Intracellular Accumulation of Linezolid and Florfenicol in OptrA-Producing Enterococcus faecalis and Staphylococcus aureus
Abstract
1. Introduction
2. Results
2.1. MIC Values
2.2. Time Course Study of Accumulation Rules
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates and Growth Conditions
4.2. Antimicrobial Susceptibility Testing
4.3. Accumulation and Extraction
4.4. LC-MS/MS Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Bush, K. The ABCD’s of beta-lactamase nomenclature. J. Infect. Chemother. 2013, 19, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Rennie, R.P. Current and future challenges in the development of antimicrobial agents. Handb. Exp. Pharmacol. 2012, 211, 45–65. [Google Scholar] [CrossRef]
- Nikaido, H.; Pages, J.M. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbial. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, H.P. Understanding efflux in Gram-negative bacteria: Opportunities for drug discovery. Expert Opin. Drug Discovery 2012, 7, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Long, K.S.; Poehlsgaard, J.; Kehrenberg, C.; Schwarz, S.; Vester, B. The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob. Agents Chemother. 2006, 50, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nikaido, H. Efflux-mediated drug resistance in bacteria. Drugs 2004, 64, 159–204. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Onodera, Y.; Lee, J.; Hooper, D. NorB, an efflux pump in Staphylococcus aureus strain MW2, contributes to bacterial fitness in abscesses. J. Bacteriol. 2008, 196, 7123–7129. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.; Ross, J.I.; Cove, J.H. Msr(A) and related macrolide/streptogramin resistance determinants: Incomplete transporters? Int. J. Antimicrob. Agents 2003, 22, 228–236. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, Y.; Cai, J.; Schwarz, S.; Cui, L.; Hu, Z.; Zhang, R.; Li, J.; Zhao, Q.; He, T.; et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J. Antimicrob. Chemother. 2015, 70, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Brenciani, A.; Morroni, G.; Vincenzi, C.; Manso, E.; Mingoia, M.; Giovanetti, E.; Varaldo, P.E. Detection in Italy of two clinical Enterococcus faecium isolates carrying both the oxazolidinone and phenicol resistance gene optrA and a silent multiresistance gene cfr. J. Antimicrob. Chemother. 2016, 71, 1118–1119. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wang, Y.; Schwarz, S.; Zhang, G.; Chen, S.; Gu, D.; Shen, Y.; Li, D.; Fan, R.; Zhang, R. High detection rate of the oxazolidinone resistance gene optrA in Enterococcus faecalis isolated from a Chinese anorectal surgery ward. Int. J. Antimicrob. Agents. 2016, 48, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, L.; Wu, Z.; Wang, L. Retrospective analysis of genome sequences revealed the wide dissemination of optrA in gram-positive bacteria. J. Antimicrob. Chemoth. 2017, 72, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Rzhetsky, A.; Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome. Res. 2001, 11, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.N. The ABC of ribosome-related antibiotic resistance. MBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, L.K.; Edwards, T.A.; O’Neill, A.J. ABC-F proteins mediate antibiotic resistance through ribosomal protection. MBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Jánosi, L.; Endou, K.; Nakajima, Y. Cloning and sequences of inducible and constitutive macrolide resistance genes in Staphylococcus aureus that correspond to an ABC transporter. FEMS Microbiol. Lett. 1999, 181, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Novotna, G.; Janata, J. A new evolutionary variant of the streptogramin a resistance protein, Vga(A)LC, from Staphylococcus haemolyticus with shifted substrate specificity towards lincosamides. Antimicrob. Agents Chemother. 2006, 50, 4070–4076. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.F.; Drown, B.S.; Riley, A.P.; Garcia, A.; Shirai, T.; Svec, R.L.; Hergenrother, P.J. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 2017, 545, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Hird, S.J.; Lau, B.-P.Y.; Schuhmacher, R.; Krska, R. Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food. Trends Anal. Chem. 2014, 59, 59–72. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Sapozhnikova, Y.; Mol, H.G.J. Current issues involving screening and identification of chemical contaminants in foods by mass spectrometry. Trends Anal. Chem. 2015, 69, 62–75. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals: Second Informational Supplement VET01-S2; CLSI: Wayne, PA, USA, 2013. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-fifth Informational Supplement M100-S25; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
Sample Availability: Samples of the compounds and strains are not available from the authors. |
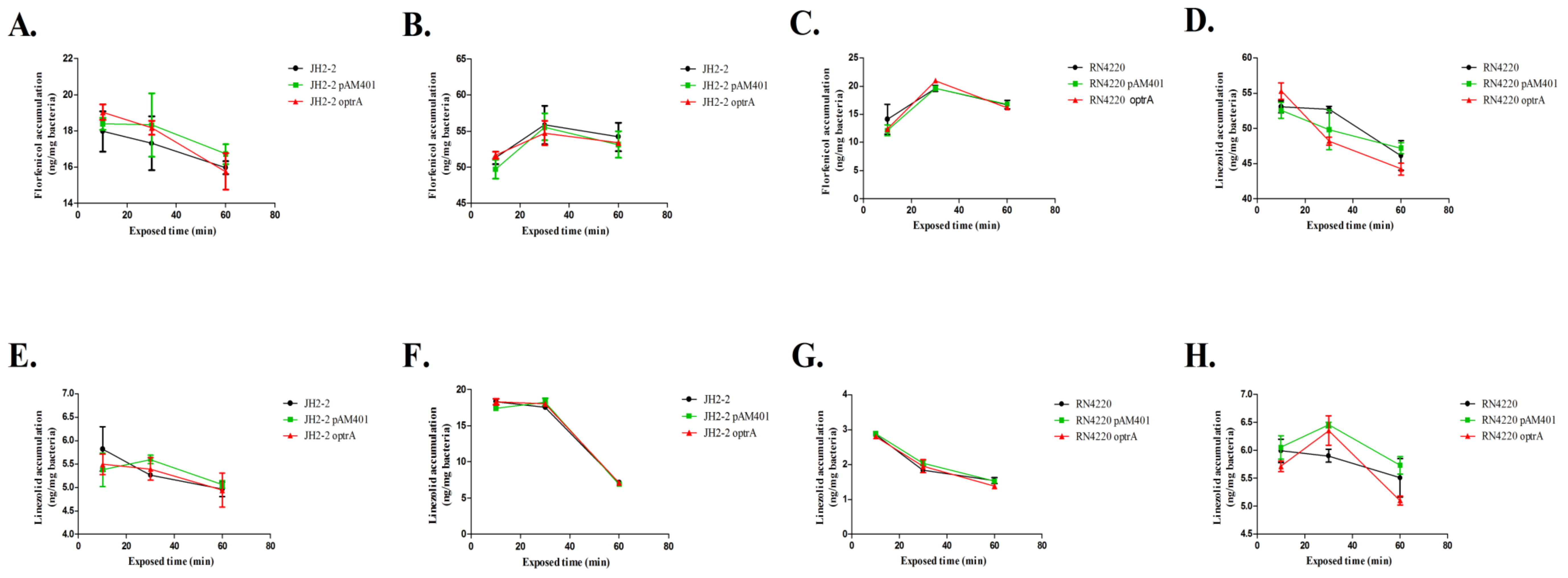
| Antibiotic | Concentration (mg/L) | Strains | Concentrations (ng/mg Bacteria) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Accumulation Time (min) | |||||||||||
| 10 | 30 | 60 | |||||||||
| Florfenicol | 16 | JH2-2 | 17.04 | 20.21 | 16.65 | 14.80 | 19.97 | 17.15 | 16.65 | 15.82 | 15.41 |
| JH2-2 pAM401 | 17.77 | 18.48 | 18.93 | 19.73 | 14.85 | 20.40 | 17.67 | 16.75 | 15.73 | ||
| JH2-2 optrA | 19.72 | 19.17 | 18.23 | 17.66 | 18.94 | 17.90 | 14.40 | 15.14 | 17.71 | ||
| 64 | JH2-2 | 51.40 | 52.70 | 49.71 | 51.28 | 55.85 | 60.51 | 52.32 | 58.19 | 52.15 | |
| JH2-2 pAM401 | 52.04 | 47.49 | 49.59 | 51.91 | 57.97 | 56.86 | 50.74 | 52.02 | 56.72 | ||
| JH2-2 optrA | 51.16 | 51.25 | 52.63 | 55.48 | 51.50 | 57.20 | 53.16 | 53.53 | 53.45 | ||
| Linezolid | 2 | JH2-2 | 6.50 | 6.06 | 4.92 | 5.33 | 5.22 | 5.24 | 5.16 | 5.08 | 4.66 |
| JH2-2 pAM401 | 5.07 | 6.09 | 4.97 | 5.76 | 5.45 | 5.60 | 4.91 | 5.11 | 5.17 | ||
| JH2-2 optrA | 5.49 | 5.87 | 5.13 | 5.69 | 5.59 | 4.91 | 5.39 | 4.23 | 5.21 | ||
| 8 | JH2-2 | 18.08 | 18.22 | 18.73 | 17.71 | 17.81 | 17.08 | 7.30 | 7.05 | 7.12 | |
| JH2-2 pAM401 | 16.92 | 17.53 | 17.81 | 19.01 | 18.51 | 17.32 | 7.19 | 7.18 | 6.38 | ||
| JH2-2 optrA | 17.57 | 18.40 | 19.00 | 17.89 | 18.29 | 17.86 | 6.87 | 7.88 | 6.70 | ||
| Florfenicol | 16 | RN4220 | 19.34 | 12.08 | 10.96 | 20.35 | 18.67 | 19.76 | 18.28 | 15.96 | 15.95 |
| RN4220 pAM401 | 13.14 | 13.13 | 10.16 | 20.48 | 19.05 | 19.30 | 16.29 | 17.01 | 17.09 | ||
| RN4220 optrA | 11.77 | 12.92 | 12.60 | 20.72 | 20.43 | 21.77 | 16.56 | 15.79 | 16.18 | ||
| 64 | RN4220 | 54.48 | 51.51 | 53.27 | 52.25 | 53.61 | 52.20 | 44.28 | 43.82 | 50.31 | |
| RN4220 pAM401 | 51.89 | 51.01 | 54.80 | 54.75 | 49.86 | 44.81 | 48.40 | 45.75 | 47.46 | ||
| RN4220 optrA | 53.24 | 57.20 | 55.49 | 47.10 | 48.51 | 49.02 | 44.32 | 42.67 | 45.67 | ||
| Linezolid | 2 | RN4220 | 2.89 | 2.96 | 2.72 | 1.78 | 1.89 | 1.84 | 1.47 | 1.43 | 1.72 |
| RN4220 pAM401 | 2.92 | 2.88 | 2.85 | 2.22 | 1.95 | 1.97 | 1.45 | 1.64 | 1.47 | ||
| RN4220 optrA | 2.82 | 2.76 | 2.83 | 1.91 | 1.66 | 2.29 | 1.42 | 1.38 | 1.34 | ||
| 8 | RN4220 | 6.12 | 5.58 | 6.27 | 5.73 | 5.85 | 6.12 | 5.00 | 6.16 | 5.37 | |
| RN4220 pAM401 | 6.20 | 5.64 | 6.32 | 6.55 | 6.42 | 6.39 | 6.04 | 5.58 | 5.57 | ||
| RN4220 optrA | 5.55 | 5.72 | 5.87 | 5.92 | 6.31 | 6.84 | 5.08 | 4.97 | 5.26 | ||
| Antibiotic | Concentration (mg/L) | Accumulation Time (min) | Statistical Significance | |||||
|---|---|---|---|---|---|---|---|---|
| JH2-2 VS JH2-2 pAM401 | JH2-2 VS JH2-2 optrA | JH2-2 pAM401 VS JH2-2 optrA | RN4220 VS RN4220 pAM401 | RN4220 VS RN4220 optrA | RN4220 pAM401 VS RN4220 optrA | |||
| Florfenicol | 16 | 10 | 0.692 | 0.337 | 0.554 | 0.424 | 0.491 | 0.905 |
| 30 | 0.614 | 0.668 | 0.938 | 0.978 | 0.072 | 0.075 | ||
| 60 | 0.474 | 0.836 | 0.365 | 0.928 | 0.449 | 0.401 | ||
| 64 | 10 | 0.288 | 0.774 | 0.193 | 0.739 | 0.189 | 0.117 | |
| 30 | 0.924 | 0.713 | 0.784 | 0.279 | 0.114 | 0.534 | ||
| 60 | 0.647 | 0.715 | 0.924 | 0.606 | 0.363 | 0.177 | ||
| Linezolid | 2 | 10 | 0.416 | 0.546 | 0.823 | 0.665 | 0.446 | 0.251 |
| 30 | 0.166 | 0.567 | 0.370 | 0.261 | 0.515 | 0.604 | ||
| 60 | 0.771 | 0.948 | 0.723 | 0.846 | 0.132 | 0.174 | ||
| 8 | 10 | 0.076 | 0.956 | 0.082 | 0.813 | 0.318 | 0.230 | |
| 30 | 0.160 | 0.340 | 0.591 | 0.060 | 0.106 | 0.697 | ||
| 60 | 0.551 | 0.983 | 0.565 | 0.503 | 0.247 | 0.093 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, X.; Wang, Y.; Schwarz, S.; Shen, J.; Xia, X. Intracellular Accumulation of Linezolid and Florfenicol in OptrA-Producing Enterococcus faecalis and Staphylococcus aureus. Molecules 2018, 23, 3195. https://doi.org/10.3390/molecules23123195
Wang Y, Li X, Wang Y, Schwarz S, Shen J, Xia X. Intracellular Accumulation of Linezolid and Florfenicol in OptrA-Producing Enterococcus faecalis and Staphylococcus aureus. Molecules. 2018; 23(12):3195. https://doi.org/10.3390/molecules23123195
Chicago/Turabian StyleWang, Yingyu, Xiaowei Li, Yang Wang, Stefan Schwarz, Jianzhong Shen, and Xi Xia. 2018. "Intracellular Accumulation of Linezolid and Florfenicol in OptrA-Producing Enterococcus faecalis and Staphylococcus aureus" Molecules 23, no. 12: 3195. https://doi.org/10.3390/molecules23123195
APA StyleWang, Y., Li, X., Wang, Y., Schwarz, S., Shen, J., & Xia, X. (2018). Intracellular Accumulation of Linezolid and Florfenicol in OptrA-Producing Enterococcus faecalis and Staphylococcus aureus. Molecules, 23(12), 3195. https://doi.org/10.3390/molecules23123195






