Induction of Apoptosis and Cytotoxicity by Raphasatin in Human Breast Adenocarcinoma MCF-7 Cells
Abstract
1. Introduction
2. Results
2.1. Effects of GRH and Raphasatin on Cell Viability of MCF-7 Cells
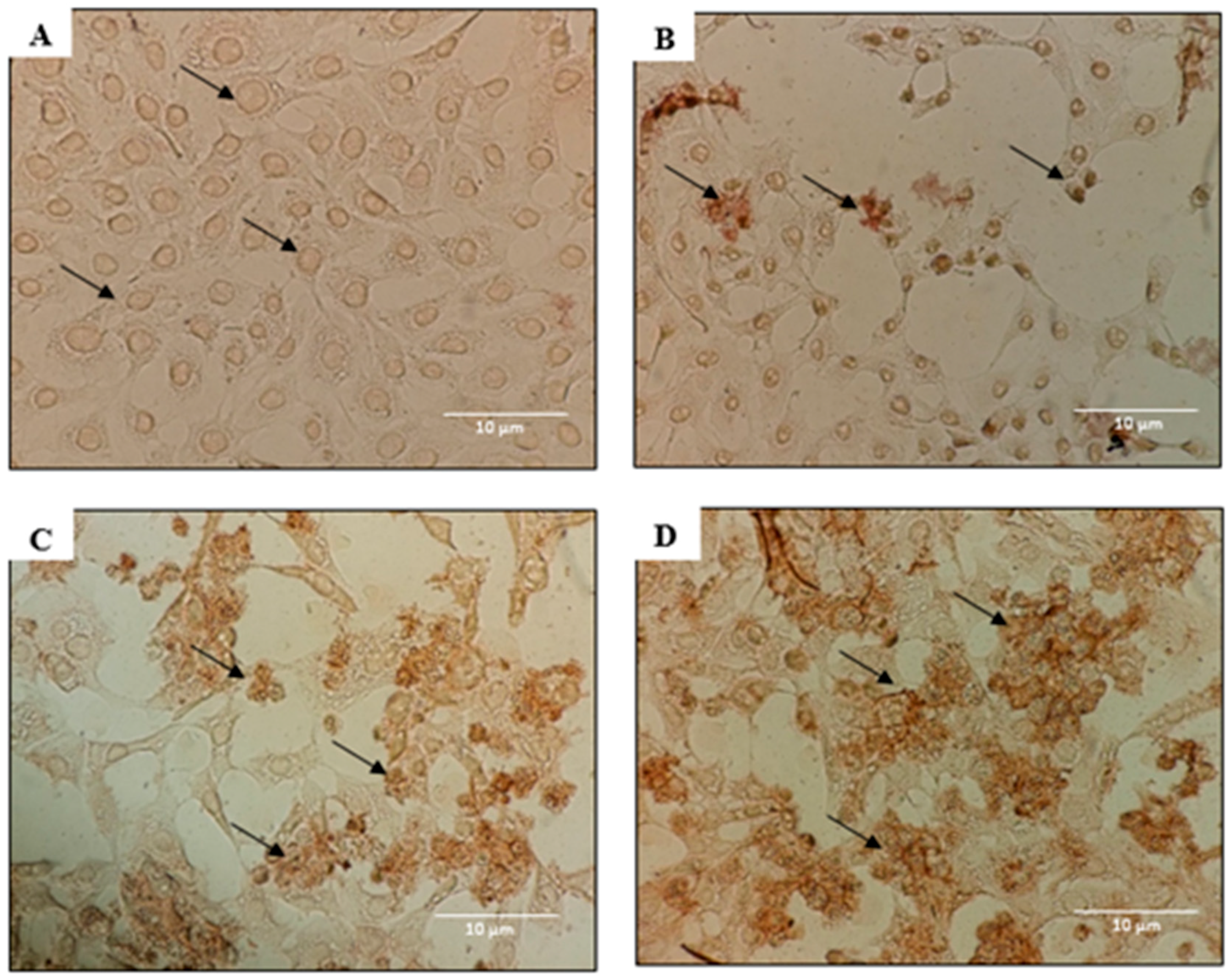
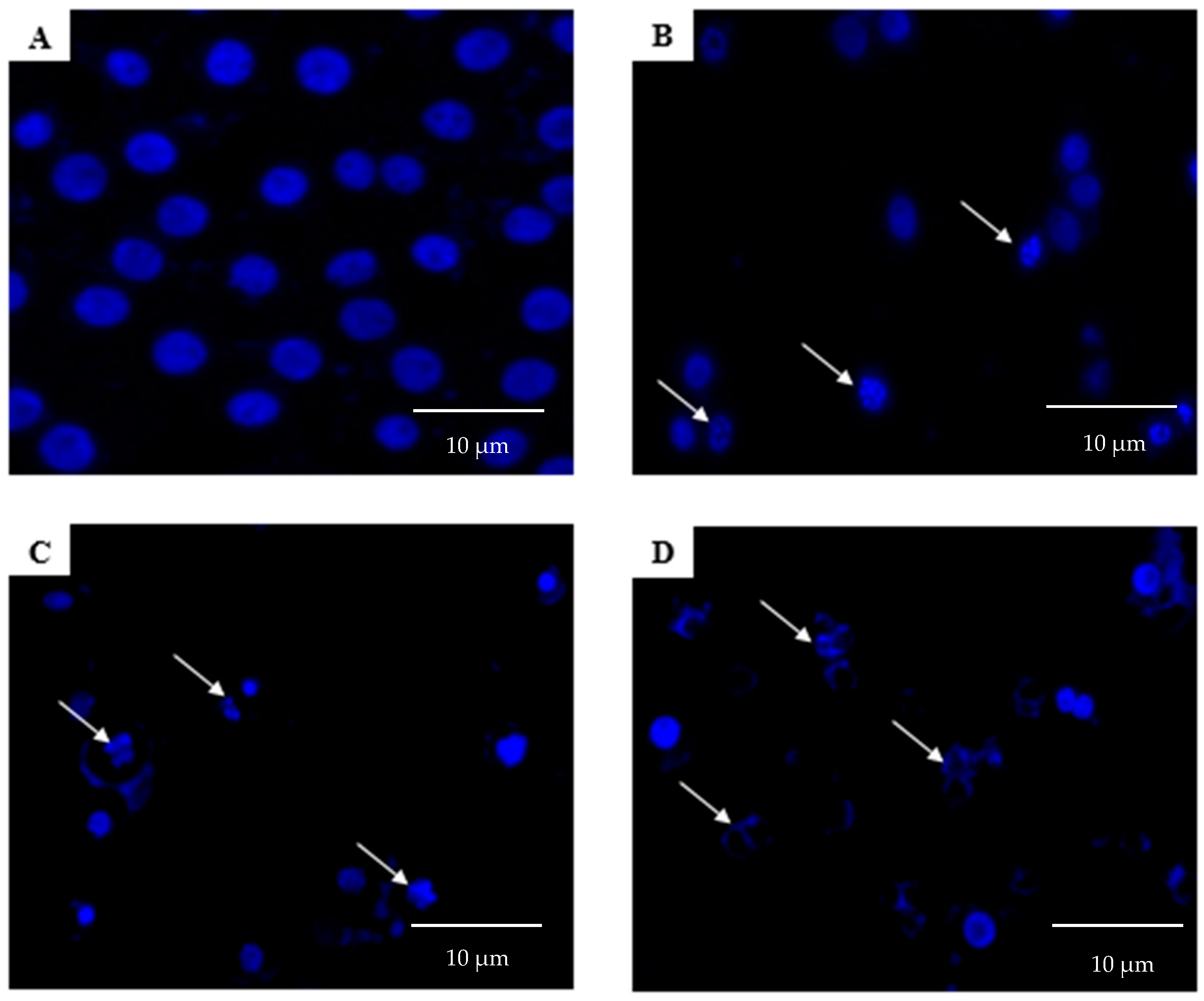
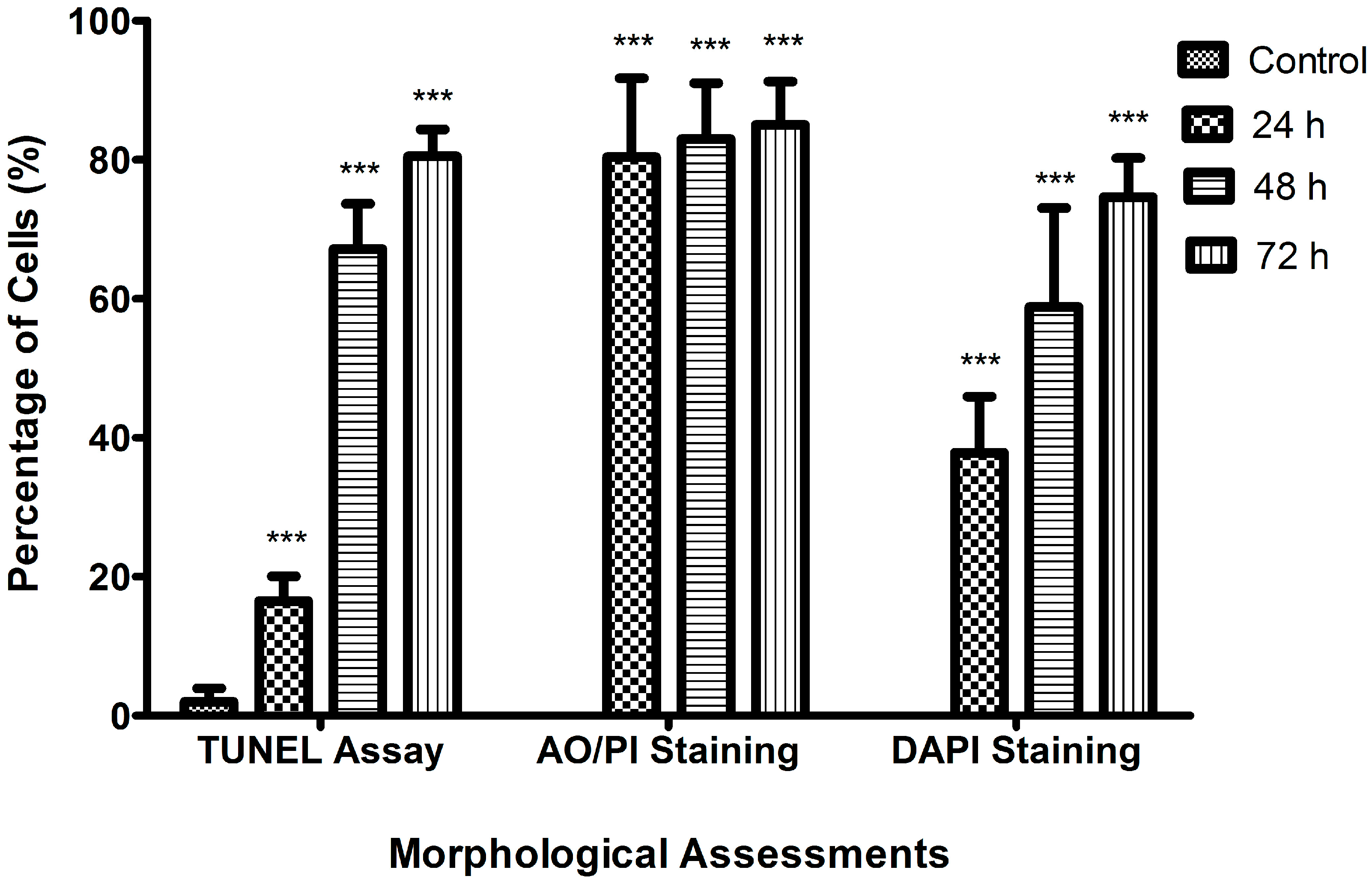
2.2. Morphological Analyses of Apoptosis Using the Terminal Deoxynucleotidyl Transferase dUTP Nick End Labelling (TUNEL) Assay, Acridine Orange/Propidium Iodide (AO/PI) Staining, and 4′,6-Diamidino-2-phenylindole (DAPI) Staining
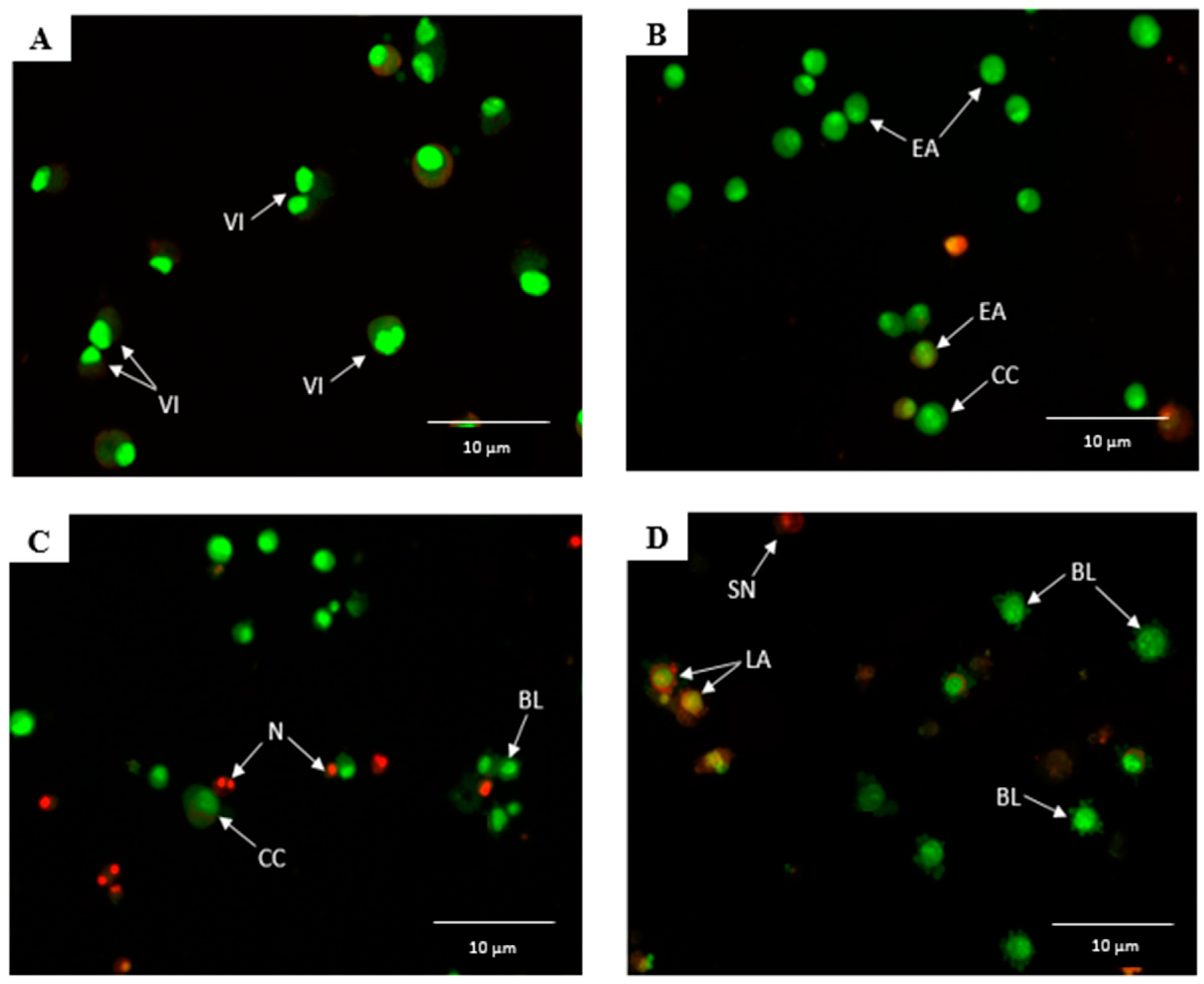
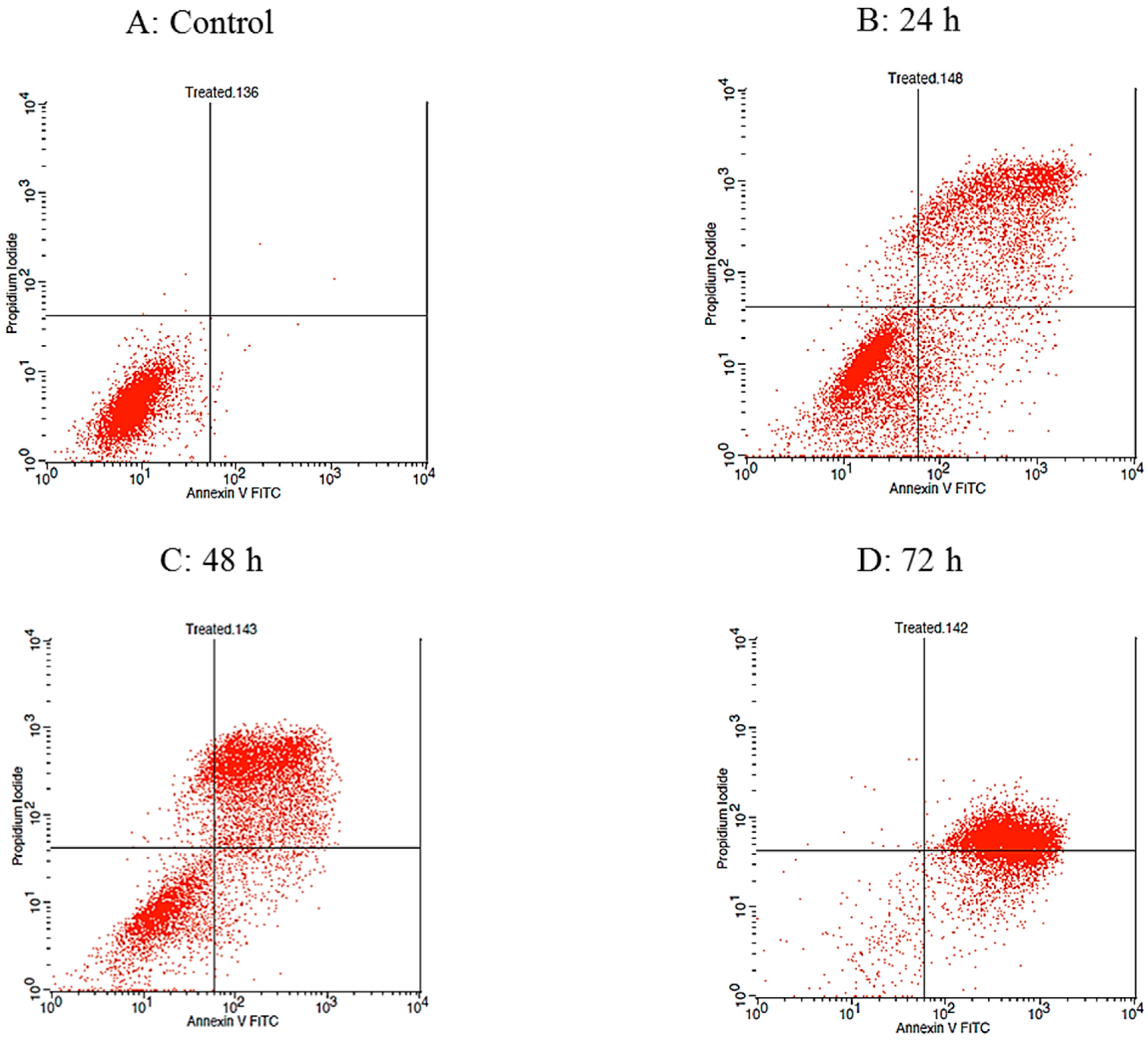
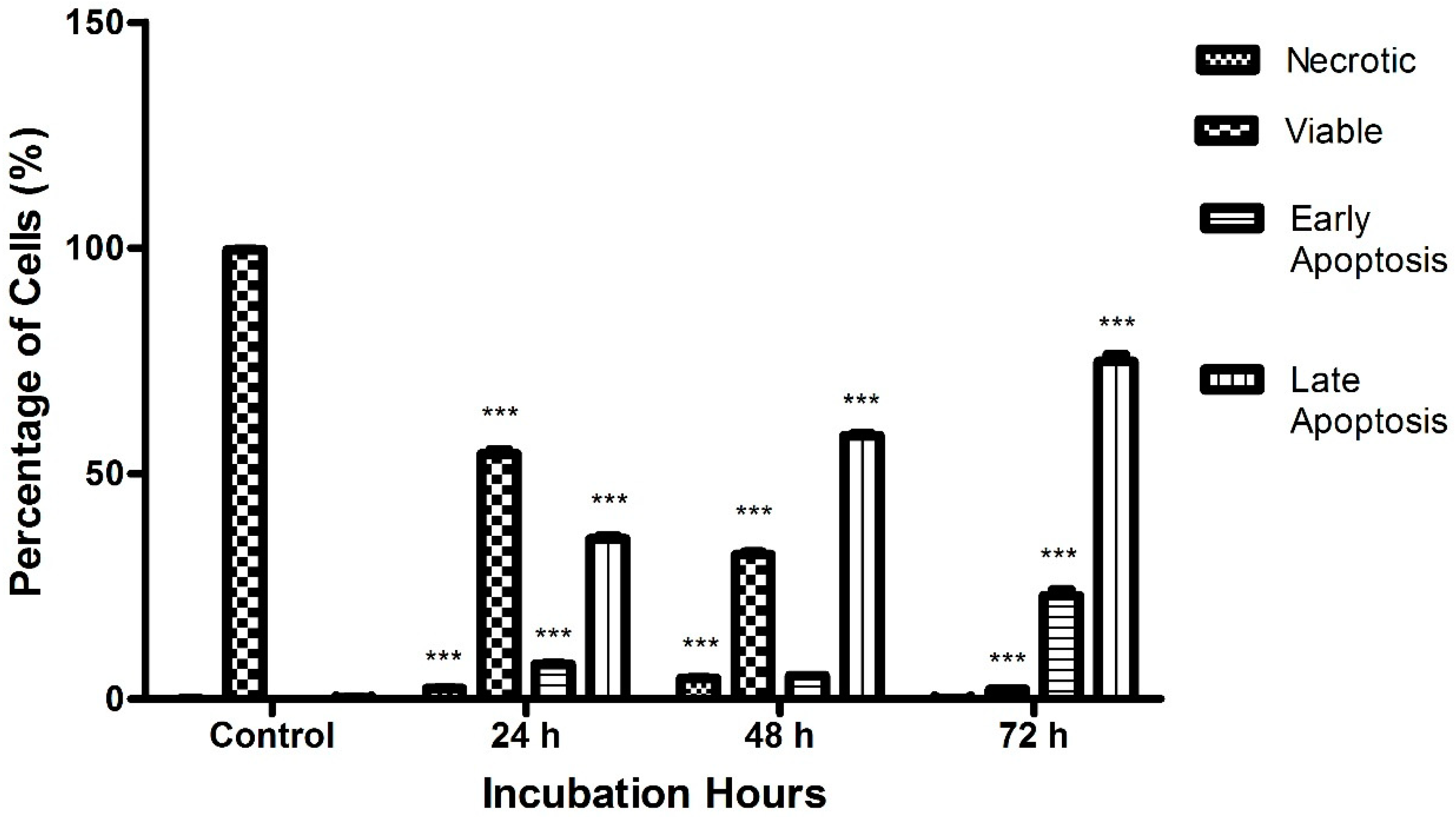
2.3. Raphasatin-Induced Apoptosis in the MCF-7 Cells Determined by Annexin V-FITC/PI Double Staining and Using Flow Cytometry
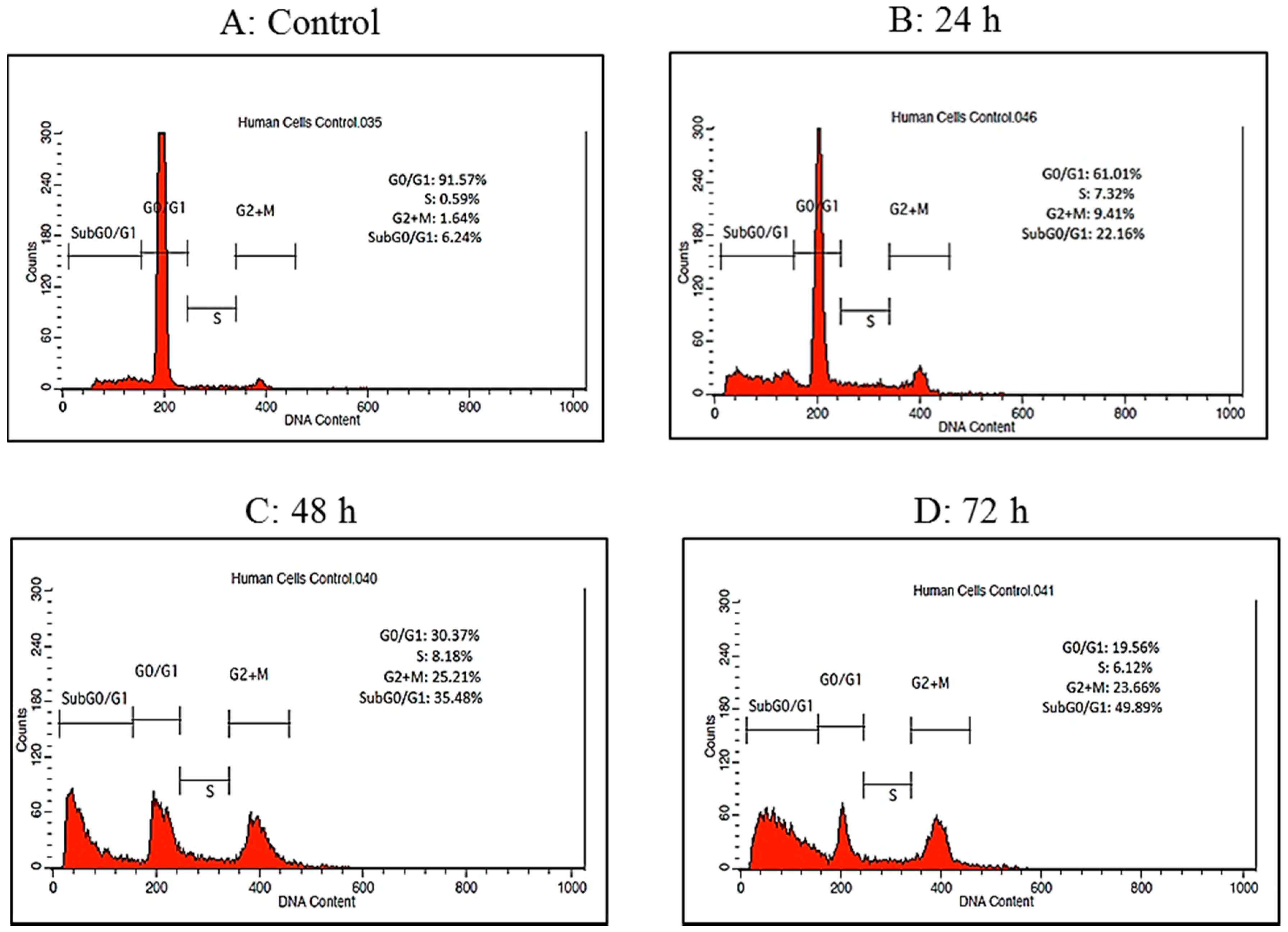
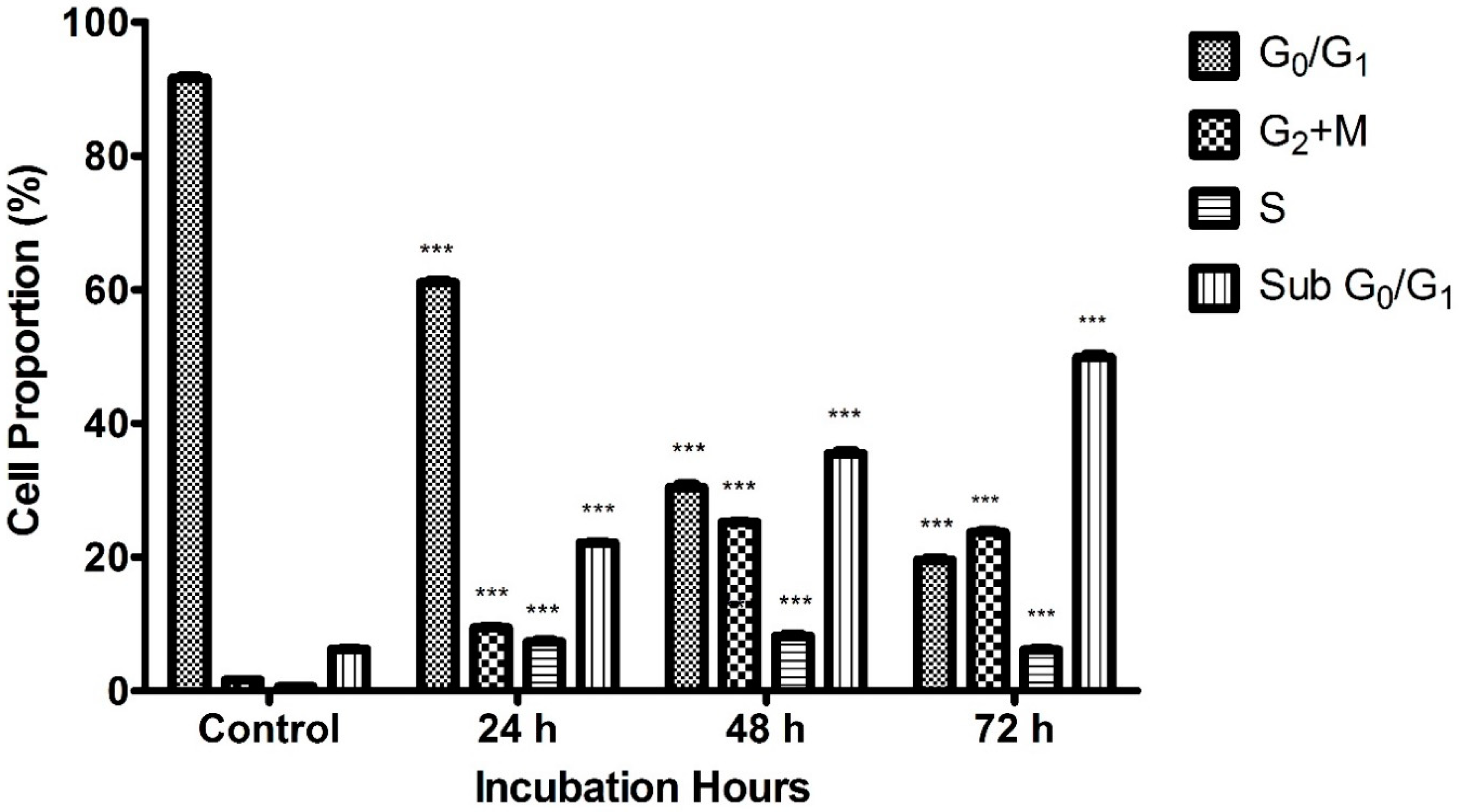
2.4. Cell Cycle Analysis by Flow Cytometry
3. Discussion
4. Materials and Methods
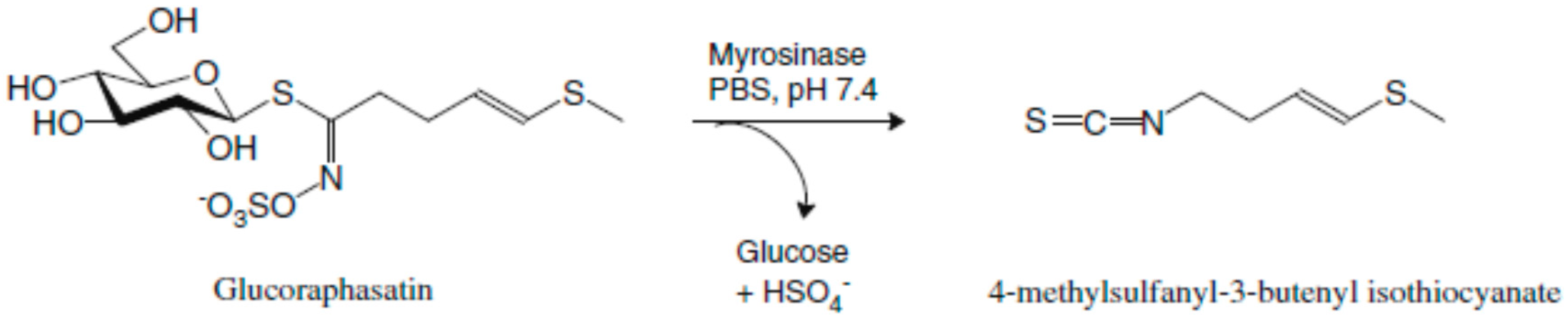
4.1. Isolation of Glucoraphasatin (GRH)
4.2. Cell Viability
4.3. Acridine Orange/Propidium Iodide Staining
4.4. DAPI Staining
4.5. DeadEnd Colorimetric TUNEL Assay
4.6. Annexin V-FITC/PI by Flow Cytometry
4.7. Cell Cycle Arrest Analysis by Flow Cytometry
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gerl, R.; Vaux, D.L. Apoptosis in the development and treatment of cancer. Carcinogenesis 2005, 26, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Dyer, N. Venom: Miracle Medicine. Sci. World 1999, 56, 5–8. [Google Scholar]
- Benjamin, C.W.; Hiebsch, R.R.; Jones, D.A. Caspase activation in MCF-7 cancer cells responding to etoposide treatment. Mol. Pharm. 1998, 53, 446–450. [Google Scholar] [CrossRef]
- Hisham, A.N.; Yip, C.H. Spectrum of breast cancer in Malaysian women: Overview. World J. Surg. 2003, 27, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Yip, C.H.; Taib, N.A.; Mohamed, I. Epidemiology of breast cancer in Malaysia. Asian Pac. J. Cancer Prev. 2006, 7, 369–374. [Google Scholar] [PubMed]
- Omar, Z.A.; Ali, Z.M.; Tamin, N.S.I. Malaysian Cancer Statistics-Data and Figure Peninsular Malaysia 2006; National Cancer Registry: Kuala Lumpur, Malaysia, 2006. [Google Scholar]
- Podsedek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. Swiss Soc. Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Zhang, N.Q.; Ho, S.C.; Mo, X.F.; Lin, F.Y.; Huang, W.Q.; Luo, H.; Zhang, C.X. Glucosinolate and isothiocyanate intakes are inversely associated with breast cancer risk: A case–control study in China. Br. J. Nutr. 2018, 119, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zirpoli, G.R.; McCann, S.E.; Moysich, K.B.; Ambrosone, C.B.; Tang, L. Trends in cruciferous vegetable consumption and associations with breast cancer risk: A case-control study. Curr. Dev. Nutr. 2017, 1, e000448. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from GLs. Eur. J. Nutr. 2008, 47, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, A.; Linseisen, L. Dietary intake of individual GLs in participants of the EIPC-Heidelberg cohort study. Ann. Nutr. Metab. 2009, 54, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Fausta, N.; Mariateresa, M.; Guido, L.; Cristina, S. Glucosinolates redox activities: Can they act as antioxidants? Food Chem. 2014, 149, 226–232. [Google Scholar]
- Talalay, P.; Fahey, J.W. Phytochemicals from cruciferous plant protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001, 131, 3027–3033. [Google Scholar] [CrossRef] [PubMed]
- Beevi, S.S.; Mangamoori, L.N.; Subathra, M.; Edula, J.R. Hexane extract of Raphanus sativus L. roots inhibits cell proliferation and induces apoptosis in human cancer cells by modulating genes related to apoptotic pathway. Plant Foods Hum. Nutr. 2010, 65, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Orlandi, M.; Bartolini, G.; Barillari, J.; Iori, R.; Paolini, M.; Ferroni, F.; Grazia Fumo, M.; Pedulli, G.F.; Valgimigli, L. Cytotoxic and antioxidant activity of 4-methylthio-3-butenyl isothiocyanate from Raphanus sativus L. (Kaiware Daikon) sprouts. J. Agric. Food Chem. 2008, 56, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, C.; Lenzi, M.; Hrelia, P. Chemoprevention of cancer by isothiocyanates and anthocyanins: Mechanisms of action and structure-activity relationship. Curr. Med. Chem. 2008, 15, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Taatjes, D.J.; Sobel, B.E.; Budd, R.C. Morphological and cytochemical determination of cell death by apoptosis. Histochem. Cell Biol. 2008, 129, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Abdull Razis, A.F.; Konsue, N.; Ioannides, C. Isothiocyanates and xenobiotic detoxification. Mol. Nutr. Food Res. 2018, 62, 1700916. [Google Scholar] [CrossRef] [PubMed]
- Abdull Razis, A.F.; De Nicola, G.R.; Pagnotta, E.; Iori, R.; Ioannides, C. A glucosinolate-rich extract of Japanese Daikon perturbs carcinogen-metabolizing enzyme systems in rat, being a potent inducer of hepatic glutathione S-transferase. Eur. J. Nutr. 2013, 52, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Razis, A.F.A.; De Nicola, G.R.; Pagnotta, E.; Iori, R.; Ioannides, C. 4-Methylsulfanyl-3-butenyl isothiocyanate derived from glucoraphasatin is a potent inducer of rat hepatic phase II enzymes and a potential chemopreventive agent. Arch. Toxicol. 2012, 86, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kntayya, S.B.; Ibrahim, M.D.; Mohd Ain, N.; Iori, R.; Ioannides, C.; Abdull Razis, A.F. Induction of apoptosis and cytotoxicity by isothiocyanate sulforaphene in human hepatocarcinoma HepG2 cells. Nutrients 2018, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, A.; Razis, A.F.A. Apoptosis as a mechanism of the cancer chemopreventive activity of glucosinolates: A review. Asian Pac. J. Cancer Prev. 2018, 19, 1439–1448. [Google Scholar] [PubMed]
- Razis, A.F.A.; Konsue, N.; Ioannides, C. Inhibitory effect of phenethyl isothiocyanate against benzo[a]pyrene-induced rise in CYP1A1 mRNA and apoprotein levels as its chemopreventive properties. Asian Pac. J. Cancer Prev. 2015, 16, 2679–2683. [Google Scholar] [CrossRef]
- Razis, A.F.A.; Mohd Noor, N.; Konsue, N. Induction of epoxide hydrolase, glucuronosyl transferase, and sulfotransferase by phenethyl isothiocyanate in male Wistar albino rats. Biomed. Res. Int. 2014, 2014, 391528. [Google Scholar] [CrossRef]
- Razis, A.F.A.; Noor, N.M. Sulforaphane is superior to glucoraphanin in modulating carcinogen-metabolising enzymes in Hep G2 cells. Asian Pac. J. Cancer Prev. 2013, 14, 4235–4238. [Google Scholar] [CrossRef]
- Razis, A.F.A.; Hanlon, N.; Soltys, E.; Krizova, V.; Iori, R.; Plant, K.E.; Ioannides, C. The naturally occurring aliphatic isothiocyanates sulforaphane and erucin are weak agonists but potent non-competitive antagonists of the aryl hydrocarbon receptor. Arch. Toxicol. 2012, 86, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Abdull Razis, A.F.; Konsue, N.; Dervetzoglou, M.; Plant, K.E.; Plant, N.; Ioannides, C. Phenethyl isothiocyanate, a naturally occurring phytochemical, is an antagonist of the aryl hydrocarbon receptor. Mol. Nutr. Food Res. 2012, 56, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Abdull Razis, A.F.; Iori, R.; Ioannides, C. The natural chemopreventive phytochemical R-sulforaphane is a far more potent inducer of the carcinogen-detoxifying enzyme systems in rat liver and lung than the S-isomer. Int. J. Cancer 2011, 128, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Razis, A.F.A.; Bagatta, M.; De Nicola, G.R.; Iori, R.; Ioannides, C. Induction of epoxide hydrolase and glucuronosyl transferase by isothiocyanates and intact glucosinolates in precision-cut rat liver slices: Importance of side-chain substituent and chirality. Arch. Toxicol. 2011, 85, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Umemura, T.; Inoue, T.; Tasaki, M.; Ishii, Y.; Nakamura, Y.; Park, E.Y.; Sato, K.; Matsuo, T.; Okamoto, S.; et al. Chemopreventive effects of 4-methylthio-3-butenyl isothiocyanate (Raphasatin) but not curcumin against pancreatic carcinogenesis in hamsters. J. Agric. Food Chem. 2013, 61, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Iwahashi, T.; Tanaka, A.; Koutani, J.; Matsuo, T.; Okamoto, S.; Sato, K.; Ohtsuki, K. 4-(Methylthio)-3-butenyl isothiocyanate, a principal antimutagen in daikon (Raphanus sativus; Japanese white radish). J. Agric. Food Chem. 2001, 49, 5755–5760. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.; Cho, Y.M.; Tadashi, H.; Takeshi, T.; Jun-ichi, A.; Yasushi, N.; Eun, Y.P.; Sasaki, A.; Nakamura, T.; Okamoto, S.; et al. 4-Methylthio-3-butenyl isothiocyanate (Raphasatin) exerts chemopreventive effects against esophageal carcinogenesis in rats. J. Toxicol. Pathol. 2016, 29, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Ben Salah-Abbès, J.; Abbès, S.; Ouanes, Z.; Abdel-Wahhab, M.A.; Bacha, H.; Oueslati, R. Isothiocyanate from the Tunisian radish (Raphanus sativus) prevents genotoxicity of Zearalenone in vivo and in vitro. Mutat. Res. 2009, 677, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Schwartz, G.K. Cyclin-dependent kinases as targets for cancer therapy. Cancer Chem. Biol. Response Mod. 2005, 22, 135–162. [Google Scholar]
- Hanlon, N.; Okpara, M.; Coldham, N.; Sauer, M.J.; Ioannides, C. Modulation of rat hepatic and pulmonary cytochromes P450 and Phase II enzyme systems by erucin, an isothiocyanate structurally related to sulforaphane. J. Agric. Food Chem. 2008, 56, 7866–7871. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, N.; Coldham, N.; Sauer, M.J.; Ioannides, C. Up-regulation of the CYP1 family in rat and human liver by the aliphatic isothiocyanates erucin and sulforaphane. Toxicology 2008, 252, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, N.; Coldham, N.; Gielbert, A.; Kuhnert, N.; Sauer, M.J.; King, L.J.; Ioannides, C. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in the rat. Br. J. Nutr. 2008, 99, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, N.; Coldham, N.; Sauer, M.J.; Ioannides, C. Modulation of rat pulmonary carcinogen-metabolising enzyme systems by the isothiocyanates erucin and sulforaphane. Chem. Biol. Interact. 2009, 177, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Konsue, N.; Ioannides, C. Tissue differences in the modulation of rat cytochromes P450 and phase II conjugation systems by dietary doses of phenethyl isothiocyanate. Food Chem. Toxicol. 2008, 46, 3677–3683. [Google Scholar] [CrossRef] [PubMed]
- Konsue, N.; Ioannides, C. Differential response of four human livers to modulation of phase II enzyme systems by the chemopreventive phytochemical phenethyl isothiocyanate. Mol. Nutr. Food Res. 2010, 54, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Konsue, N.; Ioannides, C. Modulation of carcinogen-metabolising cytochromes P450 in human liver by the chemopreventive phytochemical phenethyl isothiocyanate, a constituent of cruciferous vegetables. Toxicology 2010, 268, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Konsue, N.; Kirkpatrick, J.; Kuhnert, N.; King, L.J.; Ioannides, C. Repeated oral administration modulates the pharmacokinetic behaviour of the chemopreventive agent phenethyl isothiocyanate in rats. Mol. Nutr. Food Res. 2010, 54, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis 2000, 21, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Molecular mechanism of rapid cellular accumulation of anticarcinogenic isothiocyanates. Carcinogenesis 2001, 22, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Traka, M.H.; Saha, S.; Huseby, S.; Kopriva, S.; Walley, P.G.; Barker, G.C.; Moore, J.; Mero, G.; van den Bosch, F.; Constant, H.; et al. Genetic regulation of glucoraphanin accumulation in Beneforté broccoli. New Phytol. 2013, 198, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Barillari, J.; Cervellati, R.; Paolini, M.; Tatibouet, A.; Rollin, P.; Iori, R. Isolation of 4 methylthio-3-butenyl GLs from Raphanus sativus L. sprouts (Kaiware-Daikon) and its redox properties. J. Agric. Food Chem. 2005, 53, 9890–9896. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Ahmad, B.A.; Mohd, A.S.; Rasedee, A.; Suvitha, S.; Behnam, K.; Mohamed, Y.I.; Manal, M.E.; Taha, S.I.A.; Hapipah, M.A.; et al. Dentatin isolated from Clausena excavata induces apoptosis in MCF-7 cell line through the intrinsic pathway with involvement of NF-κB signalling and G0/G1 cell cycle arrest: A bioassay guided approach. J. Ethnopharmacol. 2013, 145, 343–354. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| Compound | IC50 | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| GRH | >100 µM | >100 µM | >100 µM |
| Raphasatin | 9.84 ± 0.55 µM | 9.64 ± 0.24 µM | 5.61 ± 0.23 µM |
| Paclitaxel | 7.53 ± 0.68 nM | 5.21 ± 0.28 nM | 5.09 ± 0.07 nM |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.D.; Kntayya, S.B.; Mohd Ain, N.; Iori, R.; Ioannides, C.; Abdull Razis, A.F. Induction of Apoptosis and Cytotoxicity by Raphasatin in Human Breast Adenocarcinoma MCF-7 Cells. Molecules 2018, 23, 3092. https://doi.org/10.3390/molecules23123092
Ibrahim MD, Kntayya SB, Mohd Ain N, Iori R, Ioannides C, Abdull Razis AF. Induction of Apoptosis and Cytotoxicity by Raphasatin in Human Breast Adenocarcinoma MCF-7 Cells. Molecules. 2018; 23(12):3092. https://doi.org/10.3390/molecules23123092
Chicago/Turabian StyleIbrahim, Muhammad Din, Saie Brindha Kntayya, Nooraini Mohd Ain, Renato Iori, Costas Ioannides, and Ahmad Faizal Abdull Razis. 2018. "Induction of Apoptosis and Cytotoxicity by Raphasatin in Human Breast Adenocarcinoma MCF-7 Cells" Molecules 23, no. 12: 3092. https://doi.org/10.3390/molecules23123092
APA StyleIbrahim, M. D., Kntayya, S. B., Mohd Ain, N., Iori, R., Ioannides, C., & Abdull Razis, A. F. (2018). Induction of Apoptosis and Cytotoxicity by Raphasatin in Human Breast Adenocarcinoma MCF-7 Cells. Molecules, 23(12), 3092. https://doi.org/10.3390/molecules23123092






