Novel Rhodanine Derivative, 5-[4-(4-Fluorophenoxy) phenyl]methylene-3-{4-[3-(4-methylpiperazin-1-yl) propoxy]phenyl}-2-thioxo-4-thiazolidinone dihydrochloride, Induces Apoptosis via Mitochondria Dysfunction and Endoplasmic Reticulum Stress in Human Colon Cancer Cells
Abstract
:1. Introduction
2. Results
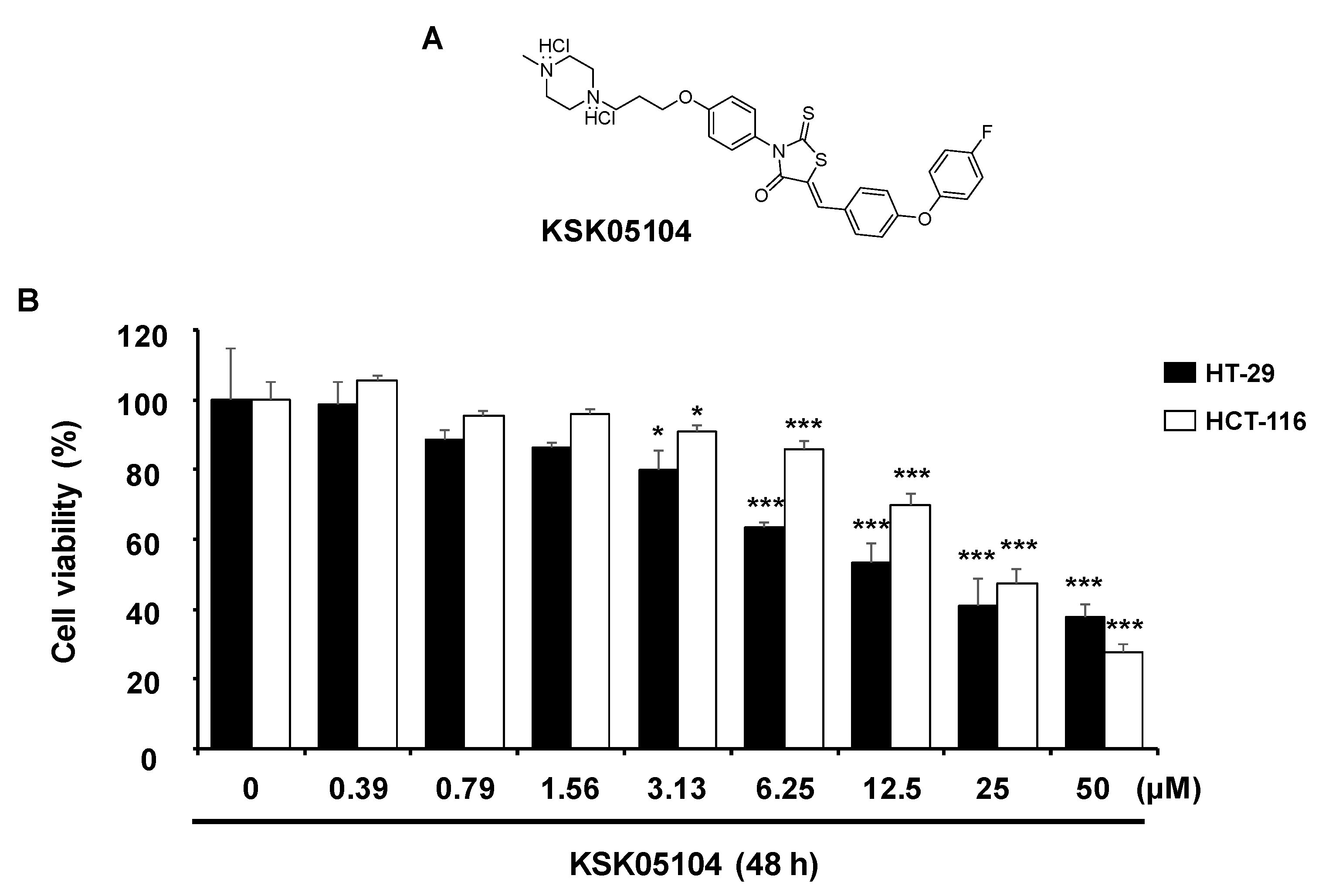
2.1. KSK05104 Induces the Growth Inhibition in HT-29 Cells
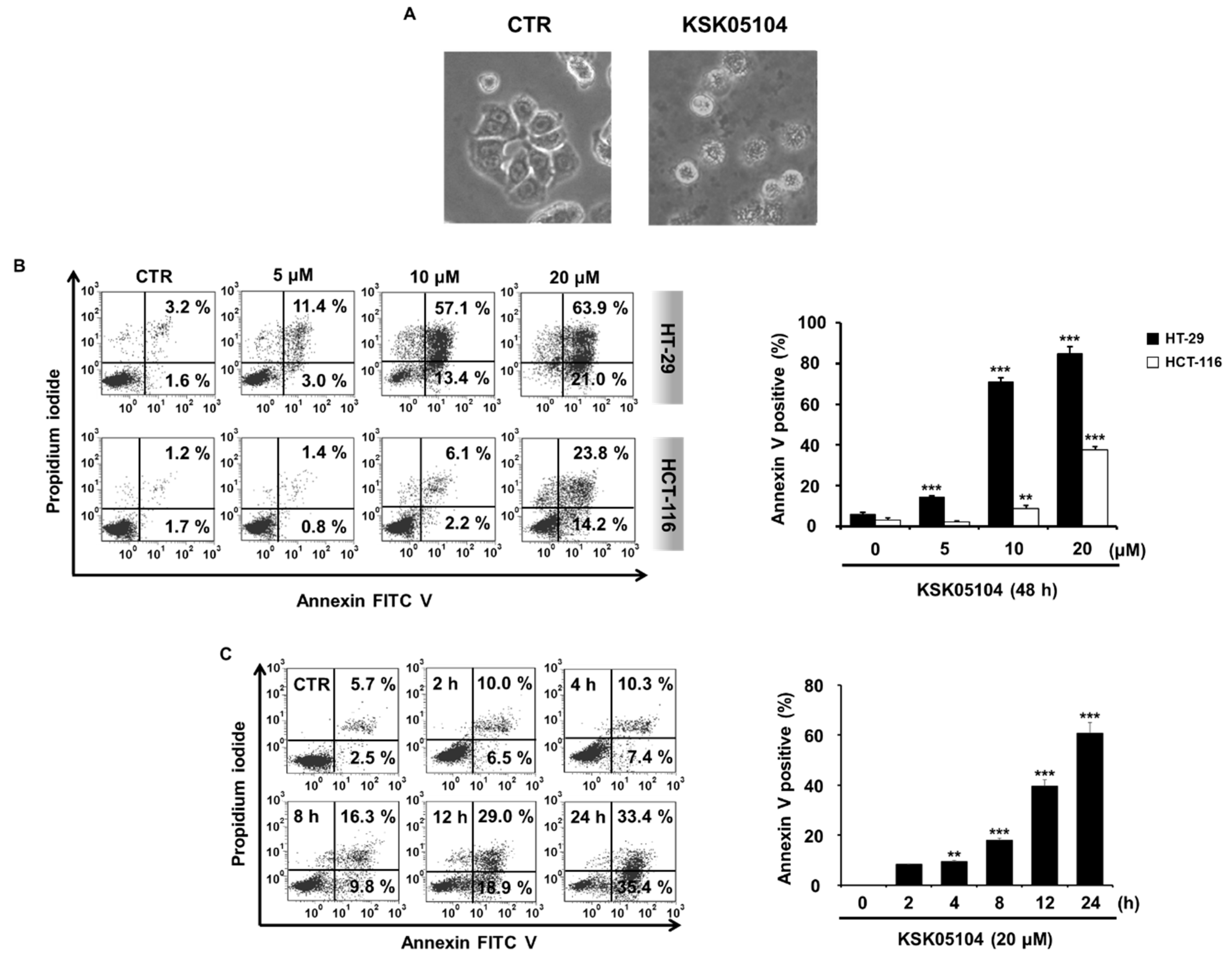
2.2. KSK05104 Induces Apoptosis in HT-29 Cells
2.3. KSK05104-Induced Apoptosis Requires Caspase Activation in HT-29 Cells
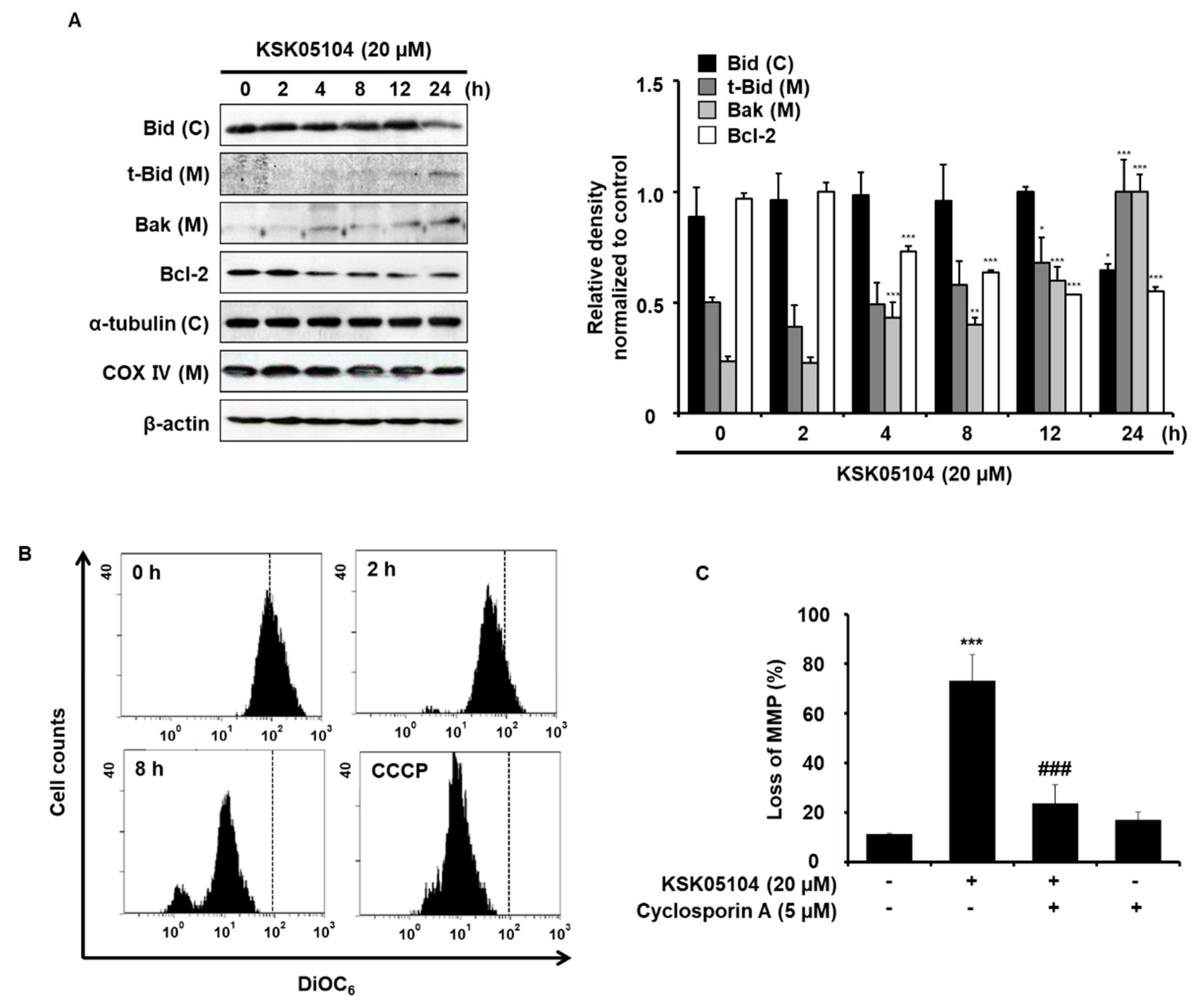
2.4. KSK05104 Modulates the Expression of Bcl-2 Family Proteins and Reduces ΔΨm in HT-29 Cells
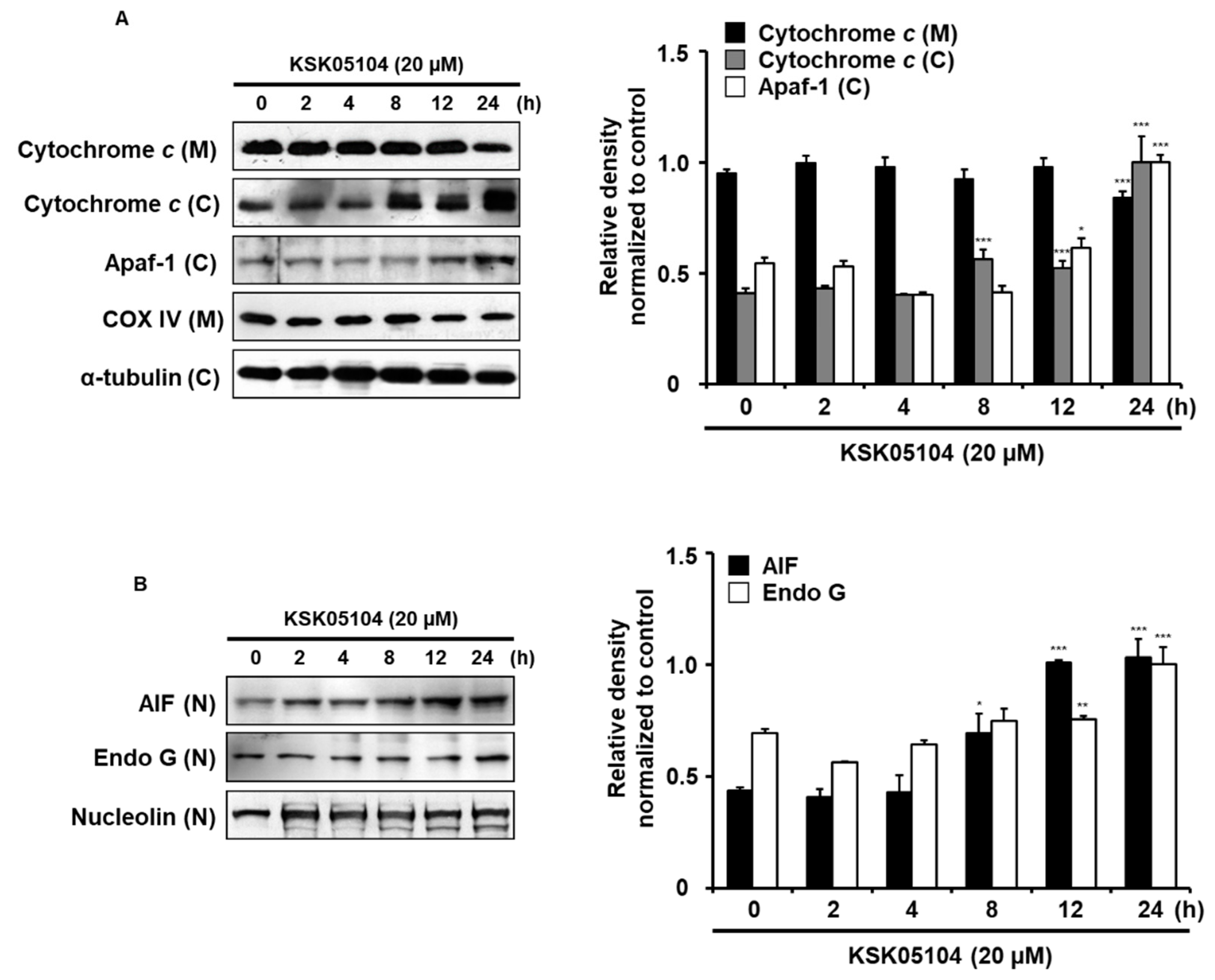
2.5. KSK05104 Induces the Translocation of Mitochondrial Apoptogenic Factors in HT-29 Cells
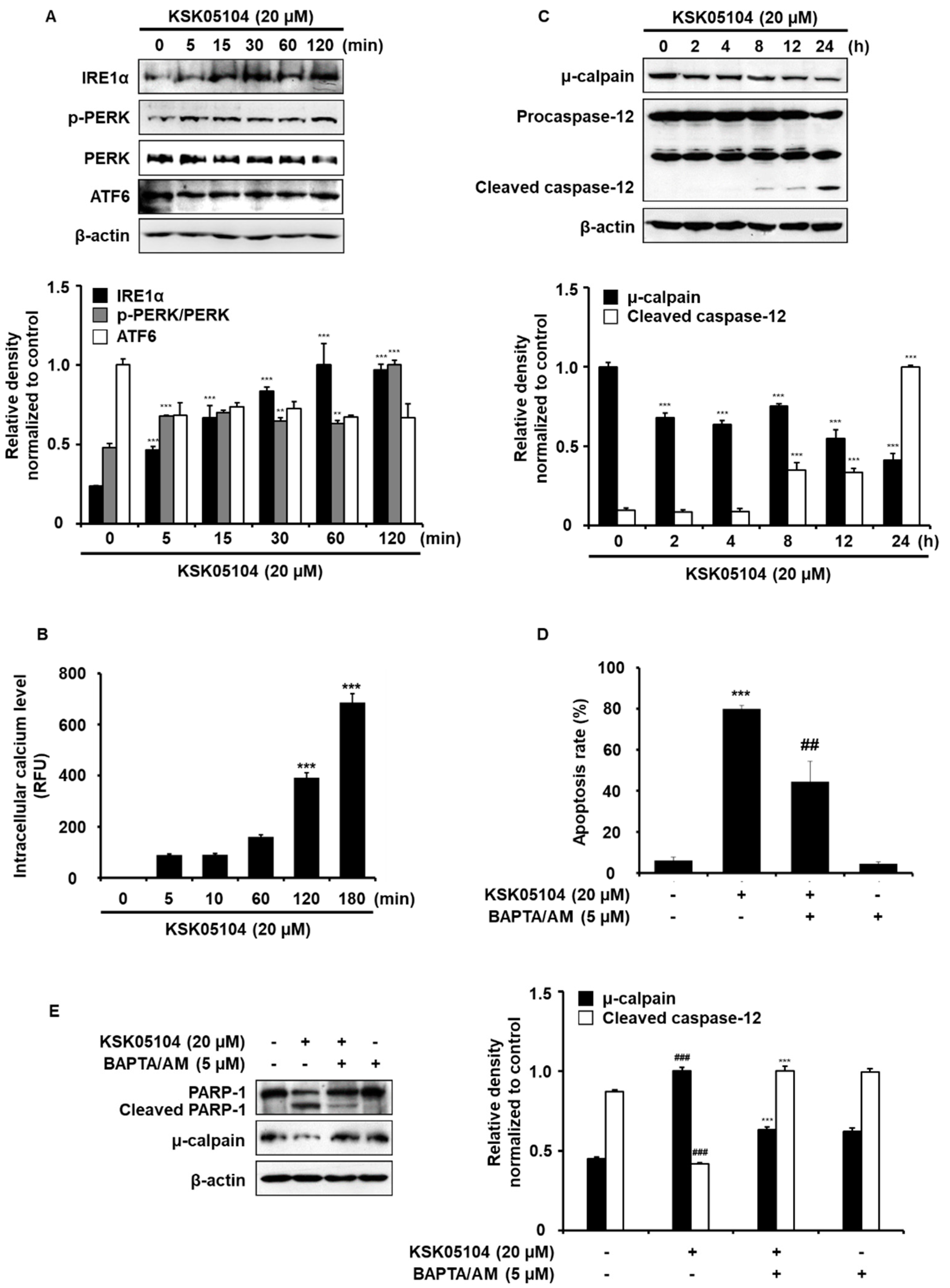
2.6. KSK05104 Increases Intracellular Calcium Level and ER Stress-Mediated Apoptosis in HT-29 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. MTT Assay
4.4. Annexin V and PI Double Staining by Flow Cytometry
4.5. Determination of the ΔΨm
4.6. Western Blot Analysis
4.7. Preparation of Cytosolic and Mitochondrial Fractionations
4.8. Determination of [Ca2+]i
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, M.Z.; Akhter, S.; Anwar, M.; Ahmad, F.J. Assam bora rice starch based biocompatible mucoadhesive microsphere for targeted delivery of 5-Fluorouracil in colorectal cancer. Mol. Pharm. 2012, 9, 2986–2994. [Google Scholar] [CrossRef] [PubMed]
- Louvet, C.; de Gramont, A. Colorectal cancer: Integrating oxaliplatin. Curr. Treat. Options Oncol. 2003, 4, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Jin, C.Y.; Kim, K.S.; Lee, Y.C.; Park, S.H.; Kim, G.Y.; Kim, W.J.; Moon, H.I.; Choi, Y.H.; Lee, J.H. Oleifolioside A mediates caspase-independent human cervical carcinoma HeLa cell apoptosis involving nuclear relocation of mitochondrial apoptogenic factors AIF and EndoG. J. Agric. Food Chem. 2012, 60, 5400–5406. [Google Scholar] [CrossRef] [PubMed]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Y.; Jones, A.F.; Sanger, R.H.; Collis, L.P.; Flannery, R.; McNay, E.C.; Yu, T.; Schwarzenbacher, R.; Bossy, B.; et al. Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 2169–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomonosova, E.; Chinnadurai, G. BH3-only proteins in apoptosis and beyond: An overview. Oncogene 2008, 27 (Suppl. 1), S2. [Google Scholar] [CrossRef] [PubMed]
- Fandy, T.E.; Shankar, S.; Srivastava, R.K. Smac/DIABLO enhances the therapeutic potential of chemotherapeutic drugs and irradiation, and sensitizes TRAIL-resistant breast cancer cells. Mol. Cancer 2008, 7, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait, S.W.; Green, D.R. Mitochondrial regulation of cell death. Cold Spring Harb. Perspect. Biol. 2013, 5, a008706. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, C.; Papas, K.A.; Constantinou, A.I. Caspase-independent pathways of programmed cell death: The unraveling of new targets of cancer therapy? Curr. Cancer Drug Targets 2009, 9, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, H.K.; Susin, S.A. Therapeutic potential of AIF-mediated caspase-independent programmed cell death. Drug Resist. Updat. 2007, 10, 235–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kaufman, R.J. Signaling the unfolded protein response from the endoplasmic reticulum. J. Biol. Chem. 2004, 279, 25935–25938. [Google Scholar] [CrossRef] [PubMed]
- Aridor, M.; Balch, W.E. Integration of endoplasmic reticulum signaling in health and disease. Nat. Med. 1999, 5, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Paschen, W. Dependence of vital cell function on endoplasmic reticulum calcium levels: Implications for the mechanisms underlying neuronal cell injury in different pathological states. Cell Calcium 2001, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, K.; Miyoshi, K.; Katayama, T.; Yoneda, T.; Taniguchi, M.; Kudo, T.; Tohyama, M. The unfolded protein response and Alzheimer’s disease. Biochim. Biophys. Acta 2001, 1536, 85–96. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Tomasic, T.; Masic, L.P. Rhodanine as a privileged scaffold in drug discovery. Curr. Med. Chem. 2009, 16, 1596–1629. [Google Scholar] [CrossRef] [PubMed]
- Momose, Y.; Meguro, K.; Ikeda, H.; Hatanaka, C.; Oi, S.; Sohda, T. Studies on antidiabetic agents. X. Synthesis and biological activities of pioglitazone and related compounds. Chem. Pharm. Bull. (Tokyo) 1991, 39, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Matsumoto, Y.; Matsushima, M.; Fujiwara, M.; Konno, K.; Shimotohno, K.; Shigeta, S.; Yokota, T. Novel hepatitis C virus protease inhibitors: Thiazolidine derivatives. Biochem. Biophys. Res. Commun. 1997, 238, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.M.; Ng, S.B.; Buss, A.D.; Crasta, S.C.; Goh, K.L.; Lee, S.K. Benzylidene rhodanines as novel inhibitors of UDP-N-acetylmuramate/L-alanine ligase. Bioorg. Med. Chem. Lett. 2002, 12, 697–699. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.G.; Seo, J.H.; Shin, K.J. Identification and characterization of potent, selective and metabolically stable IKKbeta inhibitor. Bioorg. Med. Chem. Lett. 2016, 26, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.C.; van den Brink, G.R.; Offerhaus, G.J.; van Deventer, S.J.; Peppelenbosch, M.P. NF-kappaB, p38 MAPK and JNK are highly expressed and active in the stroma of human colonic adenomatous polyps. Oncogene 2001, 20, 819–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, D.S.; Hochwald, S.N.; Malaty, J.; Rekkas, S.; Hebig, P.; Mishra, G.; Moldawer, L.L.; Copeland, E.M., 3rd; Mackay, S. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery 2001, 130, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Song, J.K.; Jo, M.R.; Park, M.H.; Song, H.S.; An, B.J.; Song, M.J.; Han, S.B.; Hong, J.T. Cell growth inhibition and induction of apoptosis by snake venom toxin in ovarian cancer cell via inactivation of nuclear factor kappaB and signal transducer and activator of transcription 3. Arch. Pharm. Res. 2012, 35, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Akl, H.; Vervloessem, T.; Kiviluoto, S.; Bittremieux, M.; Parys, J.B.; De Smedt, H.; Bultynck, G. A dual role for the anti-apoptotic Bcl-2 protein in cancer: Mitochondria versus endoplasmic reticulum. Biochim. Biophys. Acta 2014, 1843, 2240–2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiler, A.; Schneider, M.; Forster, H.; Roth, S.; Wirth, E.K.; Culmsee, C.; Plesnila, N.; Kremmer, E.; Radmark, O.; Wurst, W.; et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008, 8, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy-Berard, N.; Genestier, L.; Flacher, M.; Revillard, J.P. The phosphoprotein phosphatase calcineurin controls calcium-dependent apoptosis in B cell lines. Eur. J. Immunol. 1994, 24, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Roest, G.; La Rovere, R.M.; Bultynck, G.; Parys, J.B. IP3 Receptor Properties and Function at Membrane Contact Sites. Adv. Exp. Med. Biol. 2017, 981, 149–178. [Google Scholar] [PubMed]
- Kaufmann, S.H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: A cautionary note. Cancer Res. 1989, 49, 5870–5878. [Google Scholar] [PubMed]
- Kandasamy, K.; Srinivasula, S.M.; Alnemri, E.S.; Thompson, C.B.; Korsmeyer, S.J.; Bryant, J.L.; Srivastava, R.K. Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: Differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res. 2003, 63, 1712–1721. [Google Scholar] [PubMed]
- Micoud, F.; Mandrand, B.; Malcus-Vocanson, C. Comparison of several techniques for the detection of apoptotic astrocytes in vitro. Cell Prolif. 2001, 34, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, H.; Yang, R.; Hao, J.; Wang, J.; Sun, C.; Fesik, S.W.; Wu, J.C.; Tomaselli, K.J.; Armstrong, R.C. Regulation of the Apaf-1/caspase-9 apoptosome by caspase-3 and XIAP. J. Biol. Chem. 2003, 278, 8091–8098. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Saez, A.J. The secrets of the Bcl-2 family. Cell Death Differ. 2012, 19, 1733–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipuk, J.E.; Green, D.R. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends. Cell Biol. 2008, 18, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Westphal, D.; Dewson, G.; Czabotar, P.E.; Kluck, R.M. Molecular biology of Bax and Bak activation and action. Biochim. Biophys. Acta 2011, 1813, 521–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doran, E.; Halestrap, A.P. Cytochrome c release from isolated rat liver mitochondria can occur independently of outer-membrane rupture: Possible role of contact sites. Biochem. J. 2000, 348, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Srinivasula, S.M.; Acharya, S.; Fishel, R.; Alnemri, E.S. Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J. Biol. Chem. 1999, 274, 17941–17945. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, D.; Gaume, B.; Karbowski, M.; Sharpe, J.C.; Cecconi, F.; Youle, R.J. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 2003, 22, 4385–4399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.Y.; Luo, X.; Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 2001, 412, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N. Genistein induces Ca2+-mediated, calpain/caspase-12-dependent apoptosis in breast cancer cells. Biochem. Biophys. Res. Commun. 2004, 321, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, I.N. Calcium signaling in cancer and vitamin D. J. Steroid Biochem. Mol. Biol. 2005, 97, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.V.; Bredesen, D.E. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr. Opin. Cell Biol. 2004, 16, 653–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadowaki, H.; Nishitoh, H. Signaling pathways from the endoplasmic reticulum and their roles in disease. Genes (Basel) 2013, 4, 306–333. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Ahmed, H.; Yang, P.; Czinn, S.J.; Blanchard, T.G. Endoplasmic reticulum stress and IRE-1 signaling cause apoptosis in colon cancer cells in response to andrographolide treatment. Oncotarget 2016, 7, 41432–41444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, R.; Cook, K.L.; Hu, R.; Facey, C.O.; Tavassoly, I.; Schwartz, J.L.; Baumann, W.T.; Tyson, J.J.; Xuan, J.; Wang, Y.; et al. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res. 2012, 72, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Hsu, Y.L.; Cho, C.Y.; Ng, L.T.; Kuo, Y.H.; Lin, C.C. Apoptotic effects of Antrodia cinnamomea fruiting bodies extract are mediated through calcium and calpain-dependent pathways in Hep 3B cells. Food Chem. Toxicol. 2006, 44, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.S.; Choi, J.H.; Back, N.I.; Choi, M.S.; Kang, E.K.; Chung, H.G.; Jeong, T.S.; Lee, K.T. Eupafolin, a flavonoid isolated from Artemisia princeps, induced apoptosis in human cervical adenocarcinoma HeLa cells. Mol. Nutr. Food Res. 2010, 54, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Cell Line | Origin | IC50 (μM) |
|---|---|---|
| HT-29 | Human colorectal adenocarcinoma | 15.93 ± 2.41 |
| HCT-116 | Human colorectal carcinoma | 31.96 ± 1.56 |
| CCD-18Co | Human colon fibroblast | 88.79 ± 2.91 |
| L132 | Human lung epithelial cell | 90.06 ± 3.55 |
| IOSE-80PC | Immortalized ovarian surface epithelial cell | 45.8 ± 3.69 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.-U.; Lee, J.-H.; Chung, K.-S.; Hong, J.Y.; Choi, J.-H.; Kim, S.-D.; Roh, E.J.; Shin, K.J.; Lee, K.-T. Novel Rhodanine Derivative, 5-[4-(4-Fluorophenoxy) phenyl]methylene-3-{4-[3-(4-methylpiperazin-1-yl) propoxy]phenyl}-2-thioxo-4-thiazolidinone dihydrochloride, Induces Apoptosis via Mitochondria Dysfunction and Endoplasmic Reticulum Stress in Human Colon Cancer Cells. Molecules 2018, 23, 2895. https://doi.org/10.3390/molecules23112895
Jung H-U, Lee J-H, Chung K-S, Hong JY, Choi J-H, Kim S-D, Roh EJ, Shin KJ, Lee K-T. Novel Rhodanine Derivative, 5-[4-(4-Fluorophenoxy) phenyl]methylene-3-{4-[3-(4-methylpiperazin-1-yl) propoxy]phenyl}-2-thioxo-4-thiazolidinone dihydrochloride, Induces Apoptosis via Mitochondria Dysfunction and Endoplasmic Reticulum Stress in Human Colon Cancer Cells. Molecules. 2018; 23(11):2895. https://doi.org/10.3390/molecules23112895
Chicago/Turabian StyleJung, Hye-Uk, Jeong-Hun Lee, Kyung-Sook Chung, Joo Young Hong, Jung-Hye Choi, Soo-Dong Kim, Eun Joo Roh, Kye Jung Shin, and Kyung-Tae Lee. 2018. "Novel Rhodanine Derivative, 5-[4-(4-Fluorophenoxy) phenyl]methylene-3-{4-[3-(4-methylpiperazin-1-yl) propoxy]phenyl}-2-thioxo-4-thiazolidinone dihydrochloride, Induces Apoptosis via Mitochondria Dysfunction and Endoplasmic Reticulum Stress in Human Colon Cancer Cells" Molecules 23, no. 11: 2895. https://doi.org/10.3390/molecules23112895
APA StyleJung, H.-U., Lee, J.-H., Chung, K.-S., Hong, J. Y., Choi, J.-H., Kim, S.-D., Roh, E. J., Shin, K. J., & Lee, K.-T. (2018). Novel Rhodanine Derivative, 5-[4-(4-Fluorophenoxy) phenyl]methylene-3-{4-[3-(4-methylpiperazin-1-yl) propoxy]phenyl}-2-thioxo-4-thiazolidinone dihydrochloride, Induces Apoptosis via Mitochondria Dysfunction and Endoplasmic Reticulum Stress in Human Colon Cancer Cells. Molecules, 23(11), 2895. https://doi.org/10.3390/molecules23112895





