Binding Efficacy and Thermogenic Efficiency of Pungent and Nonpungent Analogs of Capsaicin
Abstract
1. Introduction
2. Results
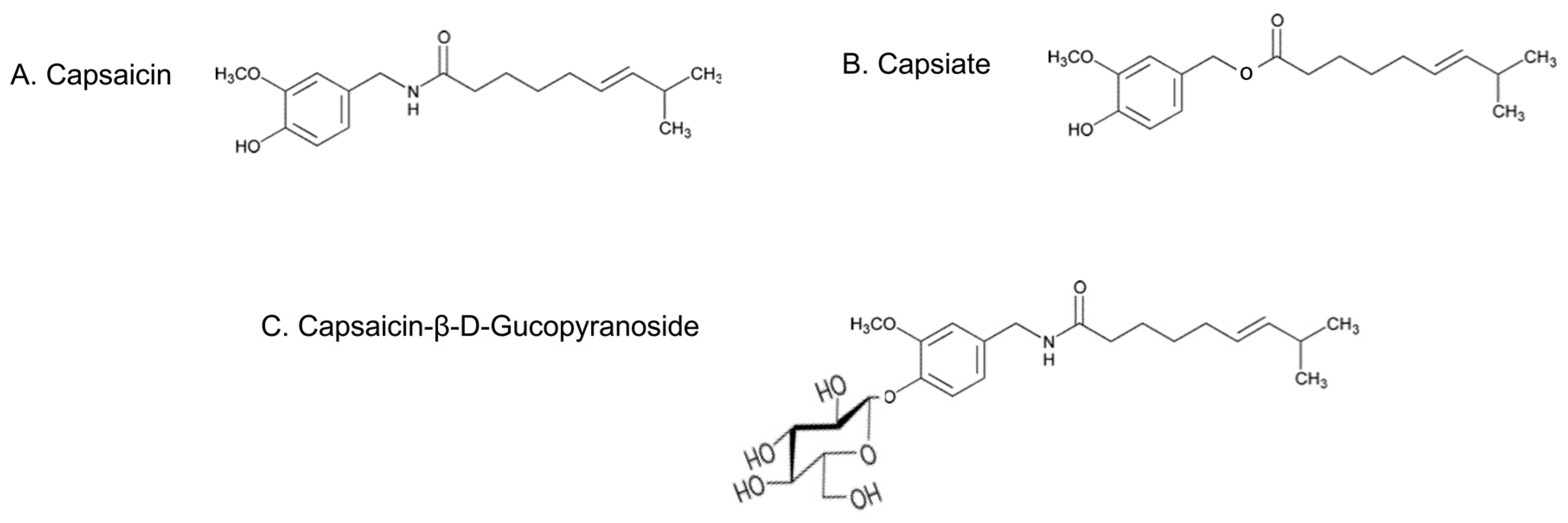
2.1. Pungent CAP and Its Non-Pungent Derivatives
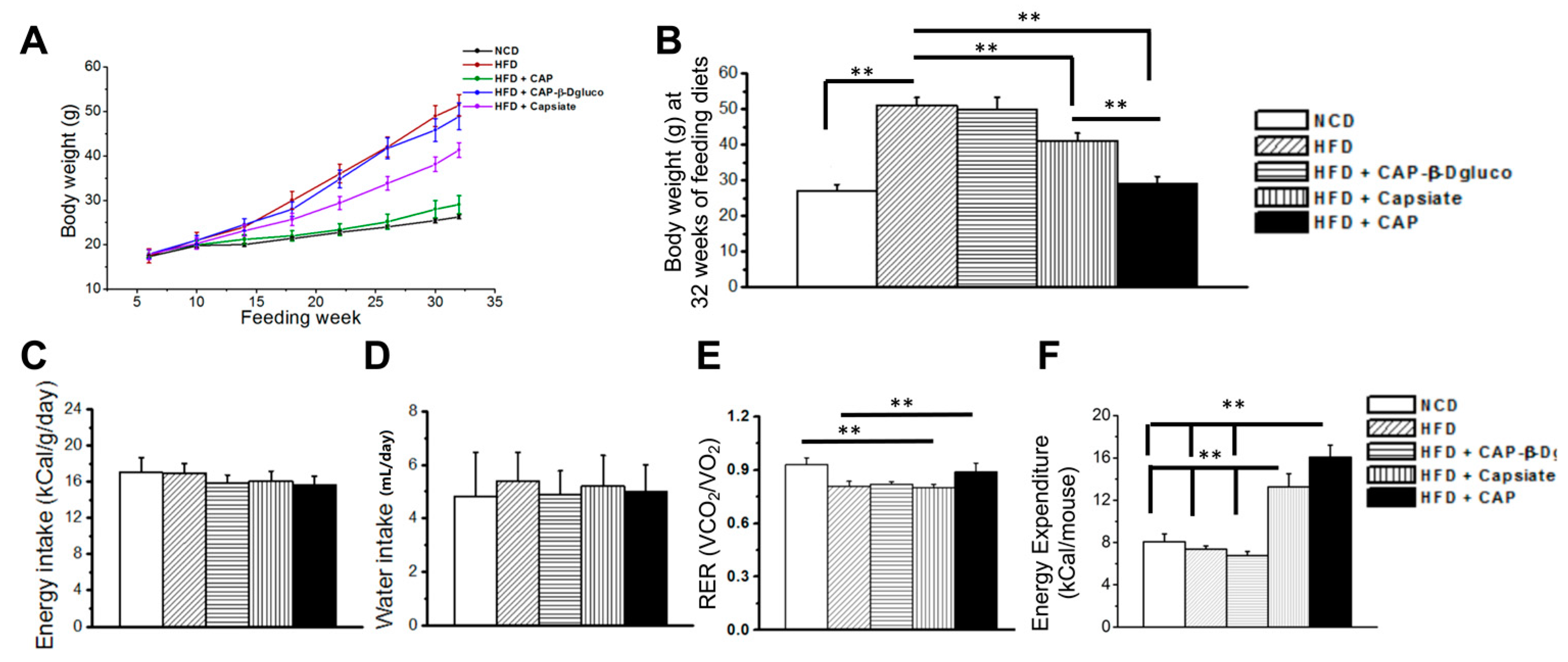
2.2. Effect of Pungent and Nonpungent Analogs of CAP on HFD-Induced Body Weight Gain, Energy, and Water Intake and Metabolic Activity
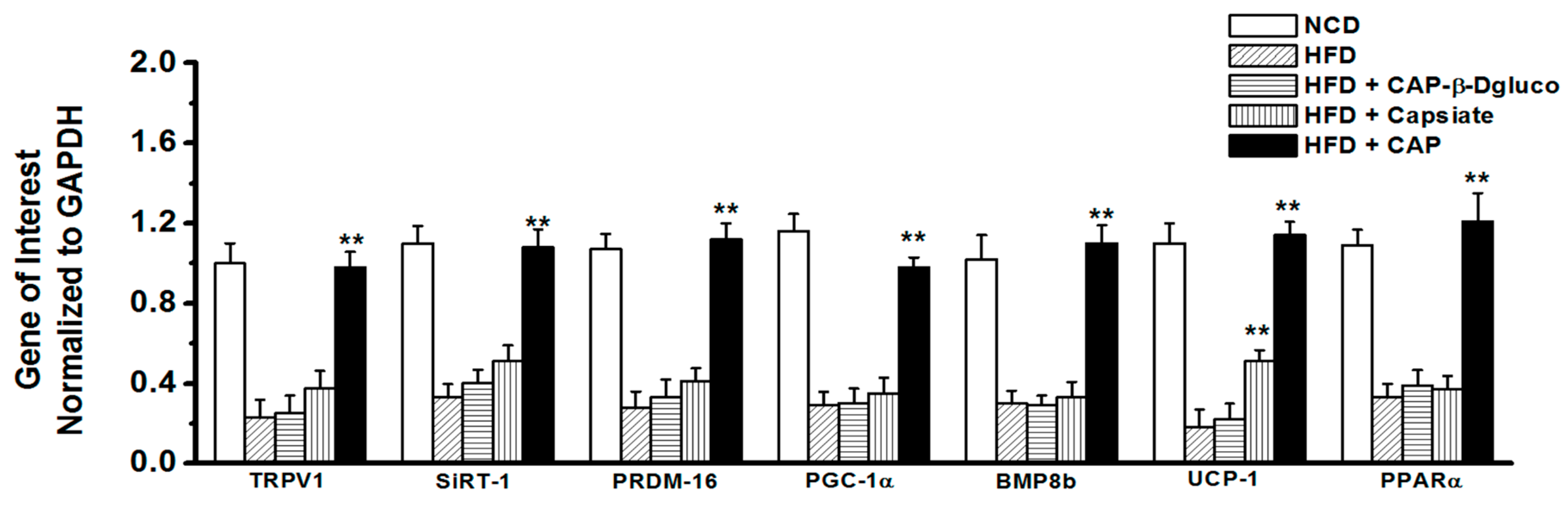
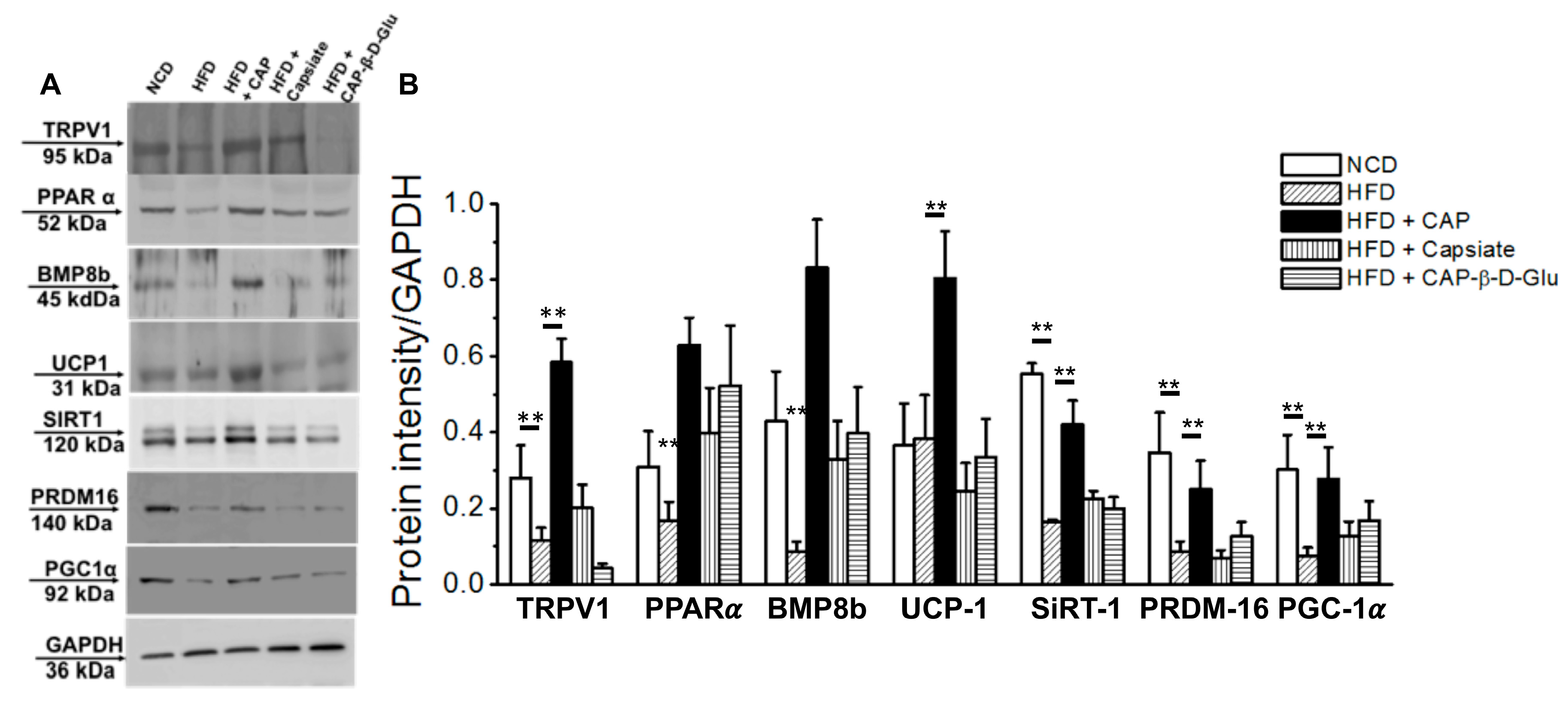
2.3. Effect of Pungent and Nonpungent Analogs of CAP on Thermogenic Genes and Proteins Expression in the Inguinal WAT of Mice
3. Discussion
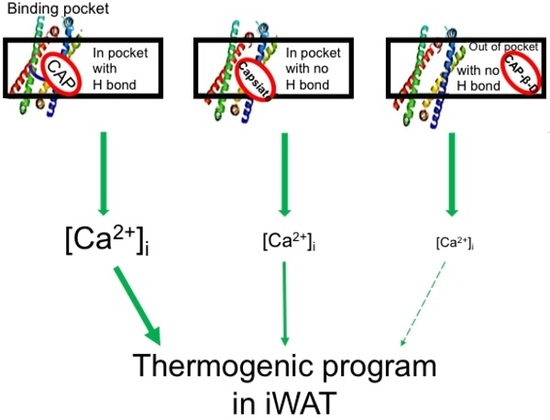
3.1. Structural Differences Impact Binding Efficacy
3.2. Pungent CAP Cy of Capsaicin Is Important for Its Anti-Obesity Effect
4. Materials and Methods
4.1. Feeding Studies
4.2. Metabolic Activity Studies
4.3. Quantitative RT-PCR Measurements
- 18s forward—5’-accgcagctaggaataatgga-3’; reverse—5’-gcctcagttccgaaaacca-3’;
- mtrpv1 forward—5’-caacaagaaggggcttacacc-3’; reverse—5’-tctggagaatgtaggccaagac-3’;
- pparα forward—5’-gtaccactacggagttcacgcat-3’; reverse—5’-cgccgaaagaagcccttac-3’;
- sirt-1 forward—5’-tcgtggagacatttttaatcagg-3’; reverse—5’-gcttcatgatggcaagtgg-3’;
- pgc-1α forward—5’-agagaggcagaagcagaaagcaat-3’; reverse—5’-attctgtccgcgttgtgtcagg-3’;
- ucp-1 forward—5’-cgactcagtccaagagtacttctcttc-3’; reverse—5’-gccggctgagatcttgtttc-3’;
- prdm-16 forward—5’—cagcacggtgaagccattc-3’; reverse—5’-gcgtgcatccgcttgtg-3’;
- bmp8b forward—5’—tccaccaaccacgccactat-3’; reverse—5’-cagtaggcacacagcacacct-3’.
4.4. Immunoblotting
4.5. Molecular Docking Simulation Studies
4.6. Chemicals and Drugs
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Antonious, G.F.; Jarret, R.L. Screening Capsicum accessions for capsaicinoids content. J. Environ. Sci. Health B 2006, 41, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Haramizu, S.; Kawabata, F.; Ohnuki, K.; Inoue, N.; Watanabe, T.; Yazawa, S.; Fushiki, T. Capsiate, a non-pungent capsaicin analog, reduces body fat without weight rebound like swimming exercise in mice. Biomed. Res. 2011, 32, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Moriyama, T.; Kobata, K.; Morita, A.; Murayama, N.; Hashizume, S.; Fushiki, T.; Yazawa, S.; Watanabe, T.; Tominaga, M. TRPV1 activation and induction of nociceptive response by a non-pungent capsaicin-like compound, capsiate. Neuropharmacology 2003, 44, 958–967. [Google Scholar] [CrossRef]
- Shintaku, K.; Uchida, K.; Suzuki, Y.; Zhou, Y.; Fushiki, T.; Watanabe, T.; Yazawa, S.; Tominaga, M. Activation of transient receptor potential A1 by a non-pungent capsaicin-like compound, capsiate. Br. J. Pharmacol. 2012, 165, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Tsurugizawa, T.; Nogusa, Y.; Ando, Y.; Uneyama, H. Different TRPV1-mediated brain responses to intragastric infusion of capsaicin and capsiate. Eur. J. Neurosci. 2013, 38, 3628–3635. [Google Scholar] [CrossRef] [PubMed]
- Ludy, M.J.; Moore, G.E.; Mattes, R.D. The effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chem. Senses 2012, 37, 103–121. [Google Scholar] [CrossRef]
- Baskaran, P.; Thyagarajan, B. Measurement of Basal and Forskolin-stimulated Lipolysis in Inguinal Adipose Fat Pads. J. Vis. Exp. 2017. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Fettel, K.; Gao, P.; Zhu, Z.; Ren, J.; Thyagarajan, B. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int. J. Obes. 2017, 41, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef]
- Thyagarajan, B.K.V.; Baskaran, P. CAP and Metabolic Diseases: A Mini Review on Preclinical Mechanisms and Clinical Efficacy. Capsaicin Hum. Ther. Dev. 2018. [Google Scholar] [CrossRef][Green Version]
- Zsiboras, C.; Matics, R.; Hegyi, P.; Balasko, M.; Petervari, E.; Szabo, I.; Sarlos, P.; Miko, A.; Tenk, J.; Rostas, I.; et al. Capsaicin and capsiate could be appropriate agents for treatment of obesity: A meta-analysis of human studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Escogido Mde, L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, X.; Cheng, W.; Yang, W.; Yu, P.; Song, Z.; Yarov-Yarovoy, V.; Zheng, J. Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nat. Chem. Biol. 2015, 11, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Hubbard, R.E. Hydrogen bonding in globular proteins. Prog. Biophys. Mol. Biol. 1984, 44, 97–179. [Google Scholar] [CrossRef]
- Bissantz, C.; Kuhn, B.; Stahl, M. A medicinal chemist’s guide to molecular interactions. J. Med. Chem. 2010, 53, 5061–5084. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Tsuji, E.; Ozawa, M.; Handa, C.; Mukaiyama, H.; Nishimura, T.; Kobayashi, S.; Okazaki, K. The importance of CH/pi hydrogen bonds in rational drug design: An ab initio fragment molecular orbital study to leukocyte-specific protein tyrosine (LCK) kinase. Bioorg. Med. Chem. 2008, 16, 10311–10318. [Google Scholar] [CrossRef]
- Pierce, A.C.; Sandretto, K.L.; Bemis, G.W. Kinase inhibitors and the case for CH...O hydrogen bonds in protein-ligand binding. Proteins 2002, 49, 567–576. [Google Scholar] [CrossRef]
- Wolber, G.; Langer, T. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef]
- Uchida, K.; Sun, W.; Yamazaki, J.; Tominaga, M. Role of Thermo-Sensitive Transient Receptor Potential Channels in Brown Adipose Tissue. Biol. Pharm. Bull. 2018, 41, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Dezaki, K.; Yoneshiro, T.; Watanabe, T.; Yamazaki, J.; Saito, M.; Yada, T.; Tominaga, M.; Iwasaki, Y. Involvement of thermosensitive TRP channels in energy metabolism. J. Physiol. Sci. 2017, 67, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Derbenev, A.V.; Zsombok, A. Potential therapeutic value of TRPV1 and TRPA1 in diabetes mellitus and obesity. Semin. Immunopathol. 2016, 38, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Ren, J. Leptin and hyperleptinemia—From friend to foe for cardiovascular function. J. Endocrinol. 2004, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tschop, M.H.; Speakman, J.R.; Arch, J.R.; Auwerx, J.; Bruning, J.C.; Chan, L.; Eckel, R.H.; Farese, R.V., Jr.; Galgani, J.E.; Hambly, C.; et al. A guide to analysis of mouse energy metabolism. Nat. Methods 2011, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Antibody | Dilution | Catalog Number and Source of the Antibody |
|---|---|---|
| PPARα | (1:500) | NB600-636; Novus Biologicals, Littleton, CO, USA |
| PRDM-16 | (1:1000) | NBPI-77096; Novus Biologicals, Littleton, CO, USA |
| BMP8b | (1:100) | SC-13086; Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
| SIRT-1 | (1:100) | SC-28766; Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
| TRPV1 | (1:100) | SC-28759; Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
| PGC-1α | (1:1000) | NBP1-04676; Novus Biologicals, Littleton, CO, USA |
| GAPDH | (1:500) | SC-365062; Santa Cruz Biotechnology, Inc., Dallas, TX, USA |
| Ligand Name | Docking Energy |
|---|---|
| Capsaicin | −27.0486 |
| Capsiate | −27.9297 |
| Capsaicin-β-d-glucopyranoside | +10.0103 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baskaran, P.; Covington, K.; Bennis, J.; Mohandass, A.; Lehmann, T.; Thyagarajan, B. Binding Efficacy and Thermogenic Efficiency of Pungent and Nonpungent Analogs of Capsaicin. Molecules 2018, 23, 3198. https://doi.org/10.3390/molecules23123198
Baskaran P, Covington K, Bennis J, Mohandass A, Lehmann T, Thyagarajan B. Binding Efficacy and Thermogenic Efficiency of Pungent and Nonpungent Analogs of Capsaicin. Molecules. 2018; 23(12):3198. https://doi.org/10.3390/molecules23123198
Chicago/Turabian StyleBaskaran, Padmamalini, Kyle Covington, Jane Bennis, Adithya Mohandass, Teresa Lehmann, and Baskaran Thyagarajan. 2018. "Binding Efficacy and Thermogenic Efficiency of Pungent and Nonpungent Analogs of Capsaicin" Molecules 23, no. 12: 3198. https://doi.org/10.3390/molecules23123198
APA StyleBaskaran, P., Covington, K., Bennis, J., Mohandass, A., Lehmann, T., & Thyagarajan, B. (2018). Binding Efficacy and Thermogenic Efficiency of Pungent and Nonpungent Analogs of Capsaicin. Molecules, 23(12), 3198. https://doi.org/10.3390/molecules23123198