Bioactivity-Guided Separation of Potential D2 Dopamine Receptor Antagonists from Aurantii Fructus based on Molecular Docking Combined with High-Speed Counter-Current Chromatography
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening of Potential D2R Antagonists
2.2. Preparation of Ethanol Extracts
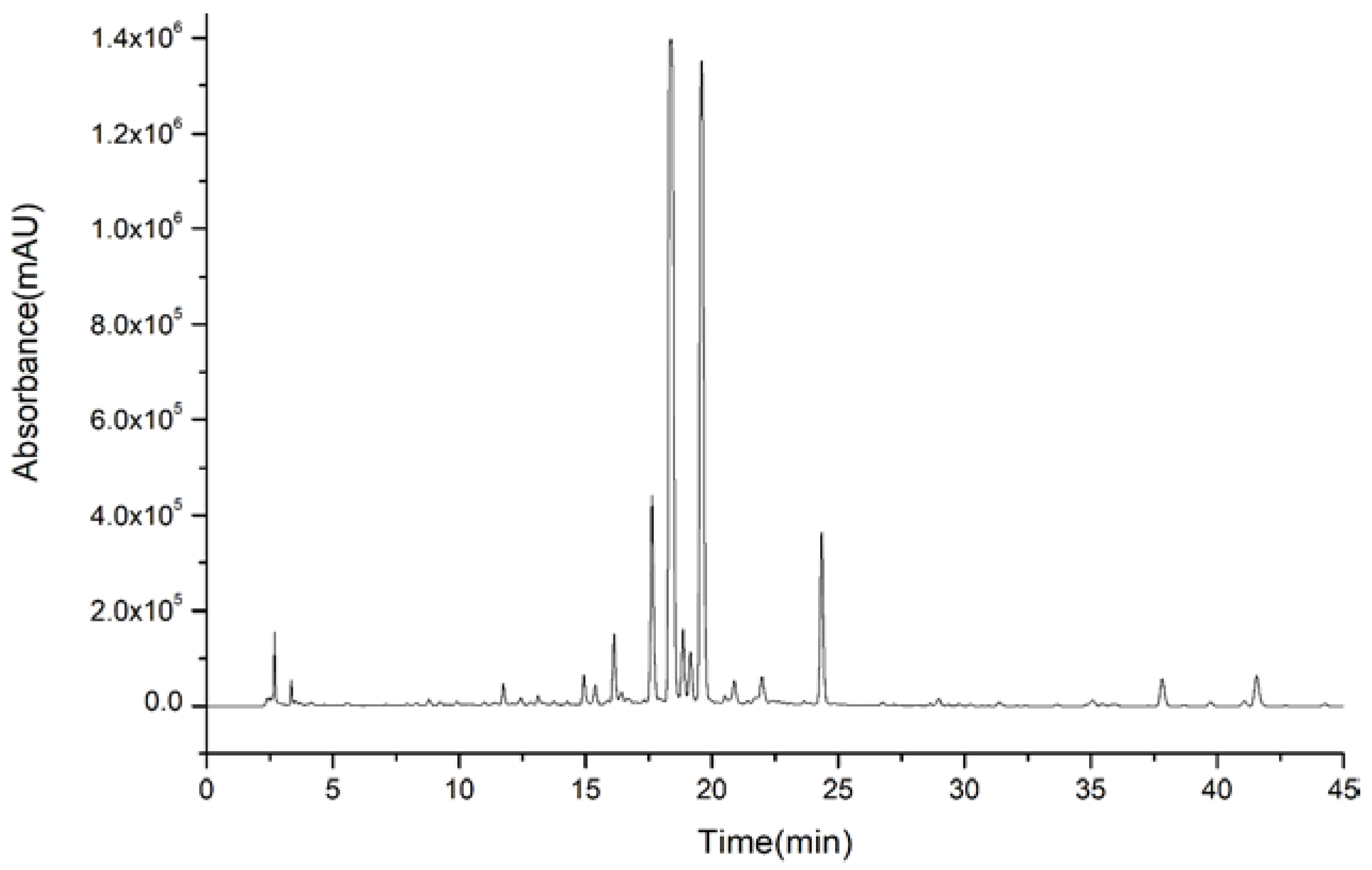
2.3. Optimization of HSCCC
2.4. Two-Phase Solvent Systems
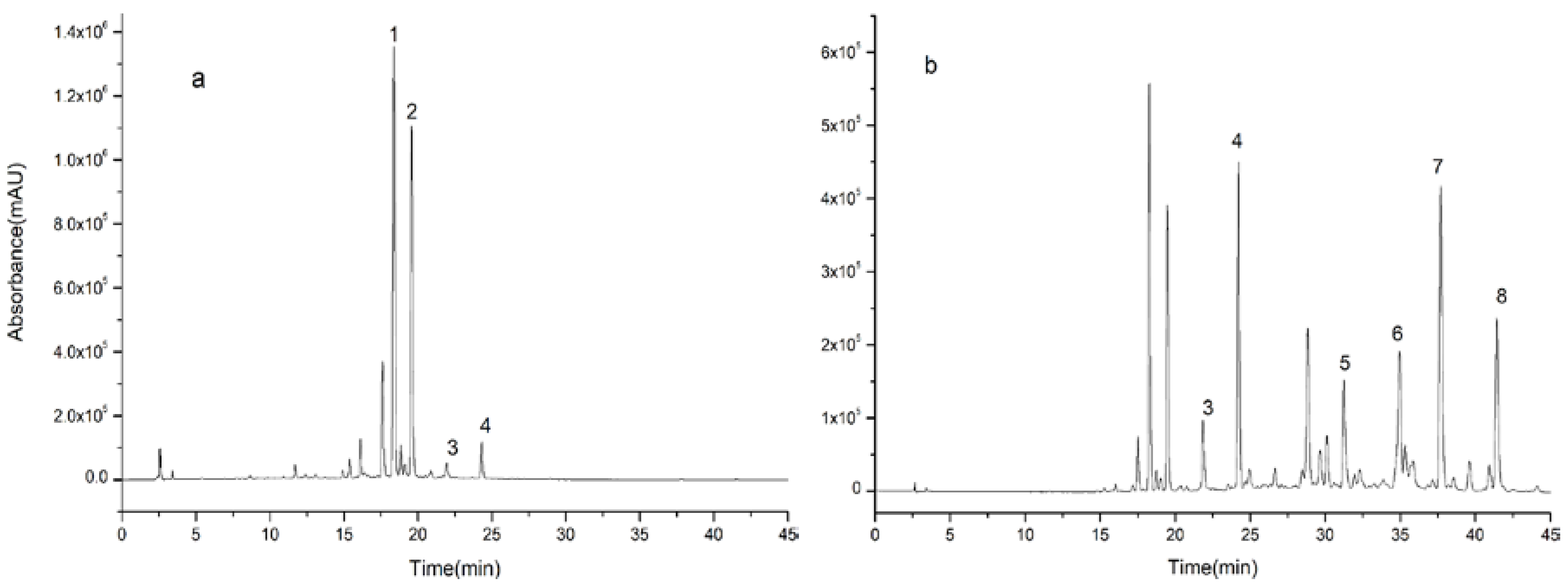
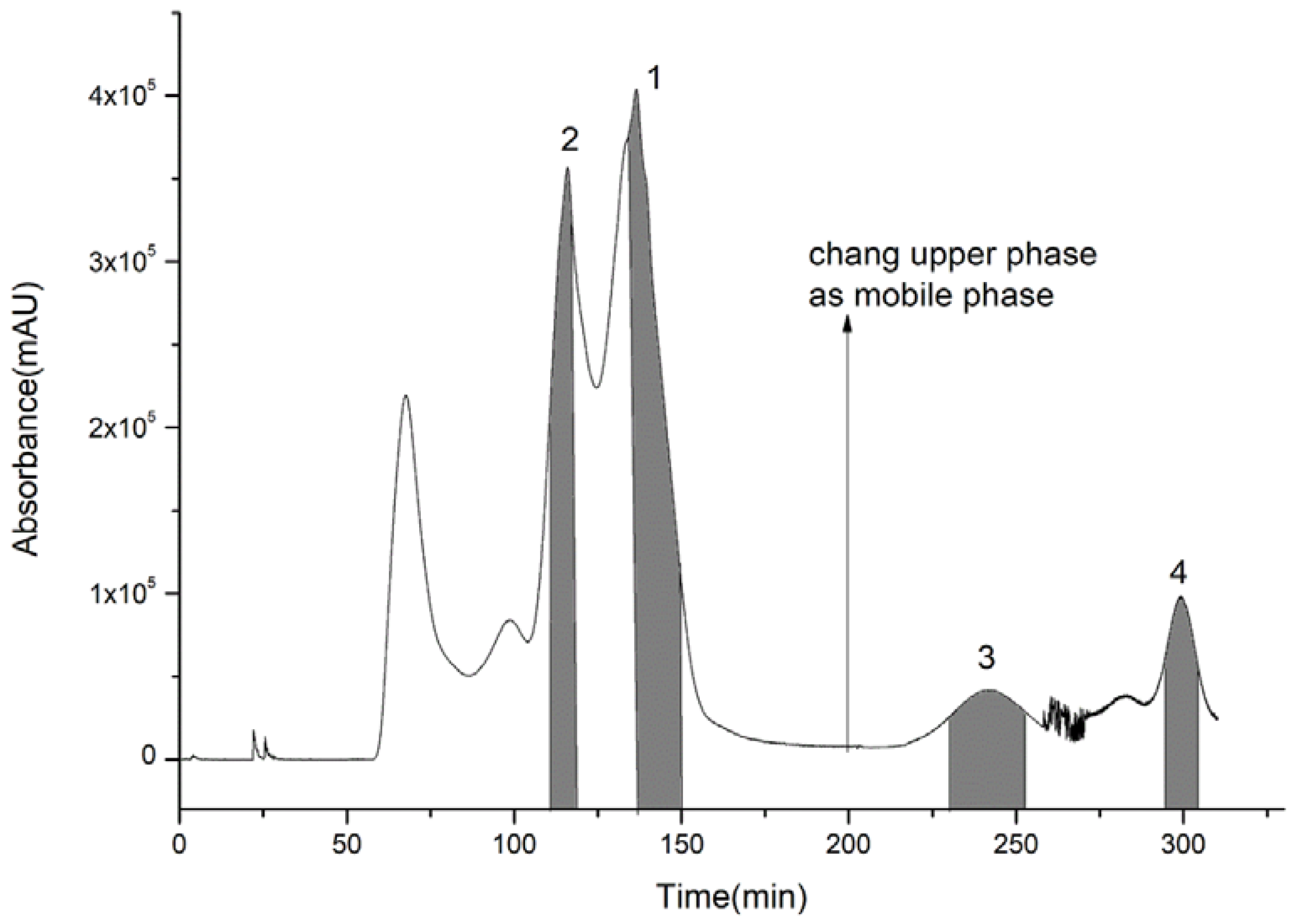
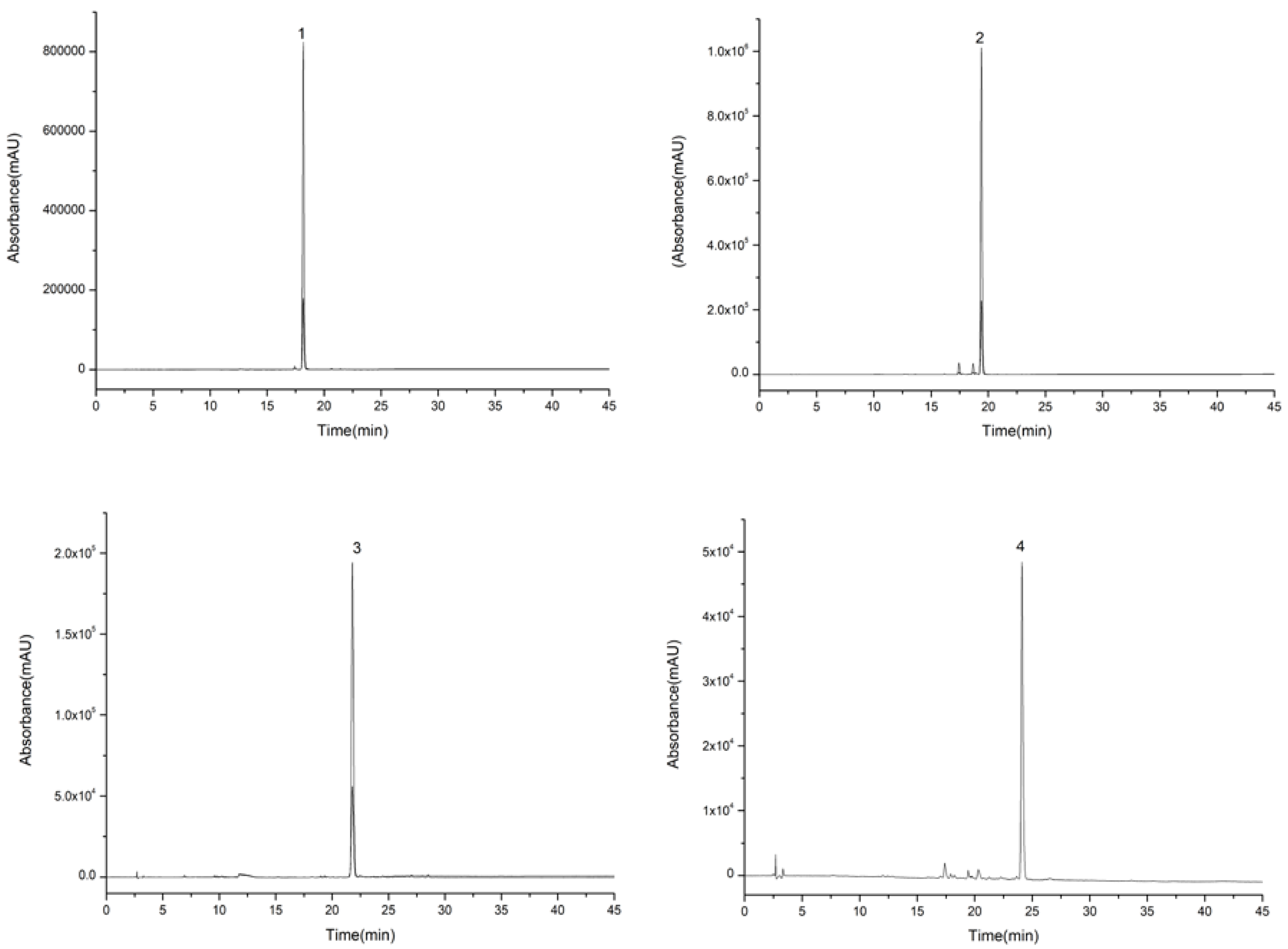
2.5. Separations of Compounds 1, 2, 3, and 4
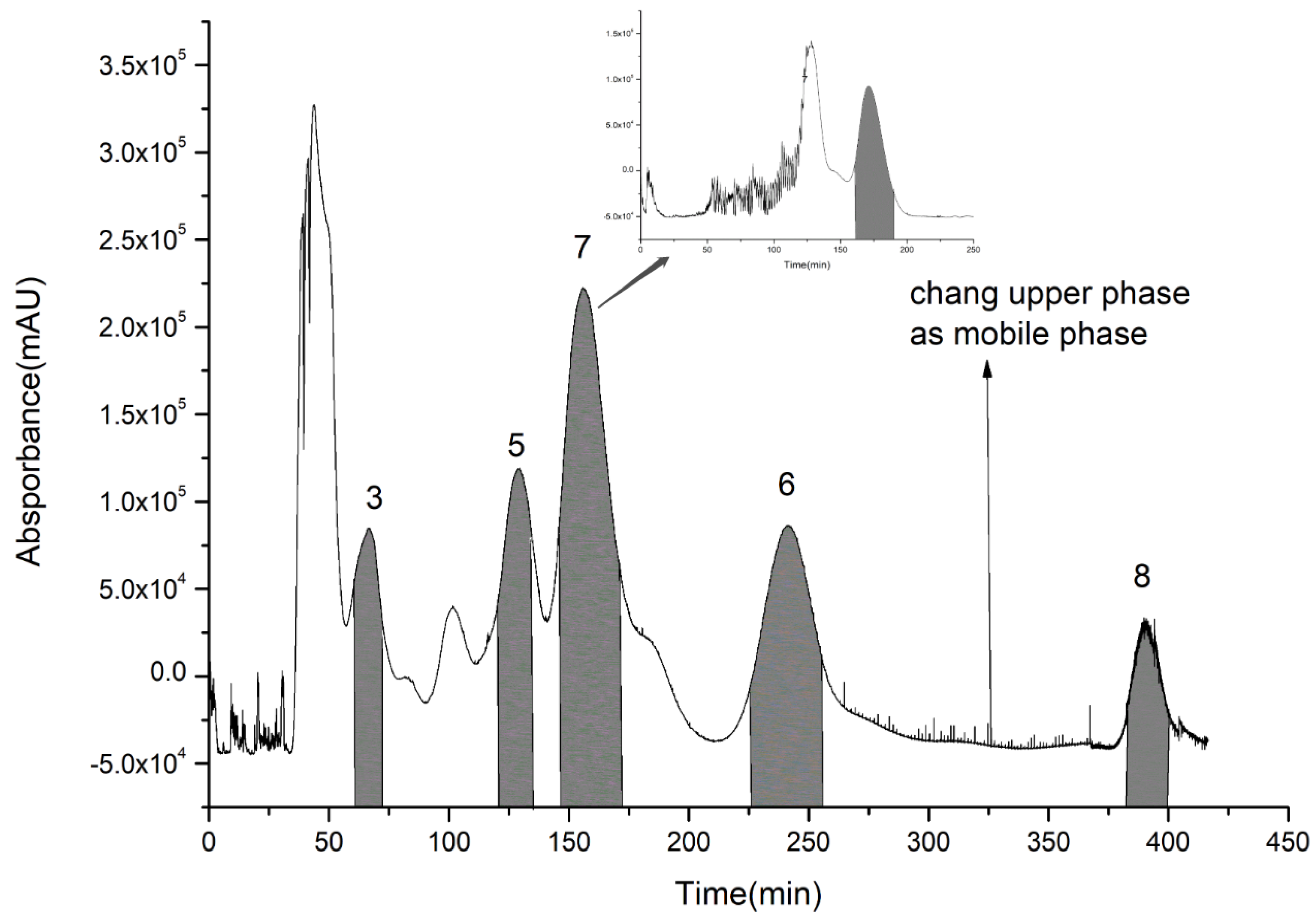
2.6. Separations of Compounds 5, 6, 7, and 8
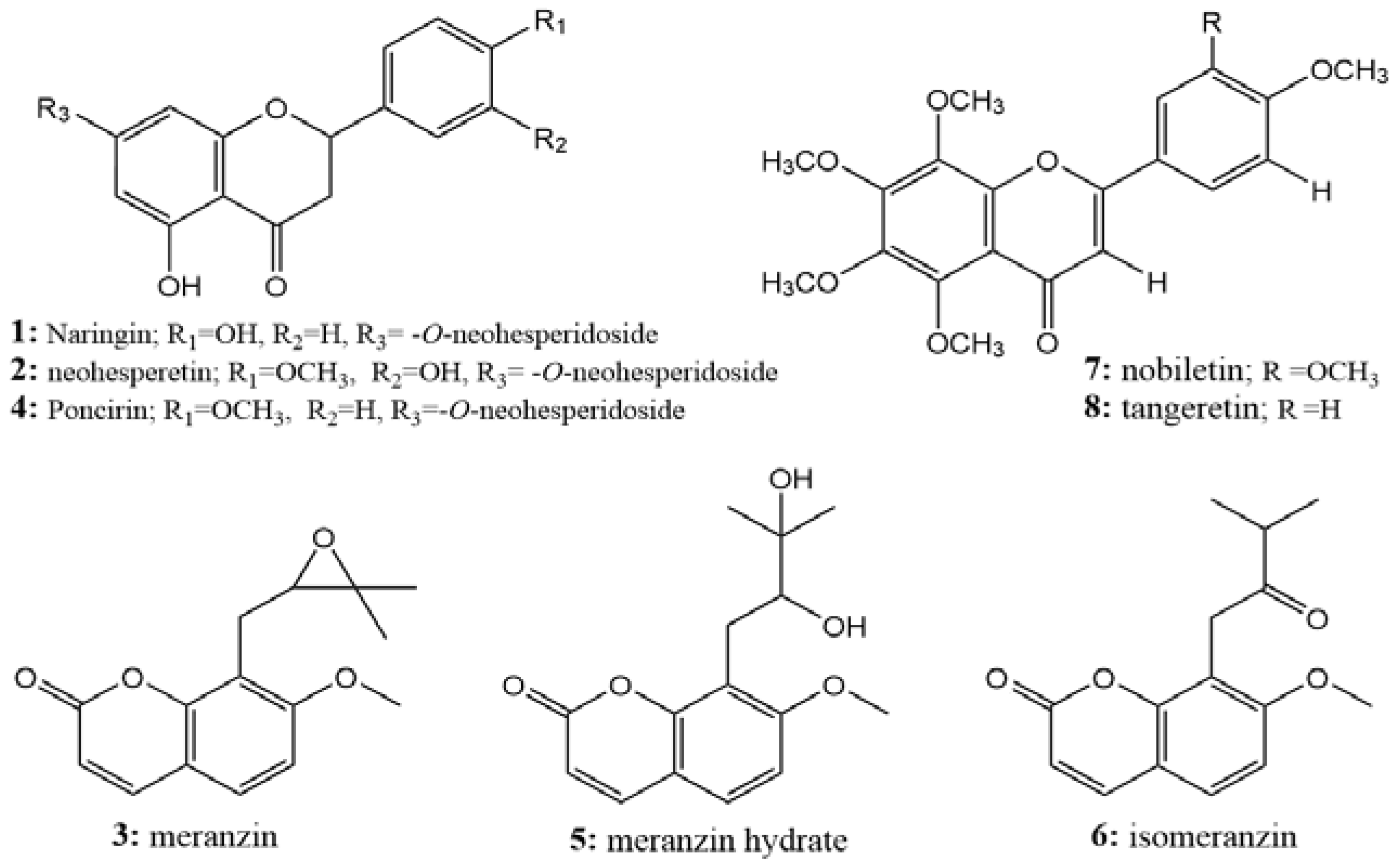
2.7. Structure Identification
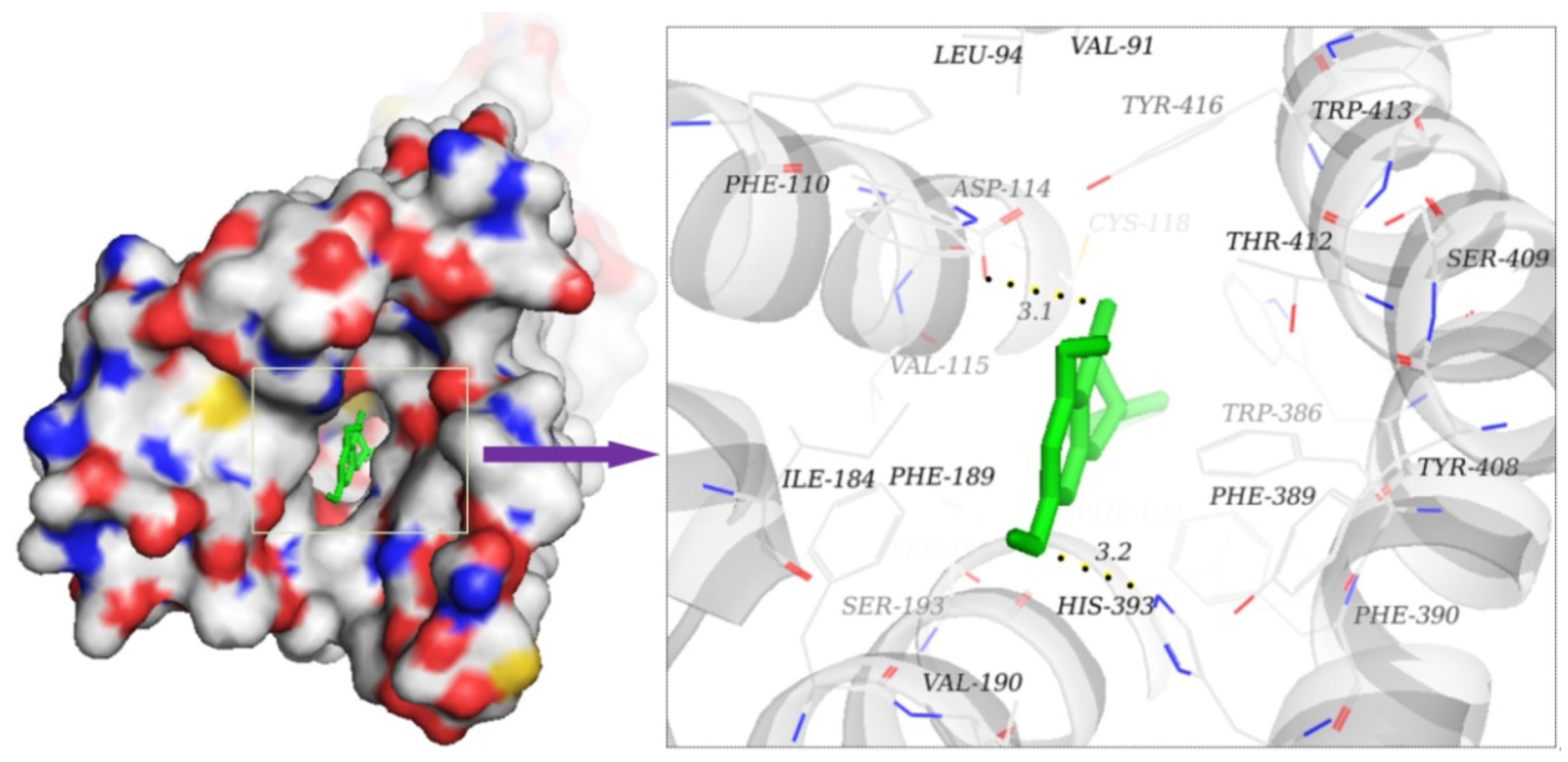
2.8. Illustration of Molecular Mechanism of Active Compounds on D2R
3. Materials and Methods
3.1. Materials and Reagents
3.2. Molecular Docking
3.3. Sample Preparation
3.4. HPLC Analysis
3.5. HSCCC Apparatus
3.6. HSCCC Separations
3.7. Structure Identification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2015. [Google Scholar]
- He, Y.; Li, Z.; Wang, W.; Sooranna, S.R.; Shi, Y.; Chen, Y.; Wu, C.; Zeng, J.; Tang, Q.; Xie, H. Chemical profiles and simultaneous quantification of aurantii fructus by use of HPLC-Q-TOF-MS combined with GC-MS and HPLC methods. Molecules 2018, 23, 2189. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Ma, H.; Mou, Y.; Xu, C.F. Association between polymorphism of dopamine D2 receptor genes and therapeutic effect of domperidone in functional dyspepsia. Turk J Gastroenterol. 2015, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tonini, M.; Cipollina, L.; Poluzzi, E.; Crema, F.; Corazza, G.R.; De Ponti, F. Review article: Clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment. Pharmacol. Ther. 2004, 19, 379–390. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cheng, P.; Wang, W.; Yan, S.; Tang, Q.; Liu, D.; Xie, H. Rapid investigation and screening of bioactive components in simo decoction via LC-Q-TOF-MS and UF-HPLC-MD methods. Molecules 2018, 23, 1792. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.Q.; Xiong, Y.; Zhou, B.; Deng, M.Z.; Deng, K.Z. Isolation and structural identification of flavonoids from Aurantii Fructus. Zhongguo Zhong Yao Za Zhi 2015, 40, 2352–2356. [Google Scholar] [PubMed]
- Han, X.; Zheng, L.L.; Qiu, Z.W.; Xu, L.N.; Xu, Y.W.; Qi, Y.; Diao, Y.P.; Peng, J.Y.; Liu, K.X. Efficient protocol for large-scale purification of naringin with high recovery from Fructus aurantii by macroporous resin column chromatography and HSCCC. Chromatographia 2008, 68, 319–326. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, Y.; Li, L.; Li, Y.; Zhou, P.; Luo, L.; Sun, B. Preparative HSCCC isolation of phloroglucinolysis products from grape seed polymeric proanthocyanidins as new powerful antioxidants. Food Chem. 2015, 188, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, Q.; Xie, Y.; Zeng, H.; Zhang, L.; Jiang, X.; Chen, X. Separation of five flavonoids from tartary buckwheat (Fagopyrum tataricum (L.) Gaertn) grains via off-line two dimensional high-speed counter-current chromatography. Food Chem. 2015, 186, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gu, D.; Wang, M.; Guo, H.; Wu, H.; Tian, G.; Li, Q.; Yang, Y.; Tian, J. A strategy based on gas chromatography-mass spectrometry and virtual molecular docking for analysis and prediction of bioactive composition in natural product essential oil. J. Chromatogr. A 2017, 1501, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, X.; Chen, Y.K.; Zhao, B.W.; Zhang, Y.L. Discover potential inhibitors of 5-LOX and LTA4H from Rhei Radix et rhizoma, Notopterygii Rhizoma et radix and Genitana Macrophyllae radix based on molecular simulation methods. Zhongguo Zhong Yao Za Zhi 2017, 42, 4494–4502. [Google Scholar] [PubMed]
- Hu, Y.B.; Peng, J.B.; Gu, S.; Pei, J.F.; Zou, Z.M. Molecular docking in Xin-Ke-Shu preparation’s multi-target effect on coronary heart disease. Acta Phys.-Chim. Sin. 2012, 28, 1257–1264. [Google Scholar]
- Lv, H.H.; Wang, H.L.; He, Y.F.; Ding, C.X.; Wang, X.Y.; Suo, Y.R. Separation and purification of four oligostilbenes from Iris lactea Pall. var. chinensis (Fisch.) Koidz by high-speed counter-current chromatography. J. Chromatogr. B 2015, 988, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Cicek, S.S.; Schwaiger, S.; Ellmerer, E.P.; Stuppner, H. Development of a fast and convenient method for the isolation of triterpene saponins from actaea racemosa by high-speed countercurrent chromatography coupled with evaporative light scattering detection. Planta Med. 2010, 76, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ma, Z.Y.; Clary, R.; Powell, J.; Knight, M.; Finn, T.M. Improved partition efficiency with threaded cylindrical column in vortex counter-current chromatography. J. Chromatogr. A 2011, 1218, 4065–4070. [Google Scholar] [CrossRef] [PubMed]
- He, C.H.; Zhao, C.X. Retention of the stationary phase for high-speed countercurrent chromatography. AIChE J. 2007, 53, 1460–1471. [Google Scholar] [CrossRef]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Liao, Y.R.; Hung, H.Y.; Chuang, C.W.; Hwang, T.L.; Huang, S.C.; Shiao, Y.J.; Kuo, D.H.; Wu, T.S. Anti-inflammatory and neuroprotective constituents from the peels of Citrus grandis. Molecules 2017, 22, 967. [Google Scholar] [CrossRef] [PubMed]
- Maltese, F.; Erkelens, C.; van der Kooy, F.; Choi, Y.H.; Verpoorte, R. Identification of natural epimeric flavanone glycosides by NMR spectroscopy. Food Chem. 2009, 116, 575–579. [Google Scholar] [CrossRef]
- Kim, C.Y.; Lee, H.J.; Lee, M.K.; Ahn, M.J.; Kim, J. One step purification of flavanone glycosides from Poncirus trifoliata by centrifugal partition chromatography. J. Sep. Sci. 2007, 30, 2693–2697. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dong, J.; Chen, H.; Hu, Y.; Tan, S.; Yang, W. Studies on separation and preparation of 3 standard materials such as meranzin hydrate from the Fructus Aurantii by high-speed counter-current chromatography. Chin. J. Pharm. Anal. 2017, 37, 137–141. [Google Scholar]
- Jiang, Z.; Liu, F.; Zhong, A.; Dugu, J.; Li, X. Determination of a new chromone from Aurantii Fructus Immaturus by DFT/GIAO method. Nat. Prod. Res. 2016, 30, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Che, T.; Levit, A.; Shoichet, B.K.; Wacker, D.; Roth, B.L. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature 2018, 555, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Compound | Docking Score (kcal/mol) | Potential Prediction a | Feasibility of Separation b |
|---|---|---|---|
| Flavonoids | |||
| Diosmetin-7-O-glucoside | −9.3 | √ | |
| Apigenin | −9.1 | √ | |
| Eriocitrin | −5.5 | √ | |
| Neoeriocitrin | −5.3 | √ | |
| Narirutin | −5.5 | √ | |
| Naringin c | −5.4 | √ | |
| Hesperidin | −2.8 | √ | |
| Neohesperidin | −3.4 | √ | |
| Poncirin | −3.2 | √ | |
| Naringenin | −9.3 | √ | √ |
| Nobiletin | −8.0 | √ | |
| 5,6,7,4′-Tetramethoxyflavone | −8.8 | √ | |
| Tangeretin | −8.6 | √ | √ |
| Coumarins | |||
| Meranzin hydrate | −8.6 | √ | √ |
| Meranzin | −7.1 | √ | |
| Epoxybergamottin | −9.2 | √ | |
| Isomeranzin | −7.4 | √ | |
| Auraptene | −9.1 | √ | |
| β-sitosterol d | −9.0 | √ | |
| No. | Solvent Systems | K1 | K2 | K3 | K4 |
|---|---|---|---|---|---|
| 1 | n-hexane: ethyl acetate: n-butanol: methanol: 0.05% acetic acid water = 1:1:1:1:3 | 0.37 | 0.34 | 2.86 | 0.90 |
| 2 | n-hexane: ethyl acetate: n-butanol: methanol: 0.05% acetic acid water = 1:3:2:1:5 | 1.47 | 0.94 | 7.30 | 3.35 |
| 3 | n-hexane: ethyl acetate: n-butanol: methanol: 0.05% acetic acid water = 1:3:1.8:1:5 | 1.01 | 0.62 | 3.80 | 2.53 |
| 4 | n-hexane: ethyl acetate: n-butanol: ethanol: 0.05% acetic acid water = 1:3:1.8:1:5 | 1.47 | 0.85 | 4.70 | 3.60 |
| No. | Solvent Systems | K5 | K6 | K7 | K8 |
|---|---|---|---|---|---|
| 5 | n-hexane: ethyl acetate: methanol: 0.05% acetic acid water = 2:1:1:3 | 4.17 | 5.77 | 4.57 | 5.54 |
| 6 | n-hexane: ethyl acetate: n-butanol: methanol: 0.05% acetic acid water = 2: 0.5:0.5:1:3 | 1.56 | 2.33 | 2.01 | 6.38 |
| 7 | n-hexane: n-butanol: ethanol: 0.05% acetic acid water = 2: 0.6:1:3 | 1.03 | 1.95 | 1.36 | 5.58 |
| 8 | n-hexane: n-butanol: methanol: 0.05% acetic acid water = 2: 0.6:1:3 | 0.90 | 1.78 | 1.28 | 5.08 |
| Compound | Score (kcal/mol) | Hydrophobic Residues (within a range of 4 Å) | H-Bond | Hydrogen Bond Residue |
|---|---|---|---|---|
| Risperidone a | −12.0 | TRP-100, PHE-110, VAL-115, ALA-122, PHE-198, PHE-382, TRP-386, PHE-389, PHE-390 | 1 | ASP-114 |
| Domperidone b | −10.7 | VAL-91, LEU-94, TRP-100, PHE-110, VAL-115, ALA-122, ILE-184, PHE-189, PHE-198, PHE-382, TRP-386, PHE-389, PHE-390, TRP-413 | 1 | ASP-114 |
| Naringenin | −9.3 | VAL-91, LEU-94, TRP-100, PHE-110, VAL-115, ALA-122, ILE-184, PHE-189, VAL-190, PHE-198, PHE-382, TRP-386, PHE-389, PHE-390, TRP-413, | 2 | ASP-114, HIS-393 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zhu, S.; Wu, C.; Lu, Y.; Tang, Q. Bioactivity-Guided Separation of Potential D2 Dopamine Receptor Antagonists from Aurantii Fructus based on Molecular Docking Combined with High-Speed Counter-Current Chromatography. Molecules 2018, 23, 3135. https://doi.org/10.3390/molecules23123135
He Y, Zhu S, Wu C, Lu Y, Tang Q. Bioactivity-Guided Separation of Potential D2 Dopamine Receptor Antagonists from Aurantii Fructus based on Molecular Docking Combined with High-Speed Counter-Current Chromatography. Molecules. 2018; 23(12):3135. https://doi.org/10.3390/molecules23123135
Chicago/Turabian StyleHe, Yingjie, Shihao Zhu, Changqiao Wu, Ying Lu, and Qi Tang. 2018. "Bioactivity-Guided Separation of Potential D2 Dopamine Receptor Antagonists from Aurantii Fructus based on Molecular Docking Combined with High-Speed Counter-Current Chromatography" Molecules 23, no. 12: 3135. https://doi.org/10.3390/molecules23123135
APA StyleHe, Y., Zhu, S., Wu, C., Lu, Y., & Tang, Q. (2018). Bioactivity-Guided Separation of Potential D2 Dopamine Receptor Antagonists from Aurantii Fructus based on Molecular Docking Combined with High-Speed Counter-Current Chromatography. Molecules, 23(12), 3135. https://doi.org/10.3390/molecules23123135





