Selenium, Selenoproteins, and Female Reproduction: A Review
Abstract
:1. Introduction
2. Transport of Se and Selenoproteins
3. Expression of Selenoproteins in Female Reproductive Tissues
4. Selenium in Follicular Development and Ovarian Function
4.1. Evidence from In Vitro and Animal Model Studies
4.2. Evidence from Human Models Studies
5. Implication of Se in Ovarian Pathologies and Assisted Reproductive Technologies-related Oxidative Stress
5.1. Human Studies
5.2. In Vitro Studies
6. Effects of Maternal Dietary Se Supplementation
7. Selenium and Placental Oxidative Stress
8. Role of Se in Pregnancy
9. Implication of Se and Selenoproteins in Reproductive Cancer
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rayman, M.P. The importance of selenium to human health. The Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, G.V.; Castellano, S.; Novoselov, S.V.; Lobanov, A.V.; Zehtab, O.; Guigó, R.; Gladyshev, V.N. Characterization of mammalian selenoproteomes. Science 2003, 300, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid. Redox Signaling 2007, 9, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, V.N.; Arnér, E.S.; Berry, M.J.; Brigelius-Flohé, R.; Bruford, E.A.; Burk, R.F.; Carlson, B.A.; Castellano, S.; Chavatte, L.; Conrad, M. Selenoprotein gene nomenclature. J. Biol. Chem. 2016, 291, 24036–24040. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.; Zoidis, E.; Surai, P.; Zervas, G. Selenoproteins and maternal nutrition. Comp. Biochem. Physiol. Part. B: Biochem. Mol. Biol. 2008, 151, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Ceko, M.J.; Hummitzsch, K.; Hatzirodos, N.; Bonner, W.M.; Aitken, J.B.; Russell, D.L.; Lane, M.; Rodgers, R.J.; Harris, H.H. X-Ray fluorescence imaging and other analyses identify selenium and GPX1 as important in female reproductive function. Metallomics 2015, 7, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ceko, M.J.; Hummitzsch, K.; Bonner, W.M.; Aitken, J.B.; Spiers, K.M.; Rodgers, R.J.; Harris, H.H. Localization of the trace elements iron, zinc and selenium in relation to anatomical structures in bovine ovaries by X-ray fluorescence imaging. Microsc. Microanal. 2015, 21, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Paszkowski, T.; Traub, A.; Robinson, S.; McMaster, D. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin. Chim. Acta 1995, 236, 173–180. [Google Scholar] [CrossRef]
- Baek, I.J.; Yon, J.M.; Lee, S.R.; Kim, M.R.; Hong, J.; Lee, B.; Yun, Y.; Nam, S.Y. Differential Expression of Gastrointestinal Glutathione Peroxidase (GI-GPx) Gene during Mouse Organogenesis. Anat. Histol. Embryo. 2011, 40, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Leng, J.-Y.; Gao, F.; Zhao, Z.-A.; Deng, W.-B.; Liang, X.-H.; Zhang, Y.-J.; Zhang, Z.-R.; Li, M.; Sha, A.-G. Differential expression and anti-oxidant function of glutathione peroxidase 3 in mouse uterus during decidualization. FEBS Lett. 2014, 588, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; García-Fuentes, E.; Callejón-Leblic, B.; García-Barrera, T.; Gómez-Ariza, J.L.; Rayman, M.P.; Velasco, I. Selenium, selenoproteins and selenometabolites in mothers and babies at the time of birth. Br. J. Nutr. 2017, 117, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Olson, G.E.; Hill, K.E.; Winfrey, V.P.; Motley, A.K.; Kurokawa, S. Maternal-fetal transfer of selenium in the mouse. FASEB J. 2013, 27, 3249–3256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayman, M.P.; Bath, S.C.; Westaway, J.; Williams, P.; Mao, J.; Vanderlelie, J.J.; Perkins, A.V.; Redman, C.W. Selenium status in UK pregnant women and its relationship with hypertensive conditions of pregnancy. Br. J. Nutr. 2015, 113, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.-G.; Ushizawa, K.; Hosoe, M.; Takahashi, T. Differential genome-wide gene expression profiling of bovine largest and second-largest follicles: identification of genes associated with growth of dominant follicles. Reprod. Biol. Endocrinol. 2010, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yant, L.J.; Ran, Q.; Rao, L.; Van Remmen, H.; Shibatani, T.; Belter, J.G.; Motta, L.; Richardson, A.; Prolla, T.A. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radical Biol. Med. 2003, 34, 496–502. [Google Scholar] [CrossRef]
- Imai, H.; Hirao, F.; Sakamoto, T.; Sekine, K.; Mizukura, Y.; Saito, M.; Kitamoto, T.; Hayasaka, M.; Hanaoka, K.; Nakagawa, Y. Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem. Biophys. Res. Commun. 2003, 305, 278–286. [Google Scholar] [CrossRef]
- Mistry, H.D.; Wilson, V.; Ramsay, M.M.; Symonds, M.E.; Pipkin, F.B. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertension 2008, 52, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Lin, Y.; Li, J.; Liu, M.; Wang, J.; Li, X.; Liu, J.; Jia, X.; Jing, Z.; Huang, Z. Evaluation of Glutathione Peroxidase 4 role in Preeclampsia. Sci. Rep. 2016, 6, 33300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinova, M.; Paskaleva, V. Selenium and glutathione peroxidase in patients with preeclampsia. Akush. Ginekol. 2013, 52, 3–7. [Google Scholar]
- Imai, H.; Hakkaku, N.; Iwamoto, R.; Suzuki, J.; Suzuki, T.; Tajima, Y.; Konishi, K.; Minami, S.; Ichinose, S.; Ishizaka, K. Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J. Biol. Chem. 2009, 284, 32522–32532. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, U.; Kamran, Z.; Raza, I.; Ahmad, S.; Babar, W.; Riaz, M.; Iqbal, Z. Role of selenium in male reproduction—A review. Anim. Reprod. Sci. 2014, 146, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chabory, E.; Damon, C.; Lenoir, A.; Kauselmann, G.; Kern, H.; Zevnik, B.; Garrel, C.; Saez, F.; Cadet, R.; Henry-Berger, J. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J. Clin. Investing. 2009, 119, 2074–2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakupoglu, C.; Przemeck, G.K.; Schneider, M.; Moreno, S.G.; Mayr, N.; Hatzopoulos, A.K.; de Angelis, M.H.; Wurst, W.; Bornkamm, G.W.; Brielmeier, M. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol. Cell. Biol. 2005, 25, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Bondareva, A.A.; Capecchi, M.R.; Iverson, S.V.; Li, Y.; Lopez, N.I.; Lucas, O.; Merrill, G.F.; Prigge, J.R.; Siders, A.M.; Wakamiya, M. Effects of thioredoxin reductase-1 deletion on embryogenesis and transcriptome. Free Radical Biol. Med. 2007, 43, 911–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, M.; Jakupoglu, C.; Moreno, S.G.; Lippl, S.; Banjac, A.; Schneider, M.; Beck, H.; Hatzopoulos, A.K.; Just, U.; Sinowatz, F. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol. Cell. Biol. 2004, 24, 9414–9423. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Novoselov, S.V.; Sun, Q.-A.; Moustafa, M.E.; Zhou, Y.; Oko, R.; Hatfield, D.L.; Gladyshev, V.N. Mammalian selenoprotein thioredoxin-glutathione reductase roles in disulfide bond formation and sperm maturation. J. Biol. Chem. 2005, 280, 26491–26498. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and adverse health conditions of human pregnancy. In Selenium; Springer: New York, NY, USA, 2011; pp. 531–544. [Google Scholar]
- Galton, V.A.; Martinez, E.; Hernandez, A.; Germain, E.A.S.; Bates, J.M.; Germain, D.L.S. Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase. J. Clin. Investing. 1999, 103, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.A.; Dorfman, D.M.; Genest, D.R.; Salvatore, D.; Larsen, P.R. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J. Clin. Endocrinol. Metab. 2003, 88, 1384–1388. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.; Vanderlelie, J.; Perkins, A. Selenium supplementation protects trophoblast cells from mitochondrial oxidative stress. Placenta 2013, 34, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, M.; Gralla, O.; Behrends, T.; Scharpf, M.; Endermann, T.; Rijntjes, E.; Pietschmann, N.; Hollenbach, B.; Schomburg, L. Selenoprotein P in seminal fluid is a novel biomarker of sperm quality. Biochem. Biophys. Res. Commun. 2014, 443, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Renko, K.; Werner, M.; Renner-Muller, I.; Cooper, T.G.; Yeung, C.H.; Hollenbach, B.; Scharpf, M.; Kohrle, J.; Schomburg, L.; Schweizer, U. Hepatic selenoprotein P (SePP) expression restores selenium transport and prevents infertility and motor-incoordination in Sepp-knockout mice. Biochem. J. 2008, 409, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.E.; Winfrey, V.P.; NagDas, S.K.; Hill, K.E.; Burk, R.F. Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J. Biol. Chem. 2007, 282, 12290–12297. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.E.; Winfrey, V.P.; NagDas, S.K.; Hill, K.E.; Burk, R.F. Selenoprotein P is required for mouse sperm development. Biol. Reprod. 2005, 73, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Searle, E.; Kelly, L.; Johnsen, S.; Bodman-Smith, K.; Bath, S.C.; Mao, J.; Redman, C.W. Effect of selenium on markers of risk of pre-eclampsia in UK pregnant women: a randomised, controlled pilot trial. Br. J. Nutr. 2014, 112, 99–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moses, E.K.; Johnson, M.P.; Tommerdal, L.; Forsmo, S.; Curran, J.E.; Abraham, L.J.; Charlesworth, J.C.; Brennecke, S.P.; Blangero, J.; Austgulen, R. Genetic association of preeclampsia to the inflammatory response gene SEPS1. Am. J. Obstet. Gynecol. 2008, 198, 20. [Google Scholar] [CrossRef] [PubMed]
- Turanov, A.A.; Malinouski, M.; Gladyshev, V.N. Selenium and male reproduction. In Selenium: Its Molecular Biology and Role in Human Health; Hatfield, D., Berry, M., Gladyshev, V., Eds.; Springer: New York, NY, USA, 2011; pp. 409–417. [Google Scholar]
- Letavayová, L.; Vlčková, V.; Brozmanová, J. Selenium: from cancer prevention to DNA damage. Toxicology 2006, 227, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Selenomethionine: A review of its nutritional significance, metabolism and toxicity. J. Nutr. 2000, 130, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-chain selenium and human health: spotlight on speciation. Br. J. Nutr. 2008, 100, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine: Panel on Dietary Antioxidants and Related Compounds: Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington (DC). Available online: https://www.ncbi.nlm.nih.gov/books/NBK225470/ (accessed on 19 November 2018).
- Council, N.R. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007; p. 384. [Google Scholar]
- Surai, P.F.; Fisinin, V.I. Selenium in Pig Nutrition and reproduction: Boars and semen quality—A Review. Asian-Australas. J. Anim. Sci. 2015, 28, 730. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Nutrient Requirements of Horses: Sixth Revised Edition; The National Academies Press: Washington, DC, USA, 2007; p. 360. [Google Scholar]
- Geor, R.J.; Coenen, M.; Harris, P. Equine Applied and Clinical Nutrition E-Book: Health, Welfare and Performance. FEMS Microbiol. Lett. 2014, 57, 73–77. [Google Scholar]
- Council, N.R. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition, 2001; The National Academies Press: Washington, DC, USA, 2001; p. 408. [Google Scholar]
- Council, N.R. Nutrient Requirements of Beef Cattle: Seventh Revised Edition: Update 2000; The National Academies Press: Washington, DC, USA, 2000; p. 248. [Google Scholar]
- Faye, B.; Seboussi, R. Selenium in camel–A review. Nutrients 2009, 1, 30–49. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The use of high-selenium yeast to raise selenium status: how does it measure up? Br. J. Nutr. 2004, 92, 557–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foresta, C.; Flohé, L.; Garolla, A.; Roveri, A.; Ursini, F.; Maiorino, M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol. Reprod. 2002, 67, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Lan, D.; Li, J.; Lin, Y.; Li, M. Selenium supplementation during in vitro maturation enhances meiosis and developmental capacity of yak oocytes. Anim. Sci. J. 2018, 89, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kommisrud, E.; Østerås, O.; Vatn, T. Blood selenium associated with health and fertility in Norwegian dairy herds. Acta Vet. Scand. 2005, 46, 229. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Dufrasne, I. Selenium in cattle: a review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef] [PubMed]
- Al-Kunani, A.; Knight, R.; Haswell, S.; Thompson, J.; Lindow, S. The selenium status of women with a history of recurrent miscarriage. BJOG: An. Int. J. Obstet. Gynaecol. 2001, 108, 1094–1097. [Google Scholar]
- Güvenç, M.; Güven, H.; Karataş, F.; Aygün, A.D.; Bektaş, S. Low levels of selenium in miscarriage. J. Trace Elements Exp. Med. 2002, 15, 97–101. [Google Scholar] [CrossRef]
- Rumiris, D.; Purwosunu, Y.; Wibowo, N.; Farina, A.; Sekizawa, A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertens. Pregnancy 2006, 25, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Van Leer, L.; Vanderlelie, J.; Perkins, A. Selenium supplementation protects trophoblast cells from oxidative stress. Placenta 2012, 33, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Morimoto, N.; Hosokawa, S.; Matsushita, T. Associations of maternal and neonatal serum trace element concentrations with neonatal birth weight. PLoS ONE 2013, 8, e75627. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ei-Samahy, M.; Fan, L.; Zheng, L.; Jin, Y.; Zhang, G.; Liu, Z.; Wang, F. In vitro influence of selenium on the proliferation of and steroidogenesis in goat luteinized granulosa cells. Theriogenology 2018, 114, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.E.; Lyons, P.R.; Burk, R.F. Differential regulation of rat liver selenoprotein mRNAs in selenium deficiency. Biochem. Biophys Res. Commun. 1992, 185, 260–263. [Google Scholar] [CrossRef]
- Bösl, M.R.; Takaku, K.; Oshima, M.; Nishimura, S.; Taketo, M.M. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp). Proc. Natl. Acad. Sci. 1997, 94, 5531–5534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.M.; Huel, G.; Godin, J.; Hellier, G.; Sahuquillo, J.; Moreau, T.; Blot, P. Inter-individual variation of selenium in maternal plasma, cord plasma and placenta. Sci. Total Environ. 1995, 159, 119–127. [Google Scholar] [CrossRef]
- Eisenmann, C.; Miller, R. The placental transfer and toxicity of selenite relative to cadmium in the human term perfused placenta. Placenta 1994, 15, 883–895. [Google Scholar] [CrossRef]
- Olson, G.E.; Whitin, J.C.; Hill, K.E.; Winfrey, V.P.; Motley, A.K.; Austin, L.M.; Deal, J.; Cohen, H.J.; Burk, R.F. Extracellular glutathione peroxidase (Gpx3) binds specifically to basement membranes of mouse renal cortex tubule cells. Am. J. Physiol. Ren. Physiol. 2009, 298, F1244–F1253. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Selenoprotein P—expression, functions, and roles in mammals. Biochim. et Biophys. Acta Gen. Subj. 2009, 1790, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, S.; Hill, K.E.; McDonald, W.H.; Burk, R.F. Long-isoform mouse selenoprotein P (Sepp1) supplies rat myoblast L8 cells with selenium via endocytosis mediated by heparin-binding properties and apolipoprotein E receptor-2 (apoER2). J. Biol. Chem. 2012, 287, 28717–28726. [Google Scholar] [CrossRef] [PubMed]
- Bou-Resli, M.; Dashti, H.; Mathew, T.; Al-Zaid, N. Pre-and postnatal tissue selenium of the rat in the growing state. Neonatology 2001, 80, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Hatzirodos, N.; Hummitzsch, K.; Irving-Rodgers, H.F.; Harland, M.L.; Morris, S.E.; Rodgers, R.J. Transcriptome profiling of granulosa cells from bovine ovarian follicles during atresia. BMC Genomics 2014, 15, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasik, J.; Rice, E. Selenoprotein P expression in liver, uterus and placenta during late pregnancy. Placenta 1995, 16, 67–74. [Google Scholar] [CrossRef]
- Galton, V.A.; Martinez, E.; Hernandez, A.; St. Germain, E.A.; Bates, J.M.; St. Germain, D.L. The type 2 iodothyronine deiodinase is expressed in the rat uterus and induced during pregnancy. Endocrinology 2001, 142, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Koopdonk-Kool, J.M.; De Vijlder, J.; Veenboer, G.J.; Ris-Stalpers, C.; Kok, J.H.; Vulsma, T.; Boer, K.; Visser, T.J. Type II and type III deiodinase activity in human placenta as a function of gestational age. J. Clin. Endocrinol. Metab. 1996, 81, 2154–2158. [Google Scholar] [PubMed]
- Ejima, K.; Koji, T.; Nanri, H.; Kashimura, M.; Ikeda, M. Expression of thioredoxin and thioredoxin reductase in placentae of pregnant mice exposed to lipopolysaccharide. Placenta 1999, 20, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Ejima, K.; Nanri, H.; Toki, N.; Kashimura, M.; Ikeda, M. Localization of thioredoxin reductase and thioredoxin in normal human placenta and their protective effect against oxidative stress. Placenta 1999, 20, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Tamanini, C. Selenium stimulates estradiol production in bovine granulosa cells: possible involvement of nitric oxide. Domes. Anim. Endocrinol. 2000, 18, 1–17. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Caton, J.S.; Arndt, W.; Burchill, K.; Thorson, C.; Borowczyk, E.; Bilski, J.J.; Redmer, D.A.; Reynolds, L.P.; Vonnahme, K.A. Cellular proliferation and vascularization in ovine fetal ovaries: Effects of undernutrition and selenium in maternal diet. Reproduction 2009, 137, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Kamada, H.; Ikumo, H. Effect of selenium on cultured bovine luteal cells. Anim. Reprod. Sci. 1997, 46, 203–211. [Google Scholar] [CrossRef]
- Tilly, J.L.; Tilly, K. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology 1995, 136, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Grabek, M.; Swies, Z.; Borzecki, A. The influence of selenium on the reproduction of rats. Eur. PMC 1991, 46, 103–105. [Google Scholar]
- Özkaya, M.O.; Nazıroğlu, M.; Barak, C.; Berkkanoglu, M. Effects of multivitamin/mineral supplementation on trace element levels in serum and follicular fluid of women undergoing in vitro fertilization (IVF). Biol. Trace Elem. Res. 2011, 139, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehdi, Y.; Hornick, J.-L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [PubMed]
- Edassery, S.L.; Shatavi, S.V.; Kunkel, J.P.; Hauer, C.; Brucker, C.; Penumatsa, K.; Yu, Y.; Dias, J.A.; Luborsky, J.L. Autoantigens in ovarian autoimmunity associated with unexplained infertility and premature ovarian failure. Fertil. Steril. 2010, 94, 2636–2641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod. Toxicol. 2013, 42, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.; Arikan, T.; Kilinc, M.; Arikan, D.C.; Ekerbiçer, H.Ç. Plasma selenium levels in Turkish women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Luddi, A.; Capaldo, A.; Focarelli, R.; Gori, M.; Morgante, G.; Piomboni, P.; De Leo, V. Antioxidants reduce oxidative stress in follicular fluid of aged women undergoing IVF. Reprod. Biol. Endocrinol. 2016, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Tuñón, J.M.; Trilles, P.P.; Molina, M.G.; Duvison, M.H.; Pastor, B.M.; Martín, P.S.; Martín, F.S.; Sánchez-Borrego, R. A Double-Blind, Randomized Prospective Study to Evaluate the Efficacy of Previous Therapy With Melatonin, Myo-inositol, Folic Acid, and Selenium in Improving the Results of an Assisted Reproductive Treatment. Clin. Med. Insight. Ther. 2017, 9, 1179559X17742902. [Google Scholar] [CrossRef]
- Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Grzeskowiak, L.E.; Dekker, G.A.; Roberts, C.T. Early pregnancy maternal trace mineral status and the association with adverse pregnancy outcome in a cohort of Australian women. J. Trace Elem. Med. Biol. 2018, 46, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Parman, T.; Wiley, M.J.; Wells, P.G. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat. Med. 1999, 5, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Winn, L.M.; Wells, P.G. Maternal administration of superoxide dismutase and catalase in phenytoin teratogenicity1. Free Radical Biol. Med. 1999, 26, 266–274. [Google Scholar] [CrossRef]
- Thompson, J. In vitro culture and embryo metabolism of cattle and sheep embryos—a decade of achievement. Anim. Reprod. Sci. 2000, 60, 263–275. [Google Scholar] [CrossRef]
- Uhm, S.J.; Gupta, M.K.; Yang, J.H.; Lee, S.H.; Lee, H.T. Selenium improves the developmental ability and reduces the apoptosis in porcine parthenotes. Mol. Reprod. Dev. 2007, 74, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- McKeehan, W.L.; Hamilton, W.G.; Ham, R.G. Selenium is an essential trace nutrient for growth of WI-38 diploid human fibroblasts. Proc. Natl. Acad. Sci. 1976, 73, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Hewlett, G. Strategies for optimising serum-free media. Cytotechnology 1991, 5, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ufer, C.; Wang, C.C. The roles of glutathione peroxidases during embryo development. Front. Mol. Neurosci. 2011, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Ishigaki, K.; Nagai, T.; Chikyu, M.; Pursel, V.G. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol. Reprod. 1993, 49, 89–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, G.B.; Parchment, R.E.; Lewellyn, A.L. Hydrogen peroxide as a mediator of programmed cell death in the blastocyst. Differentiation 1991, 46, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Parchment, R. The implications of a unified theory of programmed cell death, polyamines, oxyradicals and histogenesis in the embryo. Int. J. Dev. Biol. 2003, 37, 75–83. [Google Scholar]
- Abedelahi, A.; Salehnia, M.; Allameh, A.; Davoodi, D. Sodium selenite improves the in vitro follicular development by reducing the reactive oxygen species level and increasing the total antioxidant capacity and glutathione peroxide activity. Hum. Reprod. 2010, 25, 977–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedelahi, A.; Salehnia, M.; Allameh, A. The effects of different concentrations of sodium selenite on the in vitro maturation of preantral follicles in serum-free and serum supplemented media. J. Assist. Reprod. Genet. 2008, 25, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Ren, Y.; Zhang, C.; Yue, W.; Lei, F. Effects of organic selenium (Se-enriched yeast) supplementation in gestation diet on antioxidant status, hormone profile and haemato-biochemical parameters in Taihang Black Goats. Anim. Feed Sci. Technol. 2018, 238, 57–65. [Google Scholar] [CrossRef]
- Dalto, B.; Tsoi, S.; Audet, I.; Dyck, M.K.; Foxcroft, G.; Matte, J.J. Gene expression of porcine blastocysts from gilts fed organic or inorganic selenium and pyridoxine. Reproduction 2015, 149, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalto, D.B.; Audet, I.; Lapointe, J.; Matte, J.J. The importance of pyridoxine for the impact of the dietary selenium sources on redox balance, embryo development, and reproductive performance in gilts. J. Trace Elem. Med. Biol. 2016, 34, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Dalto, D.B.; Roy, M.; Audet, I.; Palin, M.-F.; Guay, F.; Lapointe, J.; Matte, J.J. Interaction between vitamin B6 and source of selenium on the response of the selenium-dependent glutathione peroxidase system to oxidative stress induced by oestrus in pubertal pig. J. Trace Elem. Med. Biol. 2015, 32, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.E.; Audet, I.; Giguere, A.; Laforest, J.P.; Bilodeau, J.F.; Quesnel, H.; Matte, J.J. Effect of dietary organic and inorganic selenium on antioxidant status, embryo development, and reproductive performance in hyperovulatory first-parity gilts. J. Anim. Sci. 2012, 90, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Han, J.; Guan, W.; Chen, F.; Wang, C.; Zhang, Y.; Lv, Y.; Lin, G. Selenium and vitamin E in sow diets: I. Effect on antioxidant status and reproductive performance in multiparous sows. Anim. Feed Sci. Technol. 2016, 221, 111–123. [Google Scholar] [CrossRef]
- Nogales, F.; Ojeda, M.L.; Fenutría, M.; Murillo, M.L.; Carreras, O. Role of selenium and glutathione peroxidase on development, growth, and oxidative balance in rat offspring. Reproduction 2013, 146, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khera, A.; Dong, L.-F.; Holland, O.; Vanderlelie, J.; Pasdar, E.A.; Neuzil, J.; Perkins, A.V. Selenium supplementation induces mitochondrial biogenesis in trophoblasts. Placenta 2015, 36, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.; Vanderlelie, J.J.; Holland, O.; Perkins, A.V. Overexpression of endogenous anti-oxidants with selenium supplementation protects trophoblast cells from reactive oxygen species-induced apoptosis in a Bcl-2-dependent manner. Biol. Trace Elem. Res. 2017, 177, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.; Khera, A.; Holland, O.; Vanderlelie, J. Trophoblast mitochondrial biogenesis and functionality is increased with selenium supplementation. Placenta 2016, 45, 118. [Google Scholar] [CrossRef]
- Mendelev, N.; Mehta, S.L.; Idris, H.; Kumari, S.; Li, P.A. Selenite stimulates mitochondrial biogenesis signaling and enhances mitochondrial functional performance in murine hippocampal neuronal cells. PLoS One 2012, 7, e47910. [Google Scholar] [CrossRef] [PubMed]
- Na, J.Y.; Seok, J.; Park, S.; Kim, J.S.; Kim, G.J. Effects of selenium on the survival and invasion of trophoblasts. Clin. Exp. Reprod. Med. 2018, 45, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fialova, L.; Malbohan, I.; Kalousova, M.; Soukupova, J.; Krofta, L.; Štipek, S.; Zima, T. Oxidative stress and inflammation in pregnancy. Scand. J. Clin. Lab. INVEST. 2006, 66, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Orhan, H.; Önderoglu, L.; Yücel, A.; Sahin, G. Circulating biomarkers of oxidative stress in complicated pregnancies. Arch. Gynecol. Obst. 2003, 267, 189–195. [Google Scholar]
- Karowicz-Bilinska, A.; Kędziora-Kornatowska, K.; Bartosz, G. Indices of oxidative stress in pregnancy with fetal growth restriction. Free Radical Res. 2007, 41, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Hracsko, Z.; Orvos, H.; Novak, Z.; Pal, A.; Varga, I.S. Evaluation of oxidative stress markers in neonates with intra-uterine growth retardation. Redox Rep. 2008, 13, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariath, A.B.; Bergamaschi, D.P.; Rondó, P.H.; Ana, C.A.T.; de Fragas Hinnig, P.; Abbade, J.F.; Diniz, S.G. The possible role of selenium status in adverse pregnancy outcomes. Br. J. Nutr. 2011, 105, 1418–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, K.; Holland, O.; Landers, K.; Vanderlelie, J.J.; Hofstee, P.; Cuffe, J.S.; Perkins, A.V. Effects of maternal micronutrient supplementation on placental function. Placenta 2017, 54, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Mamon, M.A.C.; Ramos, G.B. Maternal selenium-supplementation at various stages of periconception period: influence on murine blastocyst morphology and implantation status. J. Anim. Sci. Technol. 2017, 59, 7. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Berti, C.; Calabrese, S. Role of micronutrients in the periconceptional period. Hum. Reprod. Update 2009, 16, 80–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berti, C.; Biesalski, H.; Gärtner, R.; Lapillonne, A.; Pietrzik, K.; Poston, L.; Redman, C.; Koletzko, B.; Cetin, I. Micronutrients in pregnancy: current knowledge and unresolved questions. Clin. Nutr. 2011, 30, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Ambroziak, U.; Hybsier, S.; Shahnazaryan, U.; Krasnodębska-Kiljańska, M.; Rijntjes, E.; Bartoszewicz, Z.; Bednarczuk, T.; Schomburg, L. Severe selenium deficits in pregnant women irrespective of autoimmune thyroid disease in an area with marginal selenium intake. J. Trace Elem. Med. Biol. 2017, 44, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Polanska, K.; Krol, A.; Sobala, W.; Gromadzinska, J.; Brodzka, R.; Calamandrei, G.; Chiarotti, F.; Wasowicz, W.; Hanke, W. Selenium status during pregnancy and child psychomotor development—Polish Mother and Child Cohort study. Pediatr. Res. 2016, 79, 863. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.A.; Whanger, P.D.; Tripp, M.J. Blood selenium and glutathione peroxidase activity in pregnant women: comparative assays in primates and other animals. Am. J. Clin. Nutr. 19821982, 36, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zachara, B.; Wardak, C.; Didkowski, W.; Maciag, A.; Marchaluk, E. Changes in blood selenium and glutathione concentrations and glutathione peroxidase activity in human pregnancy. Gynecol. Obst. Invest. 1993, 35, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.; Glinianaia, S.V.; Torrioli, M.-G.; Platt, M.-J.; Miceli, M.; Jouk, P.-S.; Johnson, A.; Hutton, J.; Hemming, K.; Hagberg, G. Cerebral palsy and intrauterine growth in single births: European collaborative study. The Lancet 2003, 362, 1106–1111. [Google Scholar] [CrossRef]
- Dikbas, L.; Yapca, O.E.; Dikbas, N.; Gundogdu, C. Paraoxonase-2 and paraoxonase-3: comparison of mRNA expressions in the placentae of unexplained intrauterine growth restricted and noncomplicated pregnancies. J. Mat.-Fet. Neonatal Med. 2017, 30, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Nikaido, T.; Toki, T.; Kita, N.; Kanai, M.; Ashida, T.; Ohira, S.; Konishi, I. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004, 444, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mesdaghinia, E.; Rahavi, A.; Bahmani, F.; Sharifi, N.; Asemi, Z. Clinical and Metabolic Response to Selenium Supplementation in Pregnant Women at Risk for Intrauterine Growth Restriction: Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Trace Elem. Res. 2017, 178, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Barrington, J.; Lindsay, P.; James, D.; Smith, S.; Roberts, A. Selenium deficiency and miscarriage: A possible link? Int. J. Obstet. Gynaecol. 1996, 103, 130–132. [Google Scholar] [CrossRef]
- Koçak, İ.; Aksoy, E.; Üstün, C. Recurrent spontaneous abortion and selenium deficiency. Int. J. Obstet. Gynaecol. 1999, 65, 79–80. [Google Scholar] [CrossRef]
- Mistry, H.D.; Kurlak, L.O.; Young, S.D.; Briley, A.L.; Broughton Pipkin, F.; Baker, P.N.; Poston, L. Maternal selenium, copper and zinc concentrations in pregnancy associated with small-for-gestational-age infants. Mat. Child Nutr. 2014, 10, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Negro, R.; Greco, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J. Clin. End. Metab. 2007, 92, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soci. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Arnér, E.S. Selenium and selenoproteins in (redox) signaling, diseases, and animal models-200 year anniversary issue. Eur. PMC 2018, 127, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, Q.; Yin, F.; Yang, Z.; Zhang, W.; Gabra, H.; Li, L. Identification of proteomic and metabolic signatures associated with chemoresistance of human epithelial ovarian cancer. Int. J. Clin. Oncol. 2016, 49, 1651–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnani, D.; Camacho-Vanegas, O.; Camacho, C.; Lele, S.; Odunsi, K.; Cohen, S.; Dottino, P.; Martignetti, J.A. Decreased levels of serum glutathione peroxidase 3 are associated with papillary serous ovarian cancer and disease progression. J. Ovarian Res. 2011, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piekutowski, K.; Makarewicz, R.; Zachara, B. The antioxidative role of selenium in pathogenesis of cancer of the female reproductive system. Neoplasma 2007, 54, 374–378. [Google Scholar] [PubMed]
- Falck, E.; Karlsson, S.; Carlsson, J.; Helenius, G.; Karlsson, M.; Klinga-Levan, K. Loss of glutathione peroxidase 3 expression is correlated with epigenetic mechanisms in endometrial adenocarcinoma. Cancer Cell Int. 2010, 10, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saga, Y.; Ohwada, M.; Suzuki, M.; Konno, R.; Kigawa, J.; Ueno, S.; Mano, H. Glutathione peroxidase 3 is a candidate mechanism of anticancer drug resistance of ovarian clear cell adenocarcinoma. Oncol. Rep. 2008, 20, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Do, J.H.; Bae, S.; Yang, S.; Zhang, X.; Lee, A.; Choi, Y.J.; Park, D.C.; Ahn, W.S. Immunohistochemical evidence for the over-expression of Glutathione peroxidase 3 in clear cell type ovarian adenocarcinoma. Med. Oncol. 2011, 28, 010–9659. [Google Scholar] [CrossRef] [PubMed]
- Hough, C.D.; Cho, K.R.; Zonderman, A.B.; Schwartz, D.R.; Morin, P.J. Coordinately up-regulated genes in ovarian cancer. Cancer Res. 2001, 61, 3869–3876. [Google Scholar] [PubMed]
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: results from a multiple-dose, randomised controlled trial. Free Radical Biol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
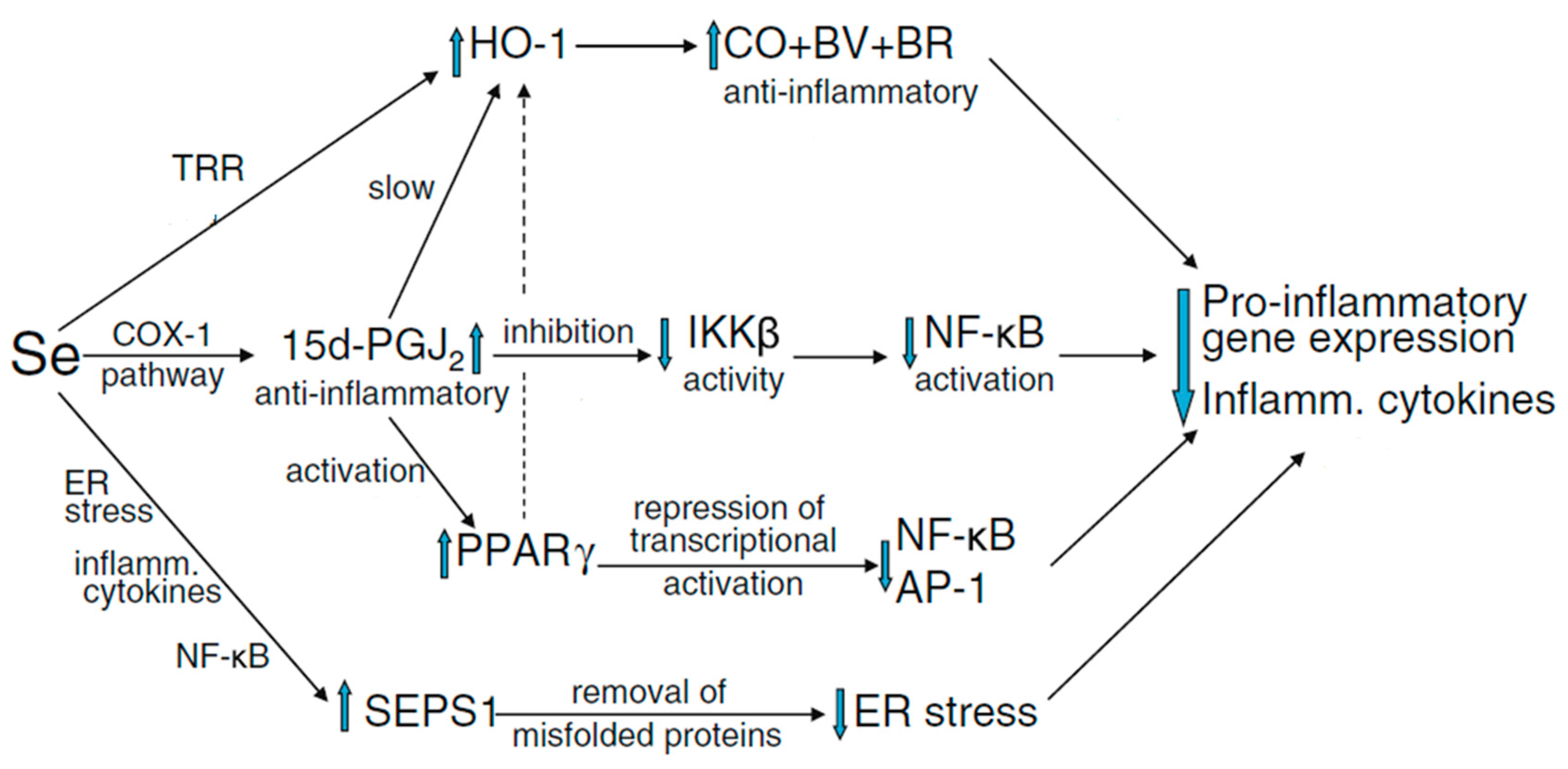
| Selenoprotein | Symbol [5] | General Description/Function [1,6,7] | Potential Implication in Reproductive Function |
|---|---|---|---|
| Glutathione peroxidase 1 | GPX1 | Antioxidant protection | Important role in female reproductive function; i.e., implication in determining the follicle growth, maturation, and dominance in both cows and women [8,9]. Implication in the follicular microenvironment [10]. Potential antioxidant role in follicle dominance protecting the dominant follicle from increasing levels of reactive oxygen species (ROS) [8,9]. |
| Glutathione peroxidase 2 | GPX2 | Antioxidant protection | Implicated in protection of embryos and extra-embryonic tissues against ROS generated in ontogenetic periods [11]. |
| Glutathione peroxidase 3 | GPX3 | Maintenance of cellular redox status Antioxidant in extracellular fluids | Gpx3 has been identified as an essential enzyme in the defense against oxidative stress during the postovulatory process of endometrial remodeling (decasualization) in preparation for implantation by reducing H2O2 in the endometrium [12]. Implicated in normal human pregnancy and birth [13]. Implicated in maternal-fetal selenium transfer mechanism [14]. Role in pre-eclampsia and pregnancy-related hypertensive conditions [15]. Implicated in preventing the oxidative stress- induced cell apoptosis during growth of large healthy follicles [16]. |
| Glutathione peroxidase 4 | GPX4 | Detoxification of lipid hydroperoxides, Antioxidant role in membranes, serves as structural protein in sperm, Apoptosis. Membrane-associated. Present at high concentrations in the testis, where it is important for sperm motility and viability, male fertility | Essential for embryonic development [17,18]. Potential implication in pre-eclampsia [19,20,21]. Implication in male fertility [22]. |
| Glutathione peroxidase 5 | GPX5 | Antioxidant role during sperm maturation, H2O2 scavenger [23] | Implicated in protection of sperm from oxidative damage that could compromise their integrity and, as a consequence, embryo viability [24]. |
| Thioredoxin reductase 1 | TXNRD1 | Part of the thioredoxin system, Antioxidant role, redox regulation, cell signaling. Controls activity of transcription factors, cell proliferation, apoptosis | Implication in early embryonic development [25,26]. |
| Thioredoxin reductase 2 | TXNRD2 | Part of the thioredoxin system, Antioxidant role, redox regulation, cell signaling | Potential role in embryogenesis [27]. |
| Thioredoxin-glutathione reductase | TXNRD3 | Part of the thioredoxin system, Antioxidant role, redox regulation, cell signaling | Role in disulfide bond formation and sperm maturation [28]. |
| Iodothyronine deiodinase 1 | DIO1 | Conversion of T4 to T3 and T4 to reverse T3, Production of T3 in the thyroid and peripheral tissues | Implication in autoimmune thyroid disease and postpartum thyroid disease [29]. Dio2 and Dio3 are highly expressed at the site of implantation in pregnant rat [30]. High expression of DIO3 at utero-plecental unit and fetal epithelia has also been reported in human, Potential role in preventing the overexposure of fetus to T3 [31]. |
| Iodothyronine deiodinase 2 | DIO2 | Conversion of T4 to T3, T3 production in peripheral tissues | |
| Iodothyronine deiodinase 3 | DIO3 | Conversion T4 to reverse T3, Production of rT3 | |
| Selenoprotein H | SELENOH | Not fully known, potential implication in upregulation of genes relevant to glutathione synthesis | Implicated in placental oxidative stress by regulating mitochondrial biogenesis in trophoblasts (Swan-71, JEG-3 and BeWo cells) [32]. |
| Selenoprotein P | SELENOP | By and large implicated in Se transportation and antioxidant defense, Regarded as a major contributor to plasma selenium and a good indicator of Se status, Essential for male fertility; its deficiency leads to infertility characterized by abnormal spermatozoa in mice | Implicated in maternal-fetal selenium transfer mechanism [14]. Implication in male fertility [33,34]. Involved in delivery of Se to spermatogenic cells [35]. Required for mouse sperm development [36]. Potential implication in pregnancy and pre-eclampsia [13,15,37]. |
| Selenoprotein S | SELENOS | Cellular redox balance, Possible influence in inflammatory response | Relevant to pre-eclampsia [29,38]. Localized to the granulosa cells of large healthy and atretic follicles and also expressed in the thecal layers. Potential role in bovine follicular development [8]. |
| Selenoprotein V | SELENOV | Unknown, possible role in redox regulation | Testis-specific expression in rodents [39], in situ hybridization experiments have shown high levels of Selenov mRNA in seminiferous tubules in mice, but its exact role in spermatogenesis remains elusive [3,39]. |
| Species | RDA |
|---|---|
| Human | Male: 55 µg/day * [43]; Female: 55 µg/day * [43] Female during pregnancy: 60 µg/day * [43] |
| Sheep | 100–200 µg/kg DM of feed/day [44] |
| Goat | 100–200 µg/kg DM of feed/day [44] |
| Growing Pigs Gestating and Lactating Sows | 150–300 µg/kg DM of feed/day [45] 150 µg/kg DM of feed/day [45] |
| Horse | 100 µg/kg DM of feed/day [46] |
| Donkey | 150 µg/100 kg body weight [47] |
| Dairy cows | 100 µg/kg DM of feed/day [48] |
| Beef cows | 300 µg/kg DM of feed/day [49] |
| Calves | 100 µg/kg DM of feed/day [48] |
| Camel | 400–800 µg/day [50] |
| Study Design | Type and Duration of Treatment | No. of Subjects Enrolled | Key Results Relevant to IVF | Ref. |
|---|---|---|---|---|
| Open label preliminary clinical trial (Italy) | Elevit, Bayer containing Se (50 μg) for three months | 18 patients aging >39 years; undergoing IVF/intracytoplasmic sperm injection (ICSI) treatment | The follicular fluid and serum proteins were protected from oxidative injury when aged women were supplemented with micronutrients ahead of IVF cycles. Significant increase in the mean values of good quality oocytes (recovered from women following the micronutrient supplement) was also observed. | [88] |
| Double-blind, randomized prospective study (Spain) | Seidivid, containing Se (27.5 μg) 2 months prior to ovarian puncture | 120 patients going through assisted reproductive interventions | Significantly improved embryonic quality was recorded. No significant improvement in the results (relating to follicle number, oocyte maturation, quality of vitrified embryos) was recorded. | [89] |
| Model | Treatment Regime | Key Findings as Reported by Authors | Ref. |
|---|---|---|---|
| Pregnant goats | 0 mg (control group), 0.5, 2.0 and 4.0 mg Se/kg DM during gestation period | Organic Se supplementation significantly improved the total antioxidant capacity (TAC), activity of GPX and SOD. The levels of estradiol, progesterone and T4 were also significantly increased | [103] |
| Gilts | Organic (0.3 mg/kg Se- enriched yeast) and inorganic (0.3 mg/kg sodium selenite) Se (combined with pyridoxine) | Organic Se (with pyridoxine) substantially induced the transcriptome of porcine expanded blastocysts compared to gilts in the control group. Organic Se (with pyridoxine) more noticeably affected the metabolism in porcine expanded blastocysts compared to those supplemented with inorganic Se (with pyridoxine). Organic Se selectively stimulated the genes implicated in antioxidant defense. In expanded blastocysts from organic Se supplemented gilts, other members of the thioredoxin family and ubiquinones seem to embellish the antioxidant activity and the modulation of cell proliferation | [104] |
| Gilts | Basal diet 0.3 mg/kg and 2.4 mg/kg of Se and pyridoxine, respectively; 0.3 mg/kg sodium selenite without pyridoxine; 0.3 mg/kg sodium selenite +10 mg/kg pyridoxine; 0.3 mg/kg of Se-enriched yeast without pyridoxine; 0.3 mg/kg of Se-enriched yeast +10 mg/kg pyridoxine | Both Se level and source significantly enhanced Se concentration in the organs of gilts and the embryos. Selenium level showed implication on total GPX activities in mitochondria | [105] |
| Hyperovulatory first-parity gilts | Control group: Se 0.2 mg/kg; control +sodium selenite 0.3 mg/kg; control +Se-enriched yeast 0.3 mg/kg | The Se content of individual embryos was higher in the Se-supplemented group compared to the control. The uterine transfer of Se to embryos was ameliorated and this was concomitant with an improved embryo development. GPX was improved following Se supplementation | [107] |
| Multi-parous sows | sodium selenite 0.30 mg Se/kg organic Se 0.30 mg Se/kg | Organic Se significantly increased SOD activity, GPX and glutathione (GSH) content, Se level, total antioxidant activity, number of live and weaned pigs. Whereas organic Se significantly decreased malondialdehyde (MDA) contents compared to the inorganic Se | [108] |
| Rat | Se deficient (0.01 ppm) Se sufficient (0.5 ppm) | In Se deficient group, higher mortality at birth; reduced viability and survival parameters, impaired growth, and development of liver in offspring accompanied with reduced hepatic Se concentrations, GPX and CAT activities, and increased SOD activity and protein oxidation were observed. However, Se sufficient diet ameliorated all aforementioned indices. | [109] |
| Gilts | Basal diet (control group); Basal diet +0.3 mg/kg of sodium selenite; sodium selenite +10 mg/kg pyridoxine; Basal diet +0.3 mg/kg of Se-enriched yeast; Se-enriched yeast +10 mg/kg pyridoxine | Organic Se supplementation along with dietary B6 (pyridoxine) increased the expressions of GPX1, GPX3, GPX4 | [106] |
| Model | Treatment Regime | Key Findings and Implications | References |
|---|---|---|---|
| Placental trophoblast-like cell lines (BeWo, JEG-3 and Swan-71) | Sodium selenite (100 nM) for 24 h Selenomethionine (500 nM) for 24 h | Following Se supplementation, significantly higher mitochondrial respiration was observed in trophoblast-like cell lines compared to untreated control group. Interestingly, the treated Swan-71 cells were observed to have higher mitochondrial content (measured on basis of the ratio of mitochondrial DNA [mtDNA] to nuclear DNA [nDNA]) in both Se supplemented groups. The expression of four mitochondrial genes; i.e., MTRT1, MTRT2, MTRT3 and MTRT4 genes was significantly improved in cells treated with inorganic Se, whereas expression of only two out of these four genes was significantly improved in cells treated with organic Se. The other mitochondrial biogenesis markers; i.e., SELENOH, peroxisome proliferator-activated receptor γ coactivator-1alpha (PGC-1α) and nuclear respiratory factor-1 (NRF-1) were also significantly improved in Swan-71 cells after treatment with both organic and inorganic Se. | [110] |
| First trimester villous placental tissue | Sodium selenite (100 nM) for 4, 12, 14, 24, 48 or 96 h. | The mitochondrial respiration (increased oxidative phosphorylation through complex IV) was significantly improved in trimester villous placental tissue explants following four hours of culture in sodium selenate treated group. | [110] |
| Placental trophoblast cell lines (BeWo, JEG-3 and Swan-71) | Sodium selenite and Selenomethionine. (Dose-dependent manner i.e., 0, 50, 100, 200, 400, 800, 1200, 1600 or 2000 nM for 24 h). Cells were treated with mitochondrial inhibitors rotenone or antimycin to induce the oxidative stress | The expression and activity of key selenoproteins, namely, GPX and TXNRD was significantly improved (in a dose-dependent manner) in Swan-71 cells following supplementation with both sources of Se. Selenium supplementation significantly reduced the ROS- induced oxidative stress in both treated groups. Moreover, rotenone or antimycin-induced cellular toxicity was also significantly reduced in Swan-71 cells following Se supplementation. The uncontrolled apoptosis and expression of Bcl-2 in BeWo, JEG-3 and Swan-71 cells were significantly reduced. | [111] |
| Hypoxia exposed human extravillous trophoblast cell line HTR-8/Svneo | 0.5 nM selenium for 72 h | Selenium supplementation under hypoxia increased the migration and proliferation of trophoblastic cells by ameliorating the mitochondrial oxidative stress and improved the expression of antioxidant genes i.e., heme oxygenase 1, 2 (HO-1 and HO-2) and SOD. | [114] |
| Placental trophoblast cell lines BeWo, JEG-3 and Swan-71 | Sodium selenite (100 nM) | Selenium supplementation efficaciously stimulated the mitochondrial biogenesis, increasing the number of mitochondria in trophoblastic cells. The consumption of oxygen was comparatively higher in the Se treated cells compared to the control group. The mitochondrial respiration indicated that the oxygen streaming was significantly enhanced in Se treated trophoblastic cells. The ratio of mitochondrial gene/nuclear gene was higher in the cells supplemented with Se. Meanwhile, Se supplementation also improved the expression of SELENOH, NRF-1 and PGC-1α | [112] |
| Trophoblast cells (BeWo, JEG-3 and Swan-71) | Sodium selenite (100 nM) Selenomethionine (500 nM) Oxidative stress was induced in the cells with the addition of increasing doses of Antimycin C, Rotenone and Resazurin | A dose-dependent reduction in the cellular activity in BeWo, JEG-3 and Swan-71 was observed when treated for 4 h with increasing concentrations of Antimycin (40–320 μM) and Rotenone (100–800 nM). However, prior incubation with sodium selenite and selenomethionine protected the trophoblast cells from oxidative stress. Following Se supplementation, the activity of GPX and TXNRD was significantly improved (in a dose-dependent manner). Induced cellular oxidative stress was significantly ameliorated following supplementation with both organic and inorganic Se. | [32] |
| Human trophoblast cell lines BeWo and JEG-3 | Sodium selenite (100 nM) Selenomethionine (500 nM) Oxidative stress was induced using H2O2 (0–800 µM), tert-butyl hydrogen peroxide (t-butyl H2O2, 0–50 µM), cumene hydrogen peroxide (cumene H2O2, 0–50 µM) | The activity of GPX and TXNRD was significantly improved (in a dose-dependent manner). Induced cellular oxidative stress was significantly reversed following supplementation with both organic and inorganic Se. | [59] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qazi, I.H.; Angel, C.; Yang, H.; Pan, B.; Zoidis, E.; Zeng, C.-J.; Han, H.; Zhou, G.-B. Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules 2018, 23, 3053. https://doi.org/10.3390/molecules23123053
Qazi IH, Angel C, Yang H, Pan B, Zoidis E, Zeng C-J, Han H, Zhou G-B. Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules. 2018; 23(12):3053. https://doi.org/10.3390/molecules23123053
Chicago/Turabian StyleQazi, Izhar Hyder, Christiana Angel, Haoxuan Yang, Bo Pan, Evangelos Zoidis, Chang-Jun Zeng, Hongbing Han, and Guang-Bin Zhou. 2018. "Selenium, Selenoproteins, and Female Reproduction: A Review" Molecules 23, no. 12: 3053. https://doi.org/10.3390/molecules23123053
APA StyleQazi, I. H., Angel, C., Yang, H., Pan, B., Zoidis, E., Zeng, C.-J., Han, H., & Zhou, G.-B. (2018). Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules, 23(12), 3053. https://doi.org/10.3390/molecules23123053









