Isolation of Chavibetol and Methyleugenol from Essential Oil of Pimenta pseudocaryophyllus by High Performance Liquid Chromatography
Abstract
:1. Introduction
2. Results and Discussion
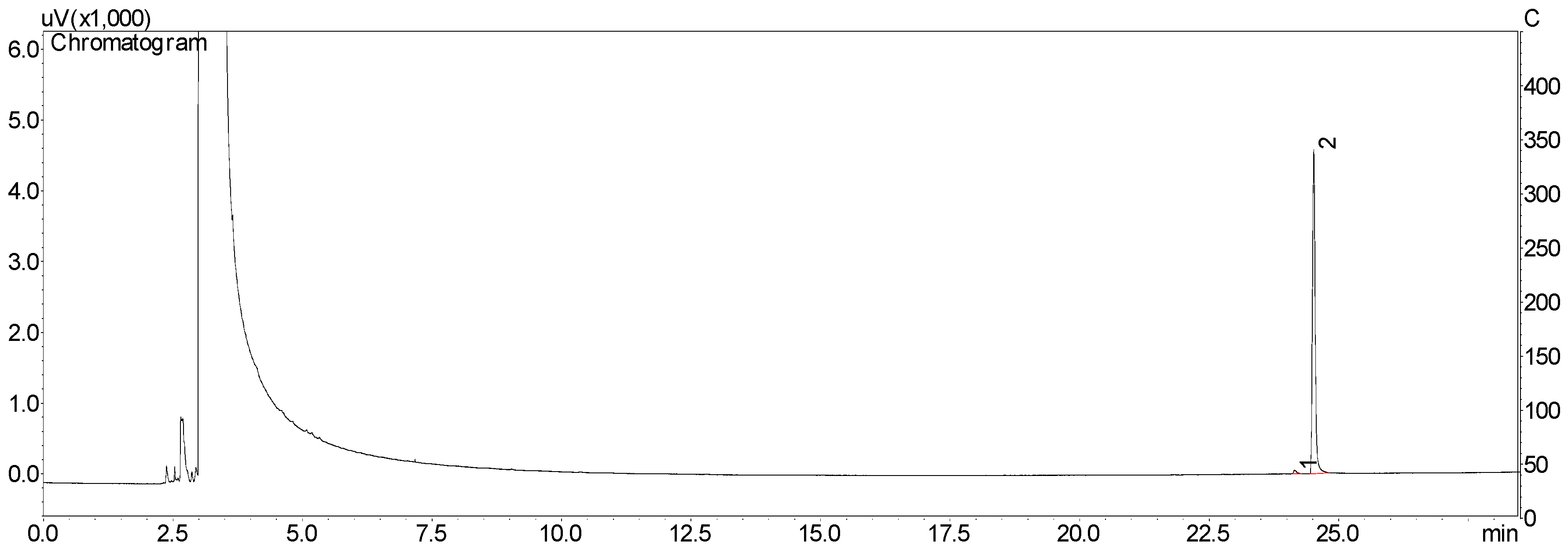
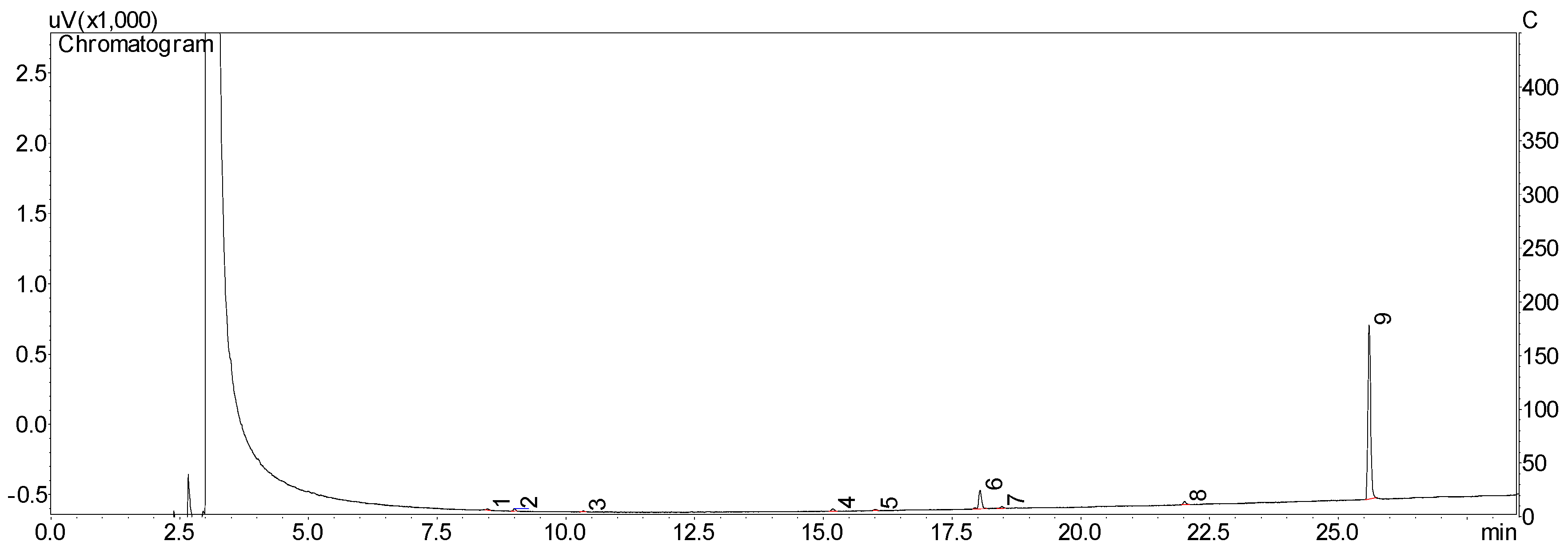
2.1. Isolation and Purity of Chavibetol and Methyleugenol
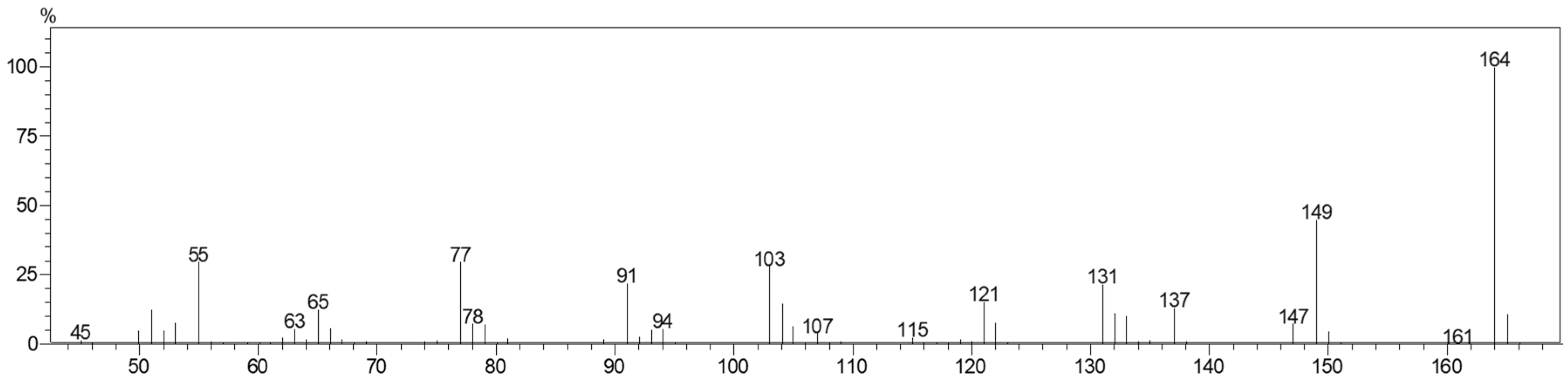
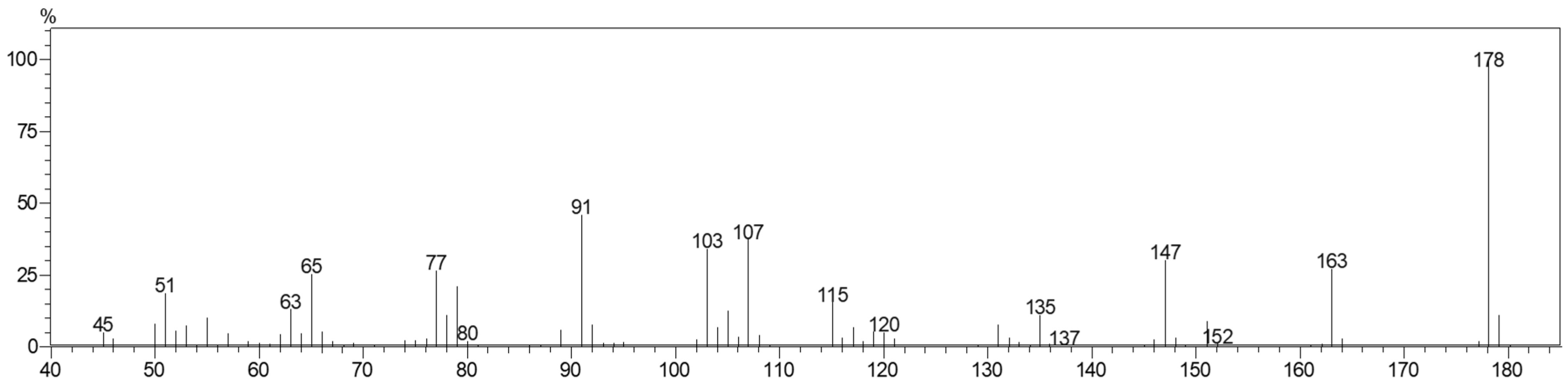
2.2. Characterization of Chavibetol and Methyleugenol
3. Materials and Methods
3.1. Chemicals and Material
3.2. Plant Material and Essential Oils Extraction
3.3. HPLC Analysis and Isolation
3.4. GC/MS Analysis
3.5. Purity Analysis of the Isolated Compound and Quantification by GC/FID Analysis
3.6. NMR Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Landrum, L.R.; Kawasaki, M.L. The genera of Myrtaceae in Brazil: An illustrated synoptic treatment and identification keys. Brittonia 1997, 49, 508–536. [Google Scholar] [CrossRef]
- Paula, J.A.M.; Rocha, J.B.; Nascimento, P.M.S.; Rezende, M.H.; Paula, J.R. Estudo farmacognóstico da casca de Pimenta pseudocaryophyllus (Gomes) L.R. Landrum-Myrtaceae. Revista Eletrônica de Farmácia 2006, 3, 1–3. [Google Scholar]
- Paula, J.A.M.; Paula, J.R.; Bara, M.T.F.; Rezende, M.H.; Ferreira, H.D. Estudo farmacológico das folhas de Pimenta pseudocaryophyllus (Gomes) L.R. Landrum-Myrtaceae. Rev. Bras. Farmacogn. 2008, 18, 265–278. [Google Scholar] [CrossRef]
- Lima, M.E.L.; Cordeiro, I.; Young, M.C.M.; Sobra, M.E.G.; Moreno, P.R.H. Antimicrobial activity of the essential oil from two specimens of Pimenta pseudocaryophyllus (Gomes) L.R. Landrum (Myrtaceae) native from São Paulo State Brazil. Pharmacologyonline 2006, 3, 589–593. [Google Scholar]
- Paula, J.A.M.; Paula, J.R.; Pimenta, F.C.; Rezende, M.H.; Bara, M.T.F. Antimicrobial activity of the crude ethanol extract from Pimenta pseudocaryophyllus. Pharm. Biol. 2009, 47, 987–993. [Google Scholar] [CrossRef]
- Custódio, D.L.; Burgo, R.P.; Moriel, B.; Barbosa, A.M.; Rezende, M.I.; Daniel, J.F.S.; Pinto, J.P.; Bianchini, E.; Faria, T.J. Antimicrobial activity of essential oils from Pimenta pseudocaryophyllus and Tynanthus micranthus. Braz. Arch. Biol. Technol. 2010, 53, 1363–1369. [Google Scholar] [CrossRef]
- Sanches, B.G.; Custódio, D.L.; Faria, T.J. Estudo do extrato em acetato de etila de Pimenta pseudocaryophyllus. In Proceedings of the XVI Encontro Anual de Iniciação Científica, Maringá, Brazil, 26 September 2017. [Google Scholar]
- Paula, J.A.M.; Silva, M.R.R.; Costa, M.P.; Diniz, D.G.A.; Sá, F.A.S.; Alves, S.F.; Costa, E.A.; Lino, R.P.; Paula, J.R. Phytochemical analysis and antimicrobial, antinociceptive, and anti-Inflammatory activities of two chemotypes of Pimenta pseudocaryophyllus (Myrtaceae). Medicine 2012, 2012. [Google Scholar] [CrossRef]
- Stefanello, M.E.A.; Pascoal, A.C.R.F.; Salvador, M.J. Essential oils from Neotropical Myrtaceae: Chemical diversity and biological properties. Chem. Biodiv. 2011, 8, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Ebadollahi, E. Essential oils isolated from Myrtaceae family as natural insecticides. Annu. Rev. Res. Biol. 2013, 3, 148–175. [Google Scholar]
- Evans, P.H.; Bowers, W.S.; Funk, E.J. Identification of fungicidal and nematocidal components in the leaves of Piper betle (Piperaceae). J. Agric. Food Chem. 1984, 32, 1254–1256. [Google Scholar] [CrossRef]
- Mula, S.; Banerjee, D.; Patro, B.S.; Bhattacharya, S.; Barik, A.; Bandyopadhyay, S.K.; Chattopadhyay, S. Inhibitory property of the Piper betel phenolics against photosensitization-induced biological damages. Bioorg. Med. Chem. 2008, 16, 2932–2938. [Google Scholar] [CrossRef] [PubMed]
- Rathee, J.S.; Patro, B.S.; Mula, S.; Gamre, S.; Chattopadhyay, S. Antioxidant activity of Piper betel leaf extract and its constituents. J. Agric. Food Chem. 2006, 54, 9046–9054. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.P.; Ansante, T.F.; Niculau, E.S.; Pavarini, R.; Silva, M.F.G.F.; Seffrin, R.C.; Vendramim, J.D. Pimenta pseudocaryophyllus Derivatives: Extraction Methods and Bioactivity Against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Neotrop. Entomol. 2015, 44, 634–642. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, B.C.B.; da Silva, J.C.T.; Guerrero, P.G., Jr.; Leitão, G.G.; Barata, L.E.S. Isolation of chavibetol from essential oil of Pimenta pseudocaryophyllus leaf by high-speed counter-current chromatography. J. Chromatogr. A 2009, 1216, 4303–4306. [Google Scholar] [CrossRef] [PubMed]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Secondary metabolites isolation in natural products chemistry: Comparison of two semipreparative chromatographic techniques (high performance liquid chromatography and high performance thin-layer chromatography). J. Chromatogr. A 2014, 1325, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Cornelio, V.E.; Maluf, F.V.; Fernandes, J.B.; da Silva, M.F.G.F.; Oliva, G.; Guido, R.V.F.; Vieira, P.C. Isolation of Tiliroside from Spiranthera odoratissima as Inhibitor of Trypanosoma cruzi Glyceraldehyde-3-phosphate Dehydrogenase by Using Bioactivity-Guided Fractionation. J. Braz. Chem. Soc. 2014, 28, 512–519. [Google Scholar] [CrossRef]
- Rabêlo, S.V.; Costa, E.V.; Barison, A.; Dutra, L.M.; Nunes, X.P.; Tomaz, J.C.; Oliveira, G.G.; Lopes, N.P.; Santos, M.F.C.; Almeida, J.R.G.S. Alkaloids isolated from the leaves of atemoya (Annona cherimola × Annona squamosa). Rev. Bras. Farmacogn. 2015, 25, 419–421. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green analytical procedure index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Marti, M.P. Chemical composition of the essential oil of Helenium amarum (Raf.) H. Rock from Cuba. J. Essent. Oil Res. 2006, 18, 438–439. [Google Scholar] [CrossRef]
- Momin, R.A.; Ramsewak, R.S.; Nair, M.G. Bioactive compounds and 1,3-Di[(cis)-9-octadecenoyl]-2-[(cis,cis)-9,12-octadecadienoyl]glycerol from Apium graveolens L. Seeds. J. Agric. Food Chem. 2000, 48, 3785–3788. [Google Scholar] [CrossRef] [PubMed]
- Meepagala, K.M.; Sturtz, G.; Wedge, D.E. Antifungal constituents of the essential oil fraction of Artemisia dracunculus L. var. dracunculus. J. Agric. Food Chem. 2002, 50, 6989–6992. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D.J. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
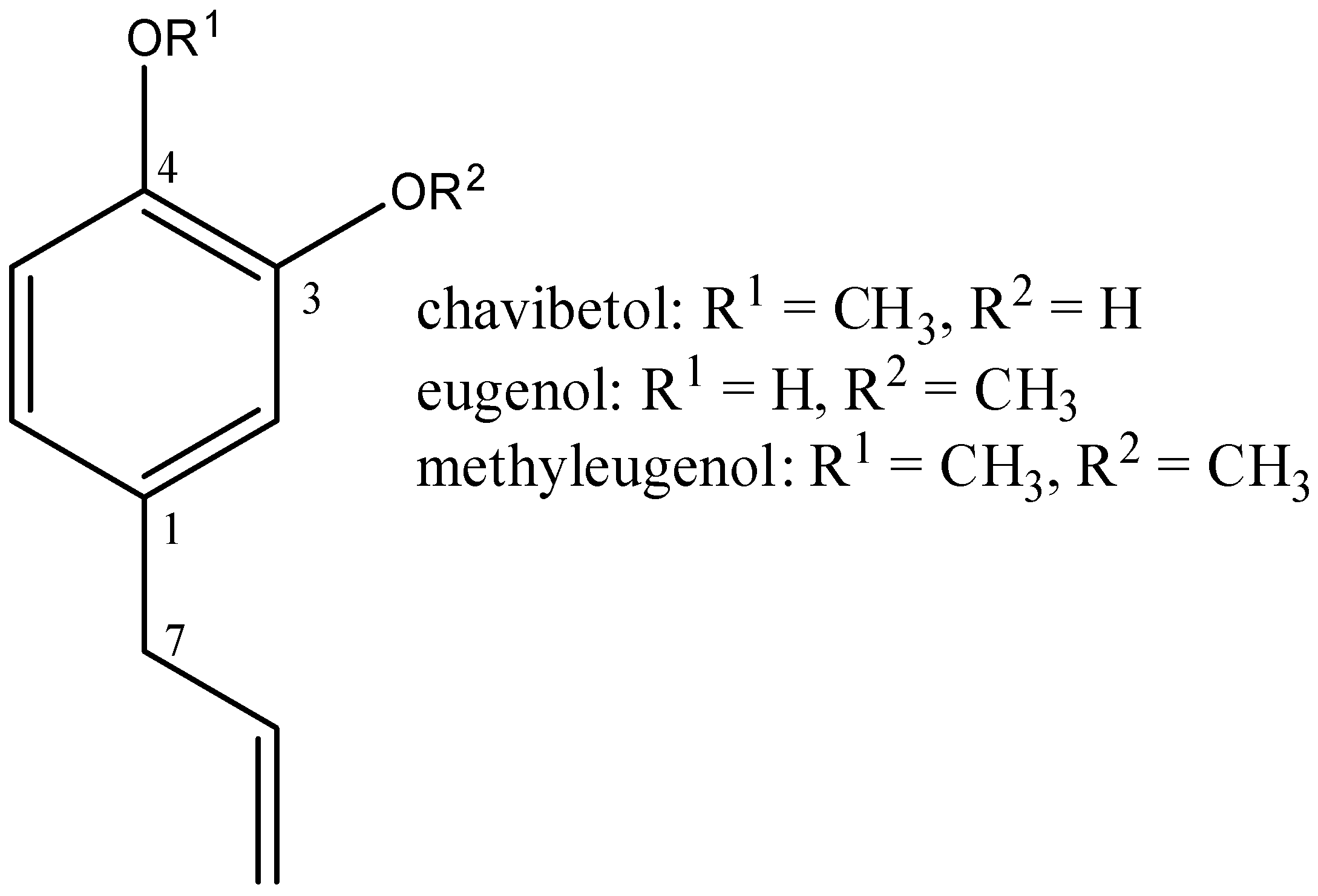

| Parameter | Chavibetol | Methyleugenol |
|---|---|---|
| Isolated mass (mg) | 102.7 | 27.9 |
| Purity (%) | 98.7 | 85.3 |
| Mass recovery (%) | 94.6 | 86.4 |
| Processing time | 11 injections: 1.5 h | 11 injections: 1.5 h |
| Solvent consumption (mL) | 528 | 528 |
| Productivity (mg/h) | 68.5 | 18.6 |
| Solvent consumption/isolated mass (mL/mg) | 5.1 | 18.9 |
| Position | Methyleugenol | Chavibetol | ||
|---|---|---|---|---|
| 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | |
| 1 | - | 132.6 | - | 133.4 |
| 2 | 6.72 (d, 1H, J = 2.0 Hz) | 111.2 | 6.79 (d, 1H, J = 2.1 Hz) | 114.8 |
| 3 | - | 147.4 | - | 144.9 |
| 4 | - | 148.9 | - | 145.5 |
| 5 | 6.81 (d, 1H, J = 7.9 Hz) | 120.4 | 6.80 (d, 1H, J = 8.2 Hz) | 119.8 |
| 6 | 6.73 (dd, 1H, J = 7.9 and 2.0 Hz) | 111.8 | 6.68 (dd, 1H, J = 8.2 and 2.1 Hz) | 110.6 |
| 7 | 3.35 (dl, 2H, J = 6.9 Hz) | 39.8 | 3.30 (d, 2H, J = 6.7 Hz) | 39.6 |
| 8 | 5.97 (tdd, 1H, J = 12.0, 10.0 and 6.9 Hz) | 137.7 | 5.95 (m, 1H) | 137.6 |
| 9 | 5.07 (m, 2H) | 115.6 | 5.07 (m, 2H) | 115.5 |
| 3-OCH3 | 3.88 | 55.9 | - | - |
| 4-OCH3 | 3.87 | 55.8 | 3.88 (s, 3H, –OCH3) | 56.0 |
| OH | - | - | 5.60 (s, 1H, –OH) | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculau, E.d.S.; Ribeiro, L.d.P.; Ansante, T.F.; Fernandes, J.B.; Forim, M.R.; Vieira, P.C.; Vendramim, J.D.; Da Silva, M.F.d.G.F. Isolation of Chavibetol and Methyleugenol from Essential Oil of Pimenta pseudocaryophyllus by High Performance Liquid Chromatography. Molecules 2018, 23, 2909. https://doi.org/10.3390/molecules23112909
Niculau EdS, Ribeiro LdP, Ansante TF, Fernandes JB, Forim MR, Vieira PC, Vendramim JD, Da Silva MFdGF. Isolation of Chavibetol and Methyleugenol from Essential Oil of Pimenta pseudocaryophyllus by High Performance Liquid Chromatography. Molecules. 2018; 23(11):2909. https://doi.org/10.3390/molecules23112909
Chicago/Turabian StyleNiculau, Edenilson dos Santos, Leandro do Prado Ribeiro, Thiago Felipe Ansante, João Batista Fernandes, Moacir Rossi Forim, Paulo Cezar Vieira, José Djair Vendramim, and Maria Fátima das Graças Fernandes Da Silva. 2018. "Isolation of Chavibetol and Methyleugenol from Essential Oil of Pimenta pseudocaryophyllus by High Performance Liquid Chromatography" Molecules 23, no. 11: 2909. https://doi.org/10.3390/molecules23112909
APA StyleNiculau, E. d. S., Ribeiro, L. d. P., Ansante, T. F., Fernandes, J. B., Forim, M. R., Vieira, P. C., Vendramim, J. D., & Da Silva, M. F. d. G. F. (2018). Isolation of Chavibetol and Methyleugenol from Essential Oil of Pimenta pseudocaryophyllus by High Performance Liquid Chromatography. Molecules, 23(11), 2909. https://doi.org/10.3390/molecules23112909







