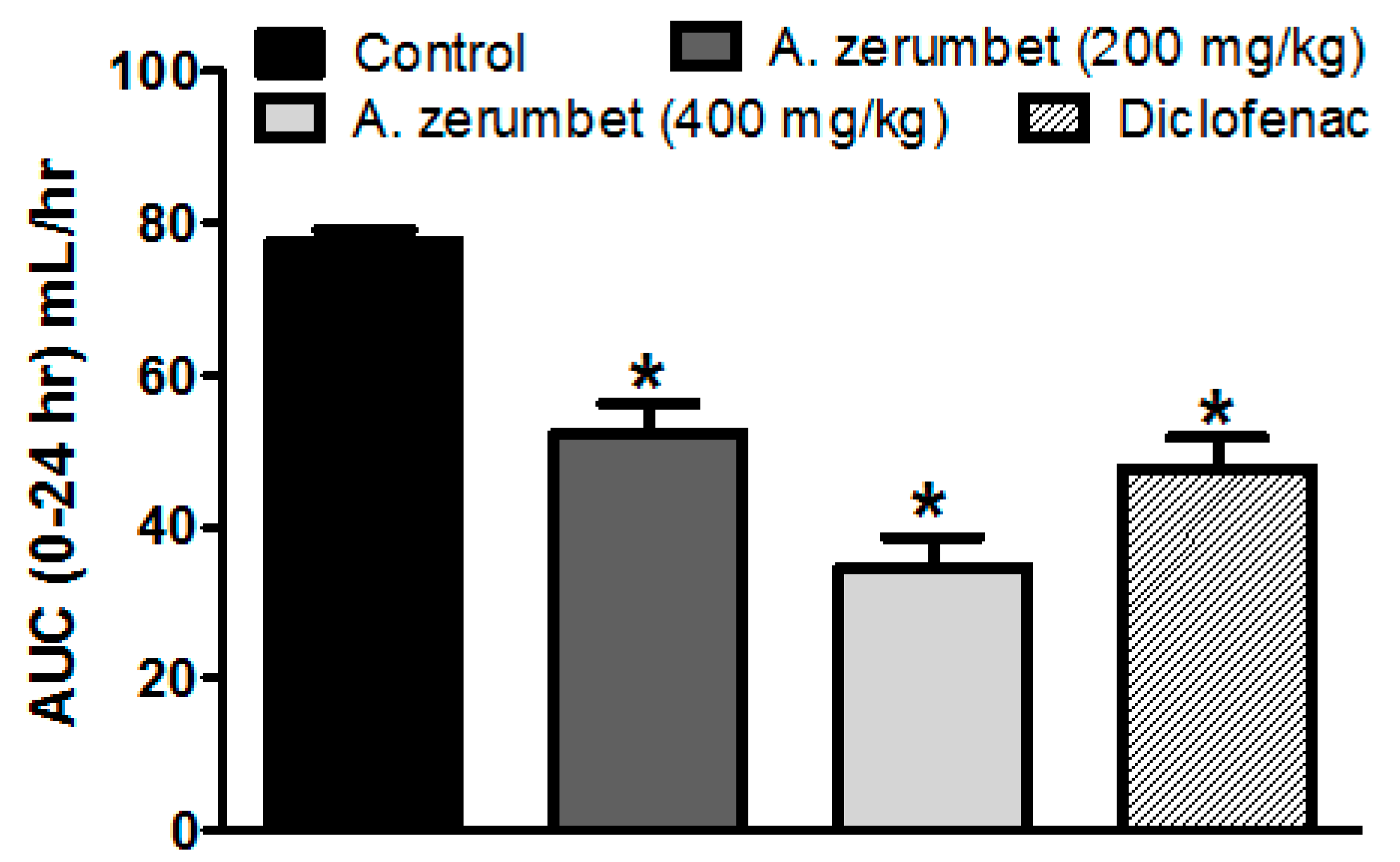
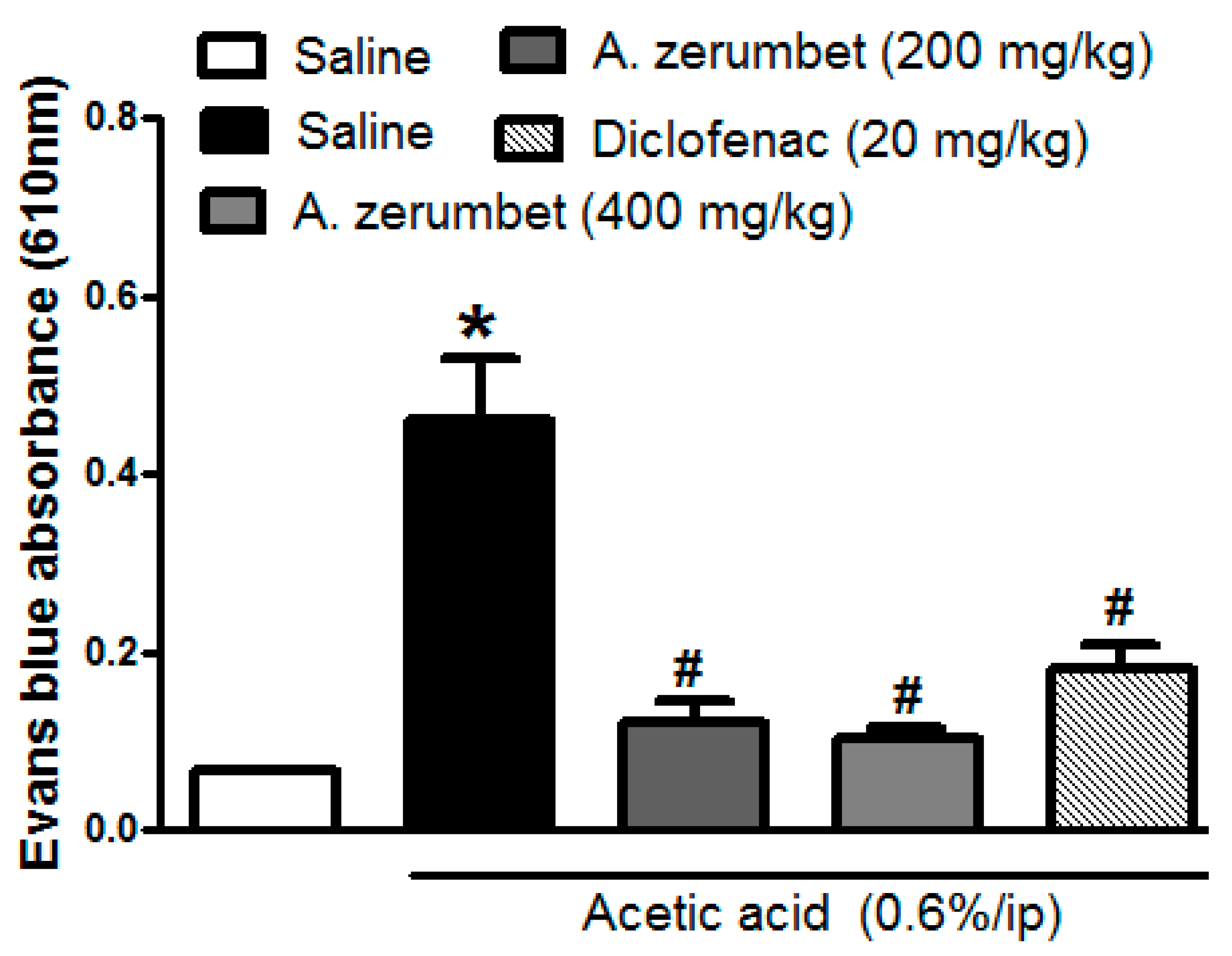
2.6. Effects of the Extract on Acetic Acid-Induced Vascular Permeability in Mice
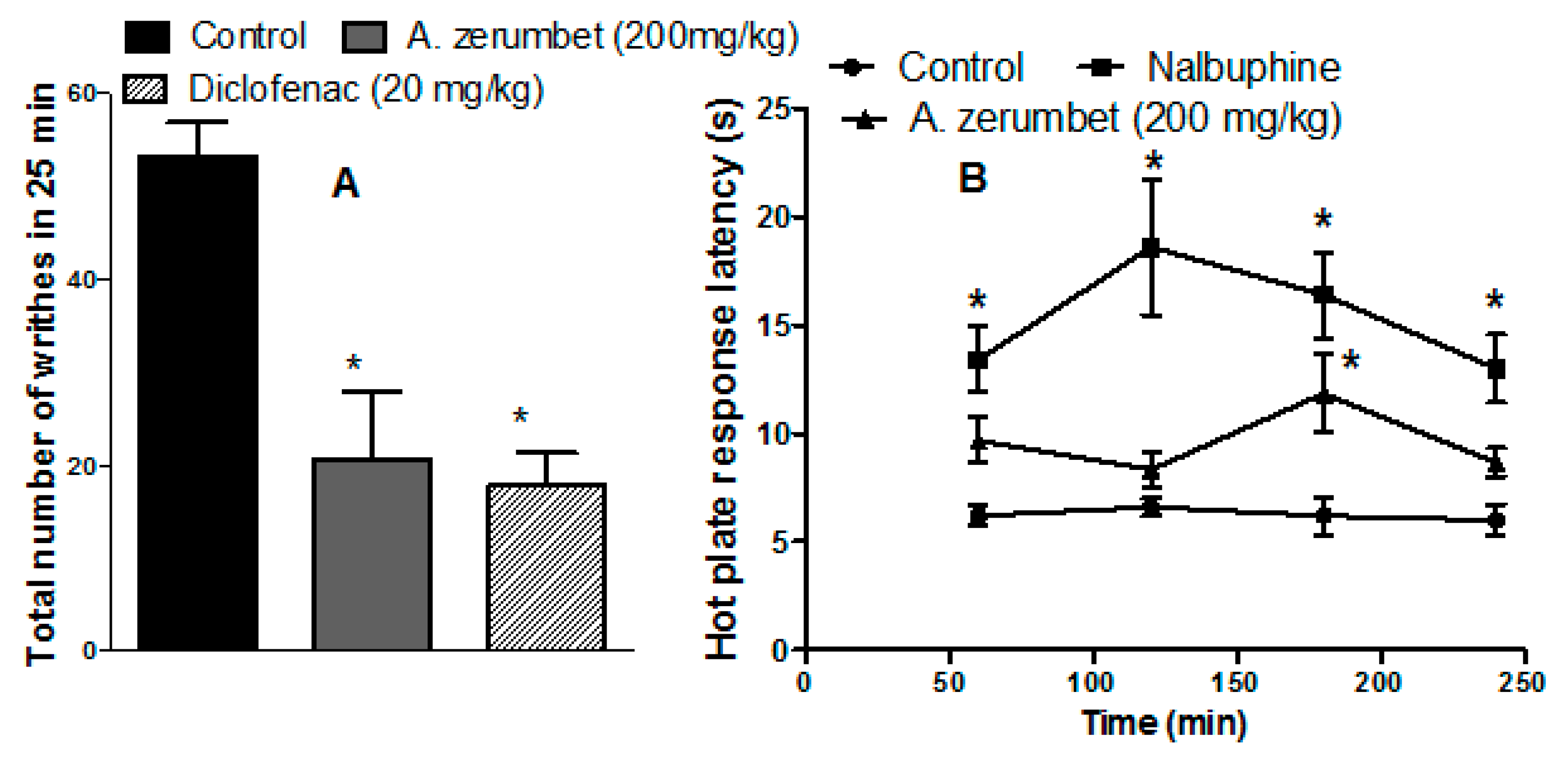
2.7. Effects of the Extract on Acetic Acid-Induced Writhing in Mice
2.8. Anti-Nociceptive Effects of the Extract in Mice on Hot Plates
2.9. Antipyretic Effect of the Extract in Mice Injected with Brewer’s Yeast
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. HPLC-ESI-MS/MS Conditions
4.3. Total Phenolic Content (TPC) and Antioxidant Assays
4.4. In Vitro Anti-Inflammatory Studies
Cyclooxygenase (COX) and Lipoxygenase (LOX) Inhibition Assays
4.5. In Vivo Anti-Inflammatory Studies
4.5.1. Anti-Inflammatory Activity in Carrageenan-Induced Hind-Paw Edema in Rats
4.5.2. Recruitment of Leukocytes to Peritoneal Cavity in Mice
4.5.3. Acetic Acid-Induced Vascular Permeability in Mice
4.5.4. Anti-Nociceptive Activity by Hot Plate Test in Mice
4.5.5. Induction of Pyrexia in Mice Using Brewer’s Yeast
4.5.6. Anti-Nociceptive Activity by Acetic Acid-Induced Abdominal Writhing in Mice
4.6. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chapple, I.L.C. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Wink, M. Phytomedicines, Herbal Drugs, and Poisons; University of Chicago Press: Chicago, IL, USA, 2015. [Google Scholar]
- Xuan, T.D.; Khanh, T.D.; Khang, D.; Quan, N.T.; Elzaawely, A.A. Changes in chemical composition, total phenolics and antioxidant activity of Alpinia (Alpinia zerumbet) leaves exposed to UV. Int. Lett. Nat. Sci. 2016, 55, 25–34. [Google Scholar] [CrossRef]
- Victório, C.P.; Alviano, D.S.; Alviano, C.S.; Lage, C.L. Chemical composition of the fractions of leaf oil of Alpinia zerumbet (Pers.) BL Burtt & RM Sm. and antimicrobial activity. Rev. Bras. Farmacogn. 2009, 19, 697–701. [Google Scholar]
- Mpalantinos, M.; Soares de Moura, R.; Parente, J.; Kuster, R. Biologically active flavonoids and kava pyrones from the aqueous extract of Alpinia zerumbet. Phytother. Res. 1998, 12, 442–444. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Changes in essential oil, kava pyrones and total phenolics of Alpinia zerumbet (Pers.) BL Burtt. & RM Sm. leaves exposed to copper sulphate. Environ. Exper. Bot. 2007, 59, 347–353. [Google Scholar]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Essential oils, kava pyrones and phenolic compounds from leaves and rhizomes of Alpinia zerumbet (Pers.) BL Burtt. & RM Sm. and their antioxidant activity. Food Chem. 2007, 103, 486–494. [Google Scholar]
- Xuan, T.D.; Teschke, R. Dihydro-5, 6-dehydrokavain (DDK) from Alpiniazerumbet: Its isolation, synthesis, and characterization. Molecules 2015, 20, 16306–16319. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.M.; Suga, R.; Oliveira, P.; Silva, F.C.; Magalhães, P.; Duarte, L.; Farias, L.M. Bioactivity of extracts from Alpinia zerumbet against sinusitis-causing bacterial pathogens. Rev. FitosEletrônica 2016, 9, 185–194. [Google Scholar]
- De Araújo Pinho, F.; Coelho-de-Souza, A.; Morais, S.; Santos, C.F.; Leal-Cardoso, J. Antinociceptive effects of the essential oil of Alpinia zerumbet on mice. Phytomedicine 2005, 12, 482–486. [Google Scholar] [CrossRef]
- Chompoo, J.; Upadhyay, A.; Fukuta, M.; Tawata, S. Effect of Alpinia zerumbet components on antioxidant and skin diseases-related enzymes. BMC Complement. Altern. Med. 2012, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Y.; Peng, C.-C.; Liang, Y.-J.; Yeh, W.-T.; Wang, H.-E.; Yu, T.-H.; Peng, R.Y. Alpinia zerumbet potentially elevates high-density lipoprotein cholesterol level in hamsters. J. Agric. Food Chem. 2008, 56, 4435–4443. [Google Scholar] [CrossRef]
- El-Hawary, S.; Kassem, H.; Motaal, A.A.; Tawfik, W.; Hassanein, H.; El-Shamy, S. GC-MS Analysis of the essential oil of Alpinia zerumbet (Pers.) BL and in vitro hepatoprotection and cytotoxicity study. MPC-4. Planta Med. 2013, 79, PI106. [Google Scholar] [CrossRef]
- De Moura, R.S.; Emiliano, A.; de Carvalho, L.C.M.; Souza, M.A.; Guedes, D.; Tano, T.; Resende, A. Antihypertensive and endothelium-dependent vasodilator effects of Alpinia zerumbet, a medicinal plant. J. Cardiovasc. Pharmacol. 2005, 46, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Victório, C.P.; Leitão, S.G.; Lage, C.L. Chemical composition of the leaf oils of Alpinia zerumbet (Pers.) Burtt et Smith and A. purpurata (Vieill) K. Schum. from Rio de Janeiro, Brazil. J. Essent. Oil Res. 2010, 22, 52–54. [Google Scholar] [CrossRef]
- Tu, P.T.B.; Tawata, S. Anti-oxidant, anti-aging, and anti-melanogenic properties of the essential oils from two varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Tachibana, M.; Noda, K.; Inoue, N.; Tanaka, M.; Kuwabara, K. Relevance of anti-reactive oxygen species activity to anti-inflammatory activity of components of Eviprostat®, a phytotherapeutic agent for benign prostatic hyperplasia. Phytomedicine 2007, 14, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.Y.; Wu, L.J.; Hong, X.X.; Tao, L.; Luo, P.; Shen, X.C. Screening of analgesic and anti–inflammatory active component in Fructus Alpiniae zerumbet based on spectrum–effect relationship and GC–MS. Biomed. Chromatogr. 2018, 32, e4112. [Google Scholar] [CrossRef]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan–induced mouse paw oedema is biphasic, age–weight dependent and displays differential nitric oxide cyclooxygenase–2 expression. Br. J. Pharmacol. 2004, 142, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, e7432797. [Google Scholar] [CrossRef]
- Liu, C.; Su, H.; Wan, H.; Qin, Q.; Wu, X.; Kong, X.; Lin, N. Forsythoside A exerts antipyretic effect on yeast-induced pyrexia mice via inhibiting transient receptor potential vanilloid 1 function. Int. J. Biol. Sci. 2017, 13, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Khan, M.R. Antipyretic, analgesic and anti-inflammatory effects of Kickxia ramosissima. J. Ethnopharmacol. 2016, 182, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Arslan, R.; Bektas, N.; Ozturk, Y. Antinociceptive activity of methanol extract of fruits of Capparis ovata in mice. J. Ethnopharmacol. 2010, 131, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Sobeh, M.; Mahmoud, M.; Hasan, R.; Cheng, H.; El-Shazly, A.; Wink, M. Senna singueana: Antioxidant, hepatoprotective, antiapoptotic properties and phytochemical profiling of a methanol bark extract. Molecules 2017, 22, 1502. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Mohamed, T.; Saad, A.M.; Refahy, L.A.-G.; Sobeh, M.; Wink, M. HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. In J. Pharm. Pharmacol.; 2018; Volume 70, pp. 133–142. [Google Scholar]
- Sobeh, M.; Mahmoud, M.F.; Petruk, G.; Rezq, S.; Ashour, M.L.; Youssef, F.S.; El-Shazly, A.M.; Monti, D.M.; Abdel-Naim, A.B.; Wink, M. Syzygium aqueum: A polyphenol–rich leaf extract exhibits antioxidant, hepatoprotective, pain-killing and anti-inflammatory activities in animal models. Front. Pharmacol. 2018, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Abdelall, E.K.A.; Lamie, P.F.; Ali, W.A.M. Cyclooxygenase-2 and 15–lipoxygenase inhibition, synthesis, anti-inflammatory activity and ulcer liability of new celecoxib analogues: Determination of region-specific pyrazole ring formation by NOESY. Bioorg. Med. Chem. Lett. 2016, 26, 2893–2899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Huang, X.D.; Wang, H.; Leung, A.K.; Chan, C.L.; Fong, D.W.; Yu, Z.L. Anti-inflammatory and analgesic effects of the ethanol extract of Rosa multiflora Thunb. hips. J. Ethnopharmacol. 2008, 118, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Silva-Comar, F.M.; Wiirzler, L.A.; Silva-Filho, S.E.; Kummer, R.; Pedroso, R.B.; Spironello, R.A.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K. Effect of estragole on leukocyte behavior and phagocytic activity of macrophages. J. Evid. Based Complementary Altern. Med. 2014, 784689. [Google Scholar] [CrossRef]
- Lee, D.Y.; Choo, B.K.; Yoon, T.; Cheon, M.S.; Lee, H.W.; Lee, A.Y.; Kim, H.K. Anti-inflammatory effects of Asparagus cochinchinensis extract in acute and chronic cutaneous inflammation. J. Ethnopharmacol. 2009, 121, 28–34. [Google Scholar]
- Nakamura, H.; Shimoda, A.; Ishii, K.; Kadokawa, T. Central and peripheral analgesic action of non-acidic non-steroidal anti-inflammatory drugs in mice and rats. Arch. Int. Pharmacodyn. Ther. 1986, 282, 16–25. [Google Scholar] [PubMed]
Sample Availability: Samples of the plant material are available from the authors. |
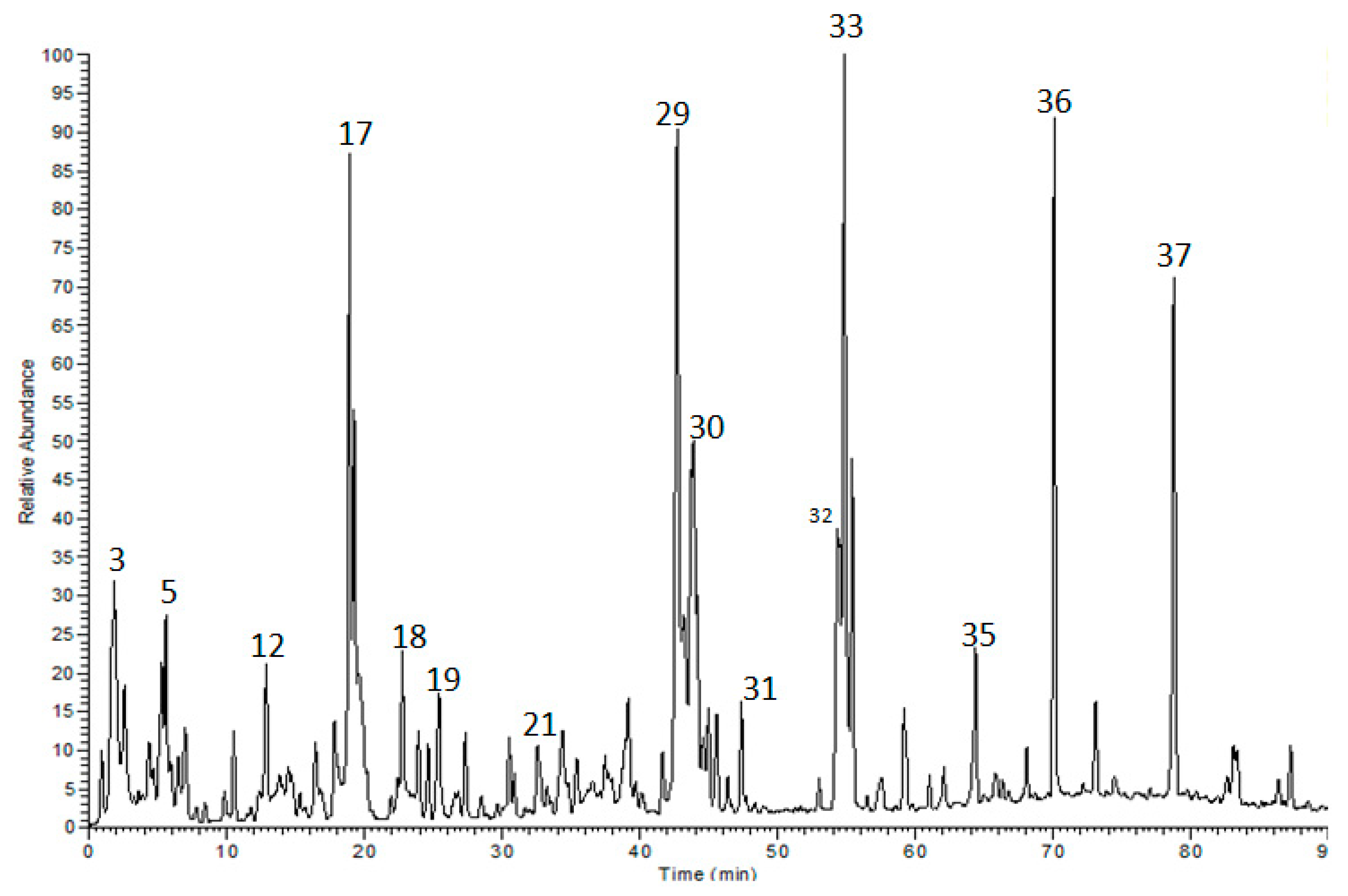
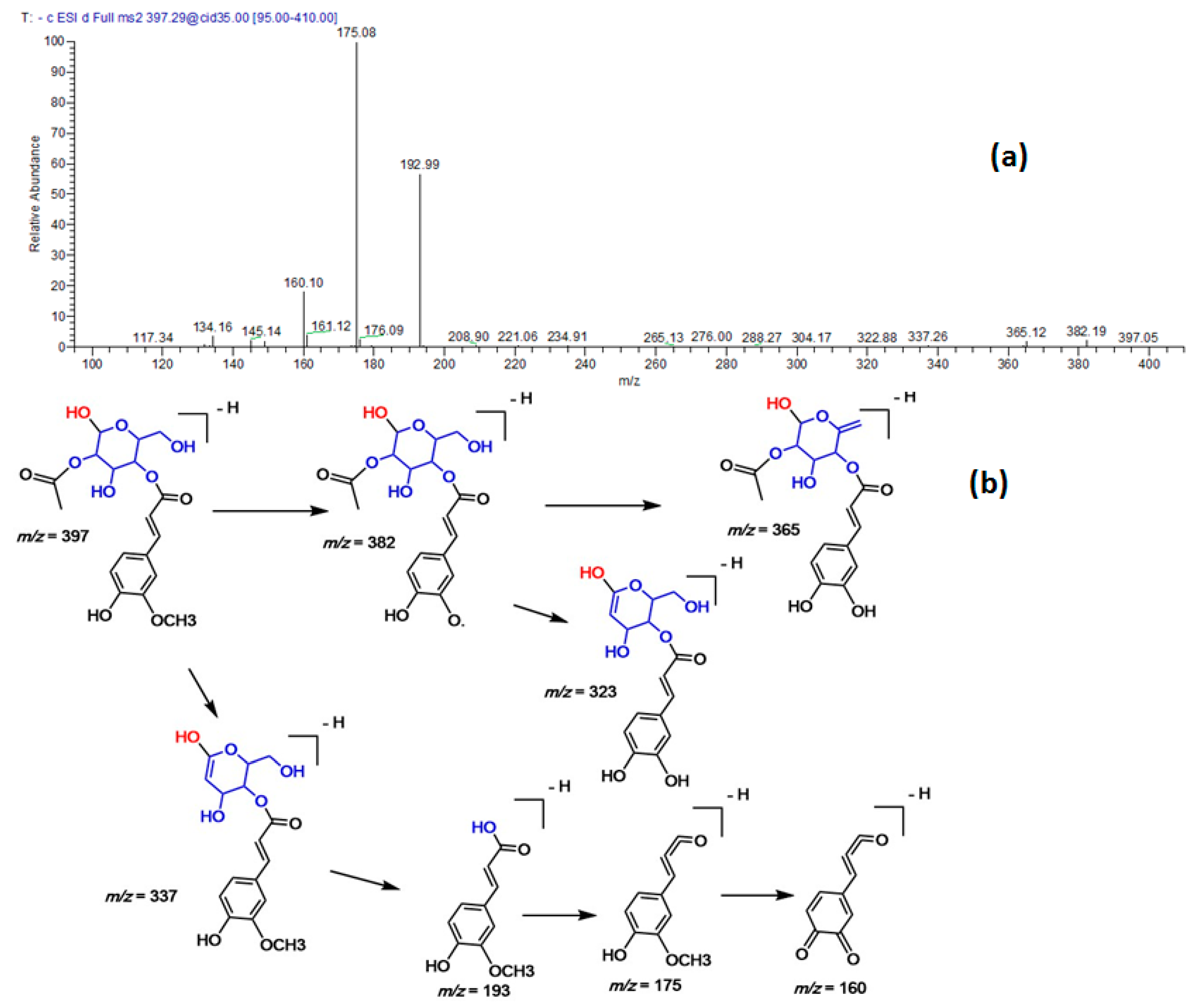
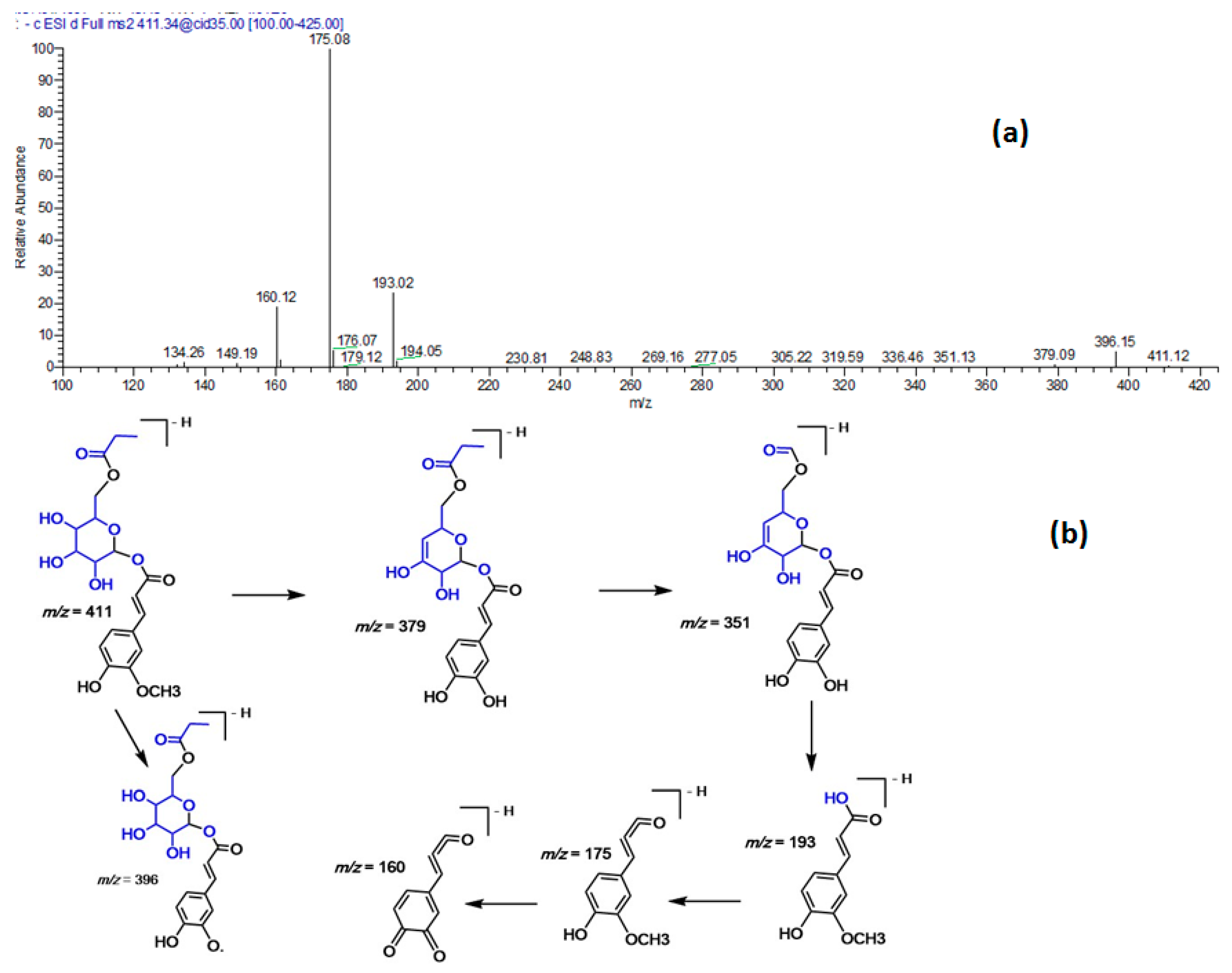
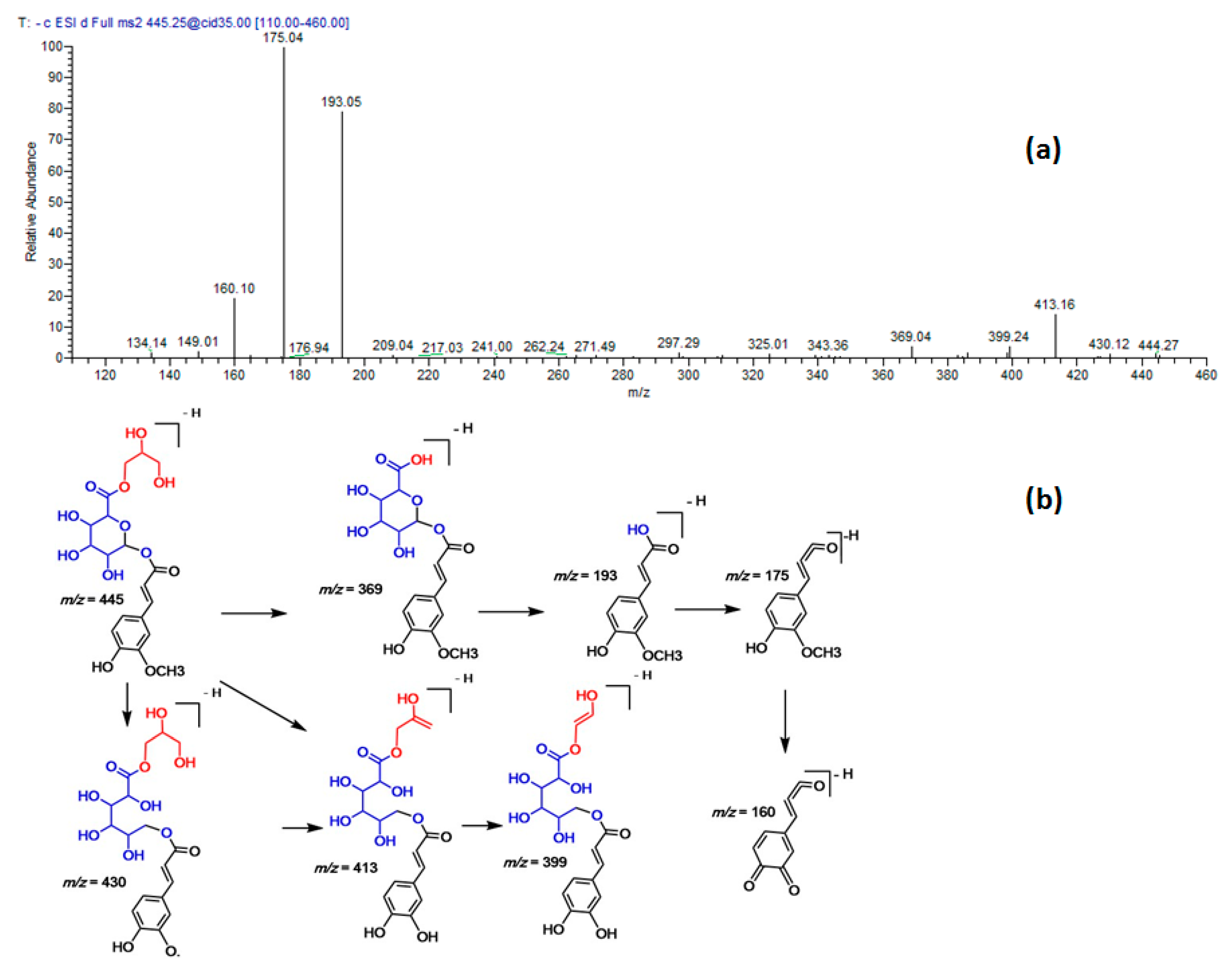

| No. | Rt | [M − H]− | MS/MS | Identified Compounds |
|---|---|---|---|---|
| 1 | 1.11 | 191 | 173, 127, 111 | Quinic acid a |
| 2 | 1.32 | 133 | 115 | Malic acid a |
| 3 | 1.52 | 169 | 169, 125 | Gallic acid a |
| 4 | 1.57 | 147 | 129, 115 | Cinnamic acid a,b |
| 5 | 4.89 | 239 | 221, 193, 179 | Eucomic acid a |
| 6 | 5.05 | 189 | 175, 157,129 | 3-Dehydroquinic acid a |
| 7 | 5.30 | 209 | 191 | 5-Hydroxyferulic acid a |
| 8 | 5.78 | 153 | 109, 97, 81 | Protocatechuic acid a |
| 9 | 7.59 | 329 | 314, 209, 167, 123 | Vanillic acid 4-β-d-glucoside a |
| 10 | 8.38 | 179 | 135, 133, 125, 107 | Caffeic acid a |
| 11 | 9.92 | 137 | 93 | Salicylic acid a |
| 12 | 12.81 | 167 | 151, 123, 109 | Vanillic acid a,b |
| 13 | 14.02 | 197 | 182, 167, 153 | Syringic acid a,b |
| 14 | 14.70 | 163 | 119 | p-Coumaric acid a,b |
| 15 | 16.47 | 385 | 223 | Sinapic acid 3-O-glucoside a |
| 16 | 17.66 | 441 | 397, 331, 330, 289 | Epicatechin 3-O-gallate a |
| 17 | 18.94 | 223 | 205, 191, 163 | Sinapic acid a |
| 18 | 22.97 | 355 | 193, 175, 160, 135 | Isoferulic acid 3-O-β-glucopyranoside a |
| 19 | 24.26 | 193 | 193, 179, 149 | Isoferulic acid a |
| 20 | 36.66 | 431 | 311, 281, 179, 151 | Isovitexin a |
| 21 | 30.16 | 463 | 301, 179 | Quercetin 3-O-glucoside a |
| 22 | 32.70 | 397 | 193, 175 | Ferulic acid acyl-glucoside |
| 23 | 34.28 | 623 | 315, 300, 255 | Isorhamnetin 3-O-rutinoside a |
| 24 | 34.33 | 593 | 327, 285 | Kaempferol 3-O-rutinoside a,c |
| 25 | 34.67 | 477 | 315, 299 | Isorhamnetin 3-O-glucoside a |
| 26 | 35.32 | 447 | 285, 255, 151 | Kaempferol 3-O-glucoside a |
| 27 | 36.45 | 433 | 301 | Quercetin 3-O-pentoside a |
| 28 | 39.05 | 491 | 315, 300, 271, 179 | Isorhamnetin-3-O-β-d-glucuronide a |
| 29 | 42.59 | 475 | 285, 271, 179 | Kaempferol-3-O-β-d-glucuronide a,c |
| 30 | 44.55 | 411 | 193, 175 | Ferulic acid propionyl-glucoside |
| 31 | 47.25 | 445 | 413, 193, 175 | Ferulyl O-glyceryl glucuronic acid |
| 32 | 53.81 | 445 | 413, 193, 175 | Ferulyl O-glyceryl glucuronic acid |
| 33 | 54.14 | 269 | 269, 255 | Apigenin a |
| 34 | 59.13 | 299 | 285 | Chrysoeriol a |
| 35 | 64.16 | 299 | 284 | Diosmetin a |
| 36 | 70.11 | 255 | 255, 213, 151 | Pinocembrin a |
| 37 | 78.74 | 269 | 269 | Genistein a |
| Treatment | TPC | DPPH | ABTS | FRAP | TAC |
|---|---|---|---|---|---|
| (mg GAE/g extract) | (IC50 µg/mL) | (mM FeSO4 equivalent/mg extract) | U/L | ||
| Extract | 226.98 ± 9.84 | 23.54 ± 1.78 | 5.52 ± 0.34 | 13.97 ± 0.43 | 30.20 ± 1.23 |
| Ascorbic acid | – | 2.92 ± 0.29 | – | – | 27.12 ± 1.11 |
| Quercetin | – | – | – | 21.45 ± 2.55 | – |
| Trolox | – | – | 1.63 ± 0.46 | – | – |
| Treatment | COX-1 | COX-2 | SI | LOX |
|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | |||
| Extract | 3.65 *,@ ± 0.45 | 0.078 @# ± 0.002 | 46.80 | 1.92 ** ± 0.37 |
| Celecoxib | 16.50 ± 1.17 | 0.046 ± 0.027 | 358.70 | – |
| Diclofenac | 4.20 ± 0.46 | 0.76 ± 0.18 | 5.52 | 2.31 ± 0.53 |
| Indomethacin | 0.042 ± 0.012 | 0.53 ± 0.20 | 0.08 | – |
| Zileuton | – | – | – | 3.21 ± 0.40 |
| Groups | Rectal Temperature # | Rectal Temperature Recorded Following Different Treatments (°C) | ||||
|---|---|---|---|---|---|---|
| 30 min | 1 h | 2 h | 3 h | 24 h | ||
| Control | 38.3 ± 0.2 | 38.4 ± 0.1 | 38.5 ± 0.1 | 38.9 ± 0.2 | 39 ± 0.3 | 38.3 ± 0.2 |
| Extract (200 mg /kg) | 38.5 ± 0.2 | 38.4 ± 0.1 | 38.4 ± 0.2 | 38.1 ± 0.2 | 38.3 ± 0.4 | 37.81 ± 0.3 |
| Extract (400 mg /kg) | 38.1 ± 0.3 | 36.6 ± 0.6 * | 36.8 ± 0.7 * | 36.8 ± 0.6 * | 36.1 ± 0.3 * | 36.7 ± 0.2 * |
| Paracetamol (150 mg/kg) | 38.5 ± 0.2 | 38.1 ± 0.2 | 37.5 ± 0.3 | 37 ± 0.3 * | 36.9 ± 0.2 * | 36.3 ± 0.2 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghareeb, M.A.; Sobeh, M.; Rezq, S.; El-Shazly, A.M.; Mahmoud, M.F.; Wink, M. HPLC-ESI-MS/MS Profiling of Polyphenolics of a Leaf Extract from Alpinia zerumbet (Zingiberaceae) and Its Anti-Inflammatory, Anti-Nociceptive, and Antipyretic Activities In Vivo. Molecules 2018, 23, 3238. https://doi.org/10.3390/molecules23123238
Ghareeb MA, Sobeh M, Rezq S, El-Shazly AM, Mahmoud MF, Wink M. HPLC-ESI-MS/MS Profiling of Polyphenolics of a Leaf Extract from Alpinia zerumbet (Zingiberaceae) and Its Anti-Inflammatory, Anti-Nociceptive, and Antipyretic Activities In Vivo. Molecules. 2018; 23(12):3238. https://doi.org/10.3390/molecules23123238
Chicago/Turabian StyleGhareeb, Mosad A., Mansour Sobeh, Samar Rezq, Assem M. El-Shazly, Mona F. Mahmoud, and Michael Wink. 2018. "HPLC-ESI-MS/MS Profiling of Polyphenolics of a Leaf Extract from Alpinia zerumbet (Zingiberaceae) and Its Anti-Inflammatory, Anti-Nociceptive, and Antipyretic Activities In Vivo" Molecules 23, no. 12: 3238. https://doi.org/10.3390/molecules23123238
APA StyleGhareeb, M. A., Sobeh, M., Rezq, S., El-Shazly, A. M., Mahmoud, M. F., & Wink, M. (2018). HPLC-ESI-MS/MS Profiling of Polyphenolics of a Leaf Extract from Alpinia zerumbet (Zingiberaceae) and Its Anti-Inflammatory, Anti-Nociceptive, and Antipyretic Activities In Vivo. Molecules, 23(12), 3238. https://doi.org/10.3390/molecules23123238







