Three Inulin-Type Fructans from Codonopsis pilosula (Franch.) Nannf. Roots and Their Prebiotic Activity on Bifidobacterium longum
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Identification of Fructan 1, 2 and 3
2.2. Prebiotic Activity of Fructan 1, 2 and 3
3. Material and Methods
3.1. Materials and Chemicals
3.2. Extraction and Isolation
3.3. Homogeneity and Molecular Weight Determination
3.4. NMR and TOF-MS Analysis of Fructan 1, 2 and 3
3.5. Prebiotic Test
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DEPT | distortionless enhancement by polarization transfer |
| DP | degree of polymerization |
| HMBC | heteronuclear multiple bond correlation |
| HPGPC | high performance gel permeation chromatography |
| HSQC | heteronuclear singular quantum correlation |
| MS | mass spectrometer |
| NMR | nuclear magnetic resonance spectrometer |
References
- Gao, S.M.; Liu, J.S.; Wang, M.; Cao, T.T.; Qi, Y.D.; Zhang, B.G.; Sun, X.B.; Liu, H.T.; Xiao, P.G. Traditional uses, phytochemistry, pharmacology and toxicology of Codonopsis: A review. J. Ethnopharmacol. 2018, 219, 50–70. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Ma, N.; Zhu, S.; Komatsu, K.; Li, Z.Y.; Fu, W.M. The genus Codonopsis (Campanulaceae): A review of phytochemistry, bioactivity and quality control. J. Nat. Med. 2015, 69, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gou, Y.; Chen, J.; An, J.; Chen, W.; Hu, F. Structural characterization and antitumor activity of a pectic polysaccharide from Codonopsis pilosula. Carbohydr. Polym. 2013, 98, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.N.; Hu, Y.L.; Wang, D.Y.; Liu, J.Z.; Guo, L.W. The comparison of immune-enhancing activity of sulfated polysaccharidses from Tremella and Condonpsis pilosula. Carbohydr. Polym. 2013, 98, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Liu, J.C. Structural characterization of a water-soluble polysaccharide from the roots of Codonopsis pilosula and its immunity activity. Int. J. Biol. Macromol. 2008, 43, 279–282. [Google Scholar]
- Zhang, Y.J.; Zhang, L.X.; Yang, J.F.; Liang, Z.Y. Structure analysis of water-soluble polysaccharide CPPS3 isolated from Codonopsis pilosula. Fitoterapia 2010, 81, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.F.; Chen, X.F.; Malterud, K.E.; Rise, F.; Barsett, H.; Inngjerdingen, K.T. Structural features and complement fixing activity of polysaccharides from Codonopsis pilosula Nannf. var. modesta L.T. Shen roots. Carbohyd. Polym. 2014, 113, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, J.; Li, E.T.; Fan, Q.; Wang, D.Y.; Li, P. The comparison of antioxidative and hepatoprotective activities of Codonopsis pilosula polysaccharide (CP) and sulfated CP. Int. Immunopharmac. 2015, 24, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G. Inulin-type prebiotics—A review Part 1. Altern. Med. Rev. 2008, 13, 315–329. [Google Scholar] [PubMed]
- Roberfroid, M.B.; Delzenne, N.M. Dietary fructans. Annu. Rev. Nutr. 1998, 18, 117–143. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G. Inulin-type prebiotics–A review Part 2. Altern. Med. Rev. 2009, 14, 36–55. [Google Scholar] [PubMed]
- Apolinário, A.C.; de Lima Damasceno, B.P.; de Macêdo Beltrão, N.E.; Pessoa, A.; Converti, A.; da Silva, J.A. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohyd. Polym. 2014, 101, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Mensink, M.A.; Frijlink, H.W.; Maarschalk, K.V.; Hinrichs, W.L.J. Inulin, a flexible oligosaccharide. II: Review of its pharmaceutical applications. Carbohyd. Polym. 2015, 134, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Wang, T.; Zhu, Z.C.; Yang, F.R.; Cao, L.Y.; Gao, J.P. Structure features and anti-gastric ulcer effects of inulin-type fructan CP-A from the roots of Codonopsis pilosula (Franch.) Nannf. Molecules 2017, 22, 2258. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.P.; Li, L.X.; Zhang, B.Z.; Paulsen, B.S.; Yin, Z.Q.; Huang, C.; Feng, B.; Chen, X.F.; Jia, R.R.; Song, X.; et al. Characterization and prebiotic activity in vitro of inulin-type fructan from Codonopsis pilosula roots. Carbohd. Polym. 2018, 193, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Caleffi, E.R.; Krausová, G.; Hyrslová, I.; Paredes, L.L.R.; Santos, M.M.; Sassaki, G.L.; Gonalvesa, R.A.C.; Oliveira, A.J.B. Isolation and prebiotic activity of inulin-type fructan extracted from Pfaffia glomerata (Spreng) Pedersen roots. Int. J. Biol. Macromol. 2015, 80, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Matulová, M.; Husárová, S.; Capek, P.; Sancelme, M.; Delort, A.M. NMR structural study of fructans produced by Bacillus sp. 3B6, bacterium isolated in cloud water. Carbohyd. Res. 2011, 346, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Cérantola, S.; Kervarec, N.; Pichon, R.; Magné, C.; Bessieres, M.A.; Deslandes, E. NMR characterisation of inulin-type fructooligosaccharides as the major water-soluble carbohydrates from Matricaria maritima (L.). Carbohyd. Res. 2004, 339, 2445–2449. [Google Scholar] [CrossRef] [PubMed]
- Aravind, N.; Sissons, M.J.; Fellows, C.M.; Blazek, J.; Gilbert, E.P. Effect of inulin soluble dietary fibre addition on technological, sensory, and structural properties of durum wheat spaghetti. Food Chem. 2012, 132, 993–1002. [Google Scholar] [CrossRef]
- Lopes, S.M.S.; Francisco, M.G.; Higashi, B.; Almeida, R.T.R.; Krausová, G.; Pilau, E.J.; Goncalves, J.E.; Goncalves, R.A.C.; Oliveira, A.J.B. Chemical characterization and prebiotic activity of fructo-oligosaccharides from Stevia rebaudiana (Bertoni) roots and in vitro adventitious root cultures. Carbohyd. Polym. 2016, 152, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.J.B.; Goncalves, R.A.C.; Chierrito, T.P.C.; Santos, M.M.; Souza, L.M.; Gorin, P.A.J.; Sassaki, G.L.; Iacomini, M. Structure and degree of polymerisation of fructooligosaccharides present in roots and leaves of Stevia rebaudiana (Bert.) Bertoni. Food Chem. 2011, 129, 305–311. [Google Scholar] [CrossRef]
- Picard, C.; Fioramonti, J.; Francois, A.; Robinson, T.; Neant, F.; Matuchansky, C. Review article: Bifidobacteria as probiotic agents—Physiological effects and clinical benefits. Aliment Pharmacol. Ther. 2005, 22, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Macfarlane, G.T. Gastrointestinal effects of prebiotics. Br. J. Nutr. 2002, 87, S145–S151. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Raskine, L.; Simoneau, G.; Vicaut, E.; Neut, C.; Flourie, B.; Brouns, F.; Bornet, F.R. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacterial in healthy humans: A double-blind, randomized, placebocontrolled, parallel-group, dose-response relation study. Am. J. Clin. Nutr. 2004, 80, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Vernazza, C.L.; Gibson, G.R.; Rastall, R.A. Carbohydrate preference, acid tolerance and bile tolerance in five strains of Bifidobacterium. J. App. Micro. 2006, 100, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Valcheva, R.; Koleva, P.; Martinez, I.; Walter, J.; Ganzle, M.G.; Dieleman, L.A. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut Microbes. 2018, 5, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Azpiroz, F.; Molne, L.; Mendez, S.; Nieto, A.; Manichanh, C.; Mego, M.; Accarino, A.; Santos, J.; Sailer, M.; Theis, S.; et al. Effect of chicory-derived inulin on abdominal sensations and bowel motor function. J. Chin. Gastroenterol. 2017, 51, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
Sample Availability: Samples of fructan 1, 2 and 3 are available from the authors. |
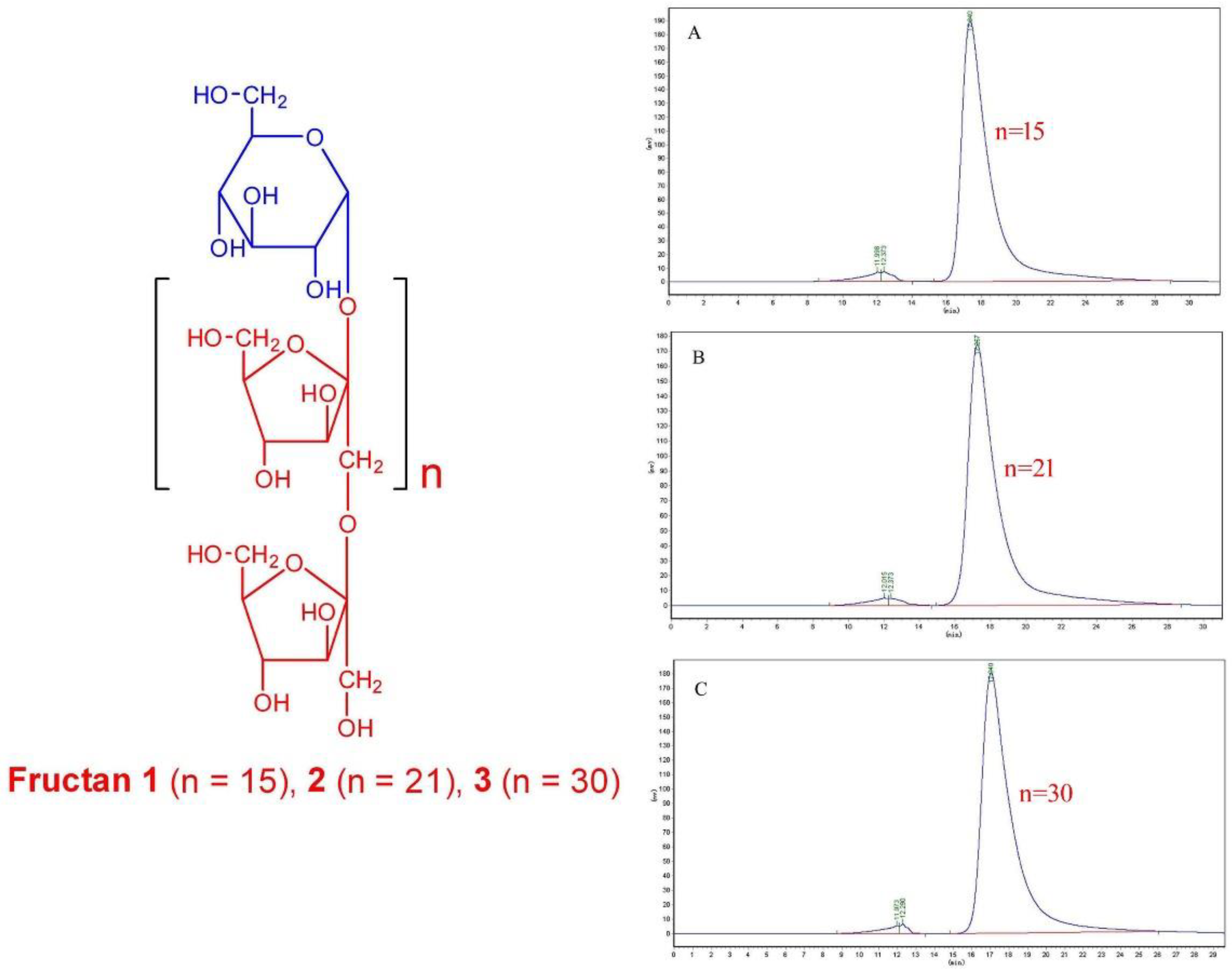
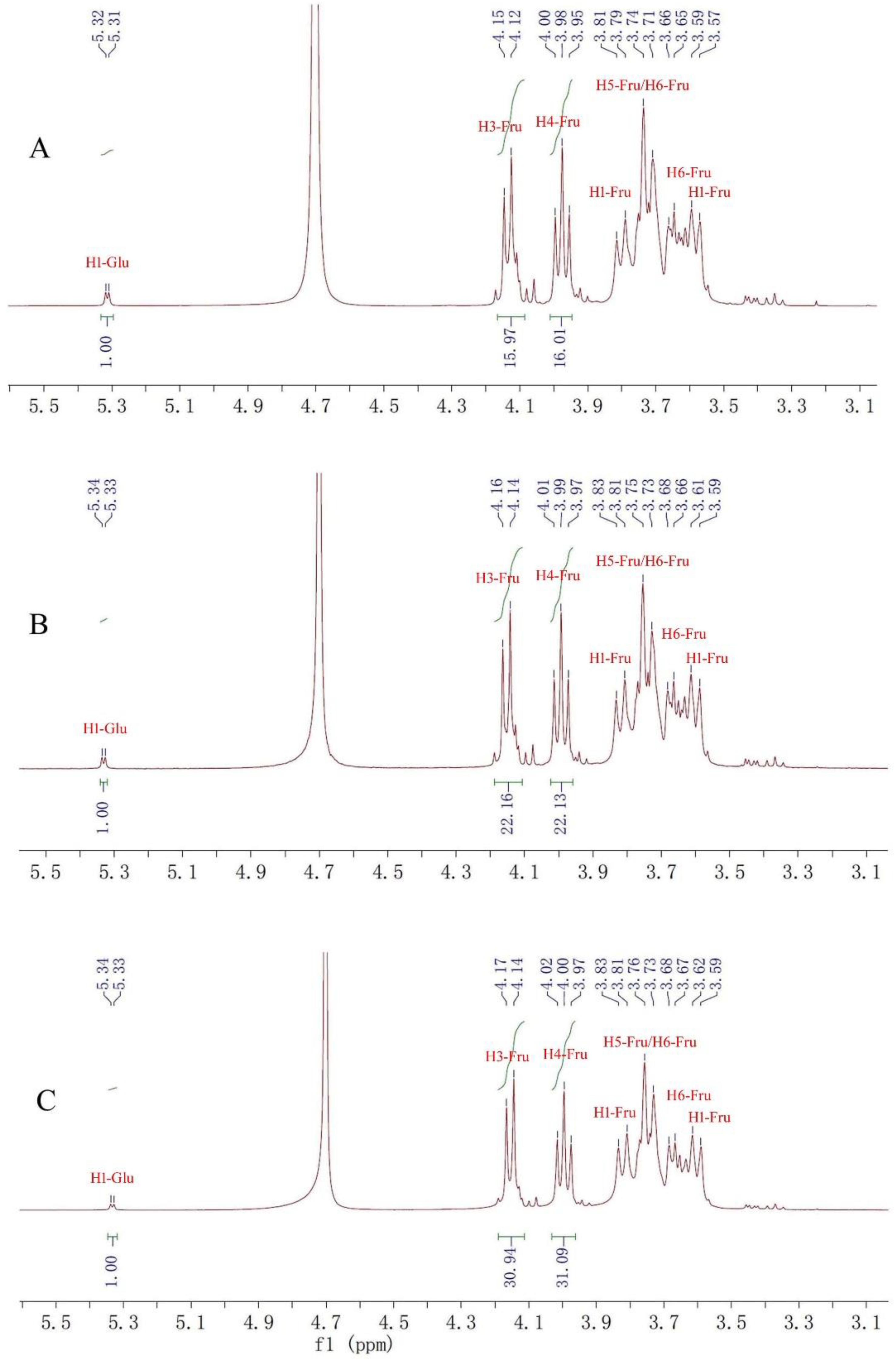
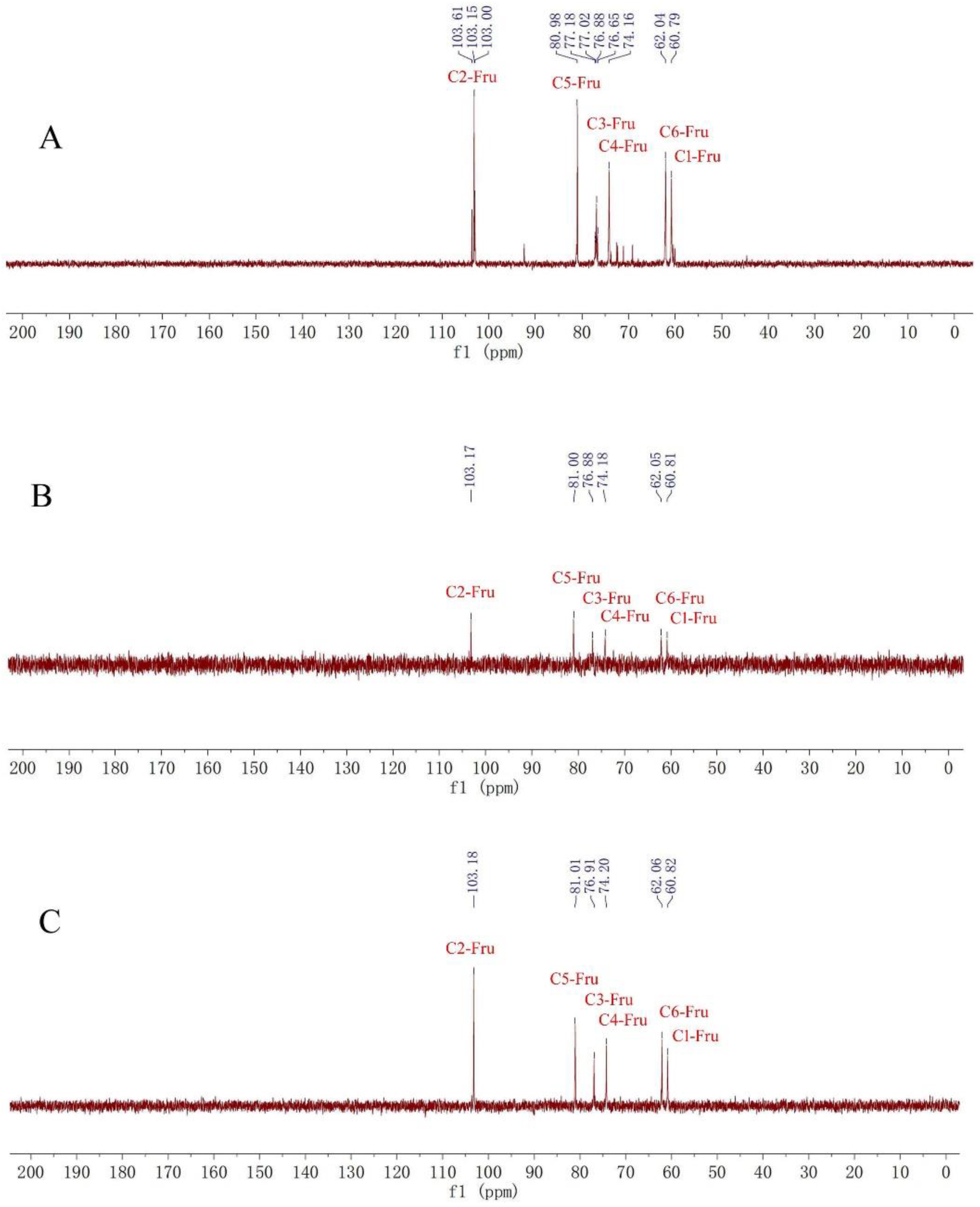

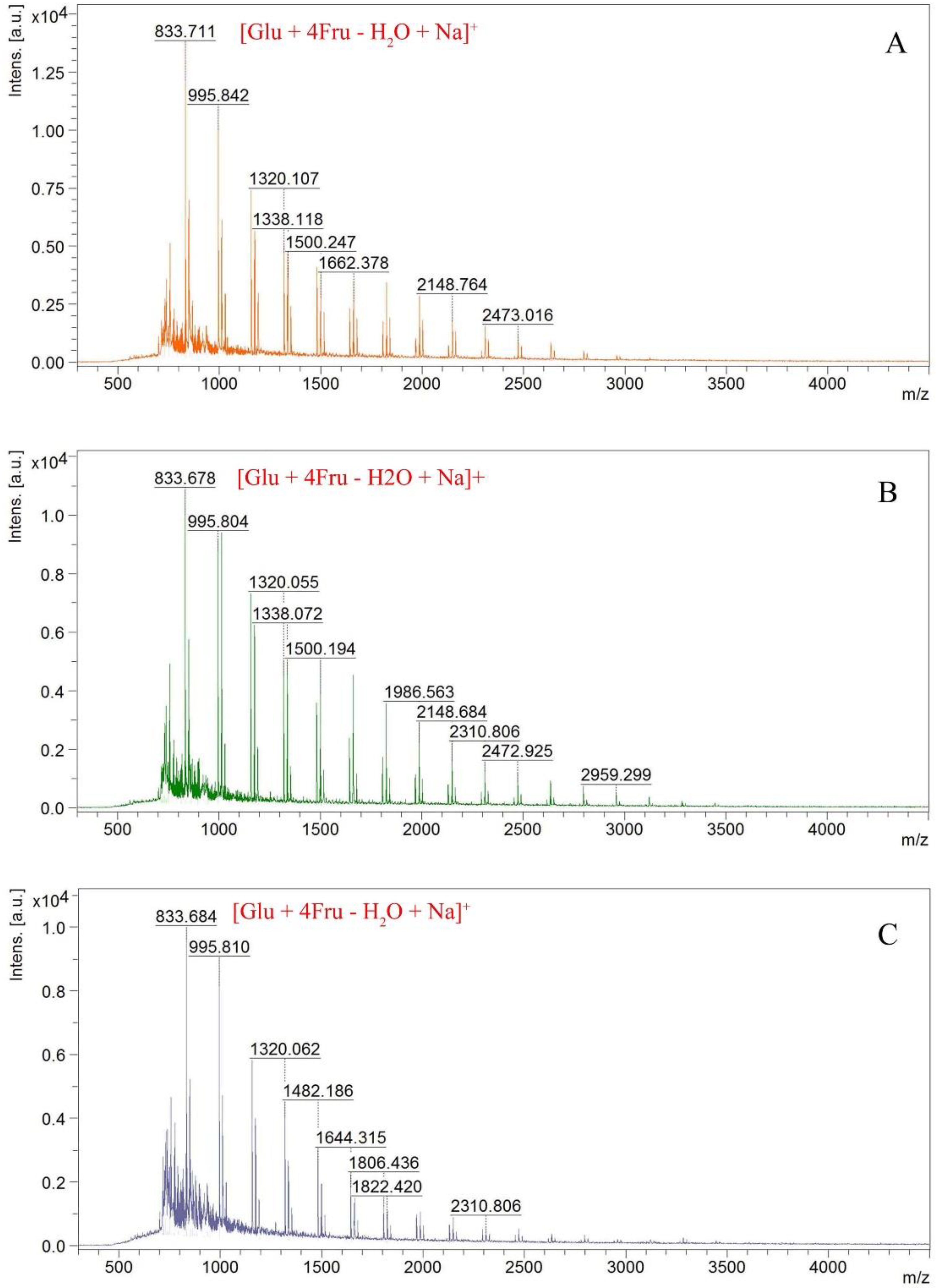

| Fructan 1 | Fructan 2 | Fructan 3 | ||||
|---|---|---|---|---|---|---|
| No. of C/H | 1H | 13C | 1H | 13C | 1H | 13C |
| 1 | 3.79, 3.59 | 60.79 | 3.81, 3.61 | 60.81 | 3.81, 3.62 | 6.82 |
| 2 | - | 103.15 | - | 103.17 | - | 103.18 |
| 3 | 4.12 | 76.88 | 4.16 | 76.88 | 4.17 | 76.91 |
| 4 | 3.98 | 74.16 | 3.99 | 74.18 | 4.00 | 74.20 |
| 5 | 3.74 | 80.98 | 3.75 | 81.00 | 3.76 | 81.01 |
| 6 | 3.71, 3.66 | 62.04 | 3.73, 3.66 | 62.05 | 3.73, 3.67 | 62.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, X.; Cao, L.; Ji, J.; Gao, J. Three Inulin-Type Fructans from Codonopsis pilosula (Franch.) Nannf. Roots and Their Prebiotic Activity on Bifidobacterium longum. Molecules 2018, 23, 3123. https://doi.org/10.3390/molecules23123123
Li J, Zhang X, Cao L, Ji J, Gao J. Three Inulin-Type Fructans from Codonopsis pilosula (Franch.) Nannf. Roots and Their Prebiotic Activity on Bifidobacterium longum. Molecules. 2018; 23(12):3123. https://doi.org/10.3390/molecules23123123
Chicago/Turabian StyleLi, Jiankuan, Xin Zhang, Lingya Cao, Jiaojiao Ji, and Jianping Gao. 2018. "Three Inulin-Type Fructans from Codonopsis pilosula (Franch.) Nannf. Roots and Their Prebiotic Activity on Bifidobacterium longum" Molecules 23, no. 12: 3123. https://doi.org/10.3390/molecules23123123
APA StyleLi, J., Zhang, X., Cao, L., Ji, J., & Gao, J. (2018). Three Inulin-Type Fructans from Codonopsis pilosula (Franch.) Nannf. Roots and Their Prebiotic Activity on Bifidobacterium longum. Molecules, 23(12), 3123. https://doi.org/10.3390/molecules23123123






