Study of Osteocyte Behavior by High-Resolution Intravital Imaging Following Photo-Induced Ischemia
Abstract
1. Introduction
2. Results
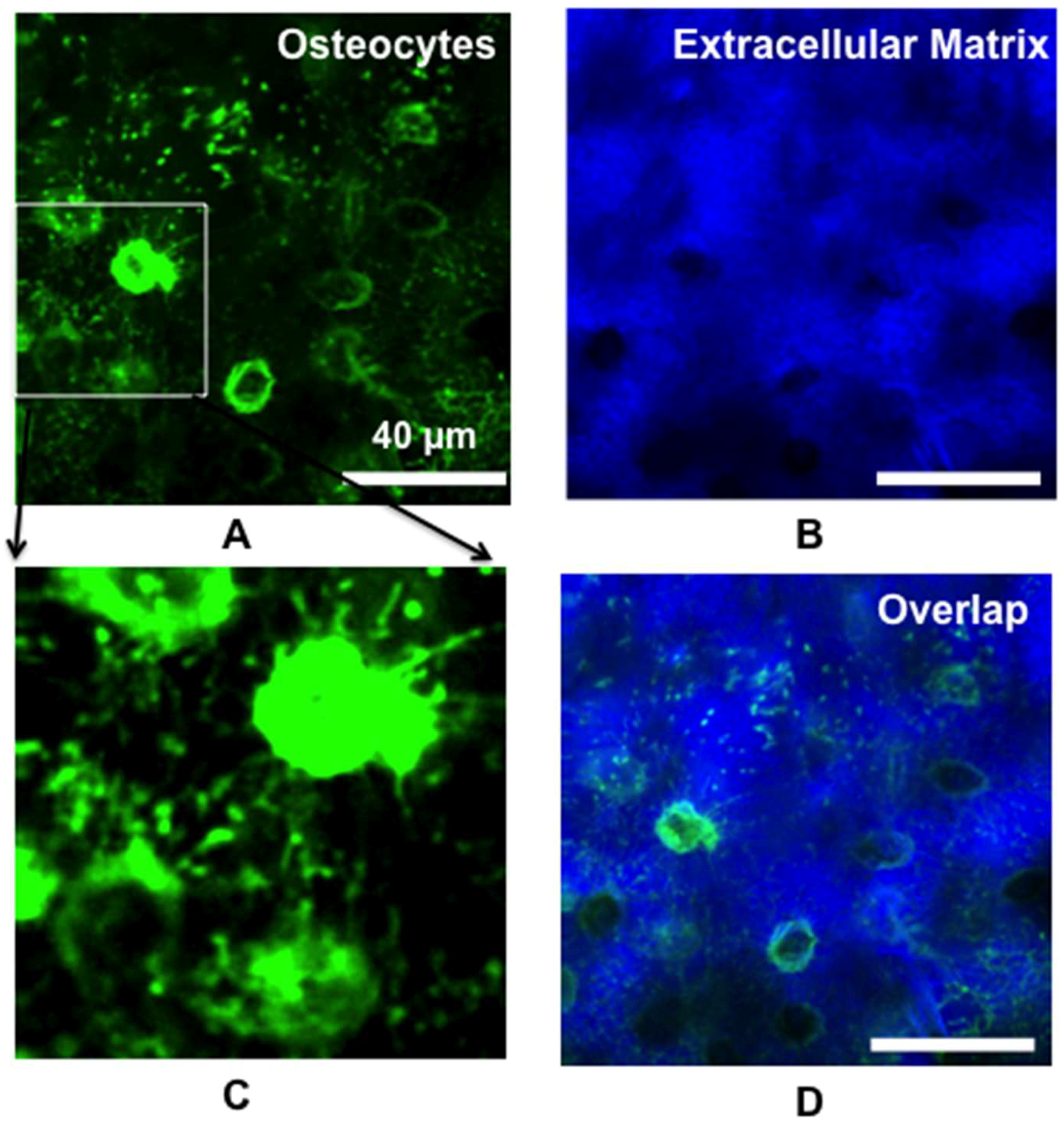
2.1. Real-Time In Vivo Imaging of Osteocyte Structure and Blood Vessels
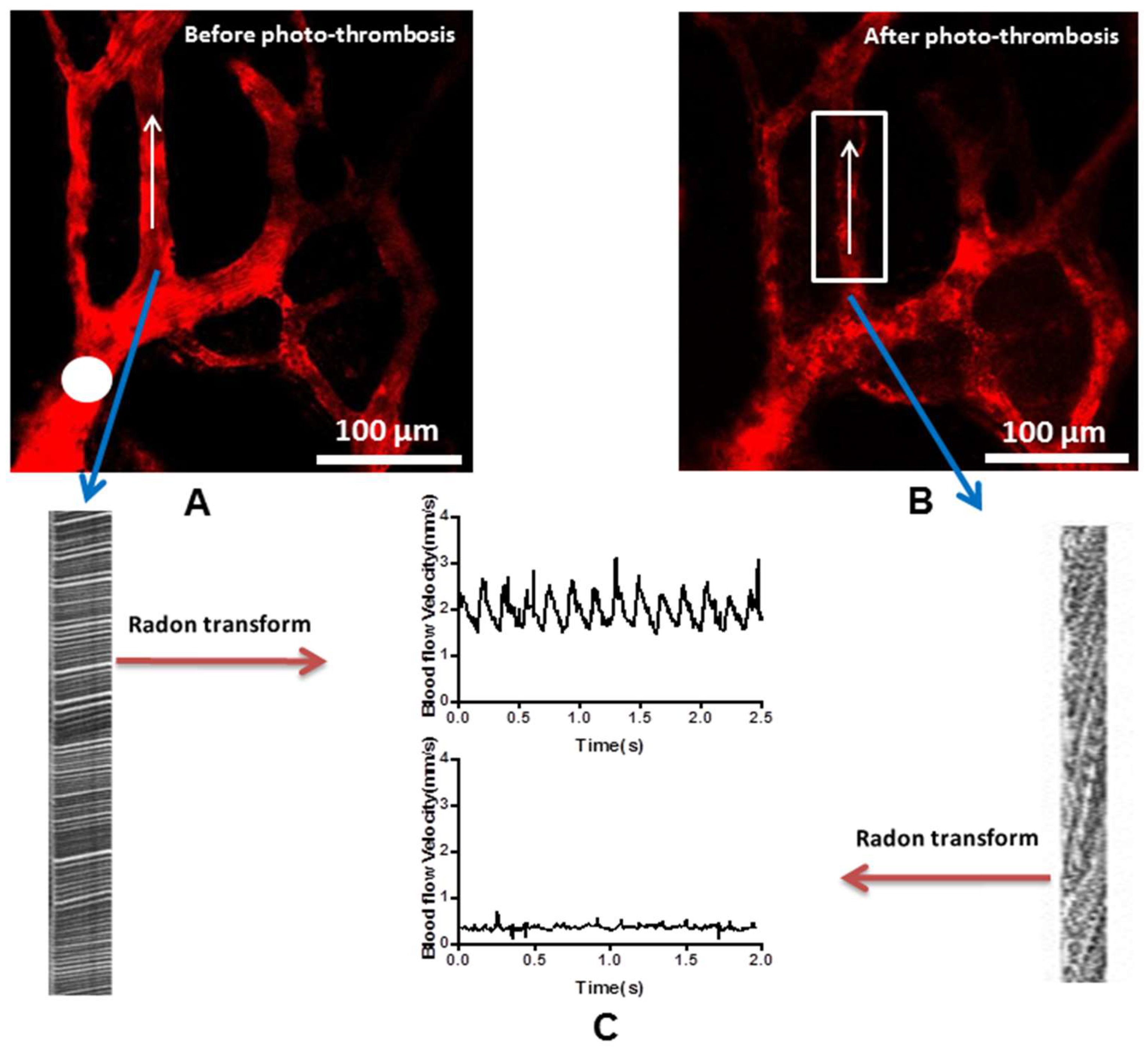
2.2. Photo-thrombosis of the Selected Blood Vessels
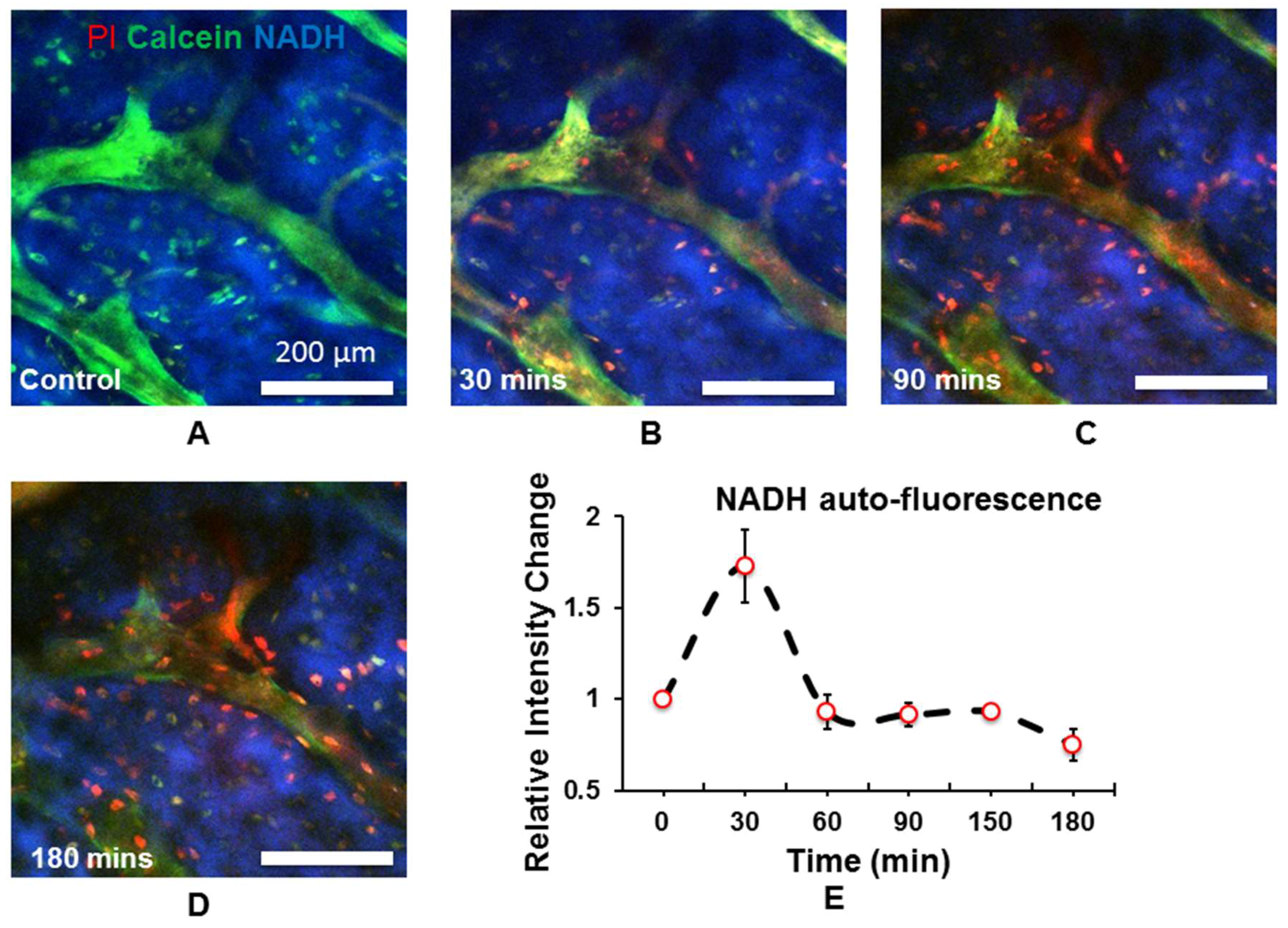
2.3. In Vivo Quantitation of Osteocyte Metabolism
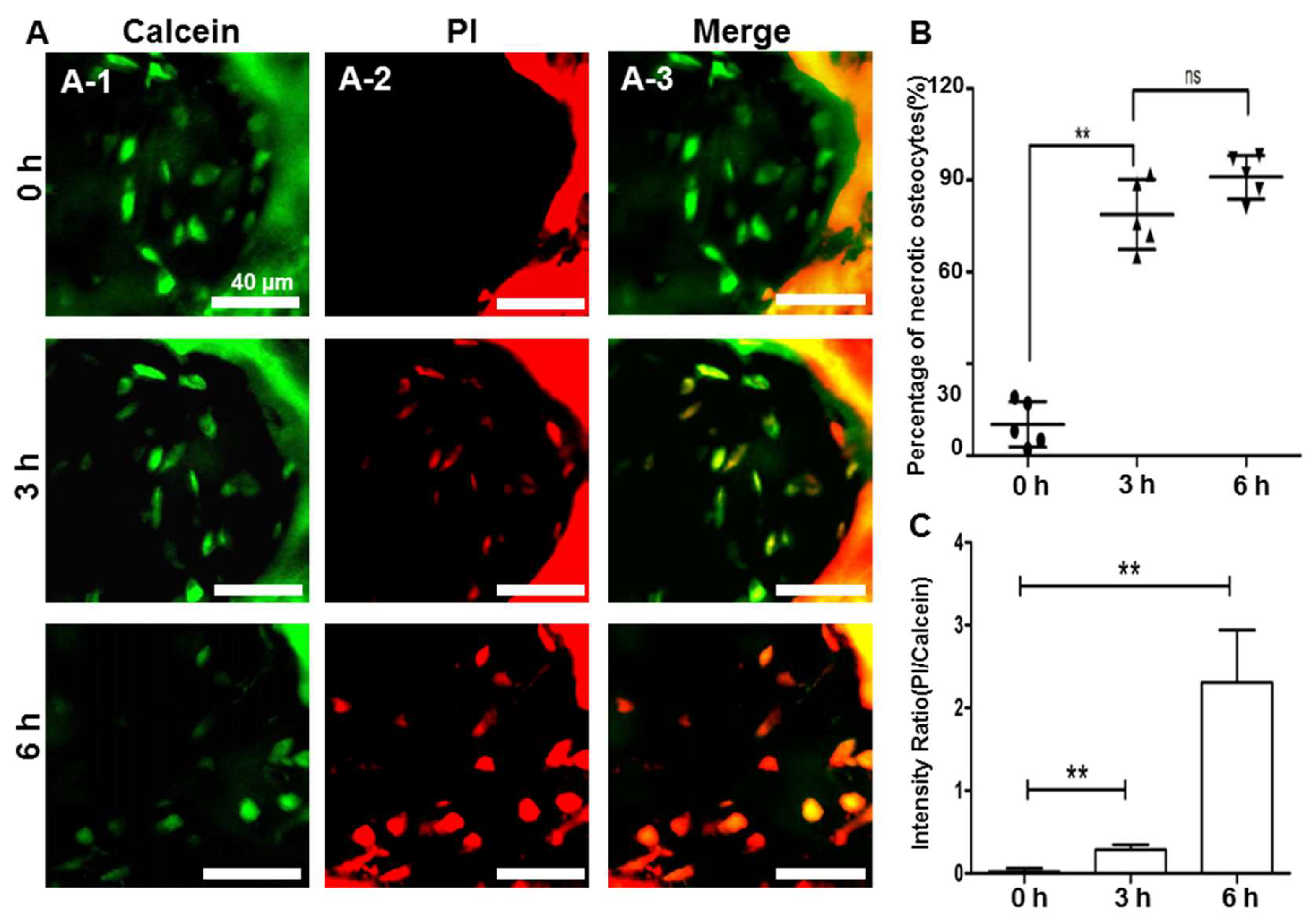
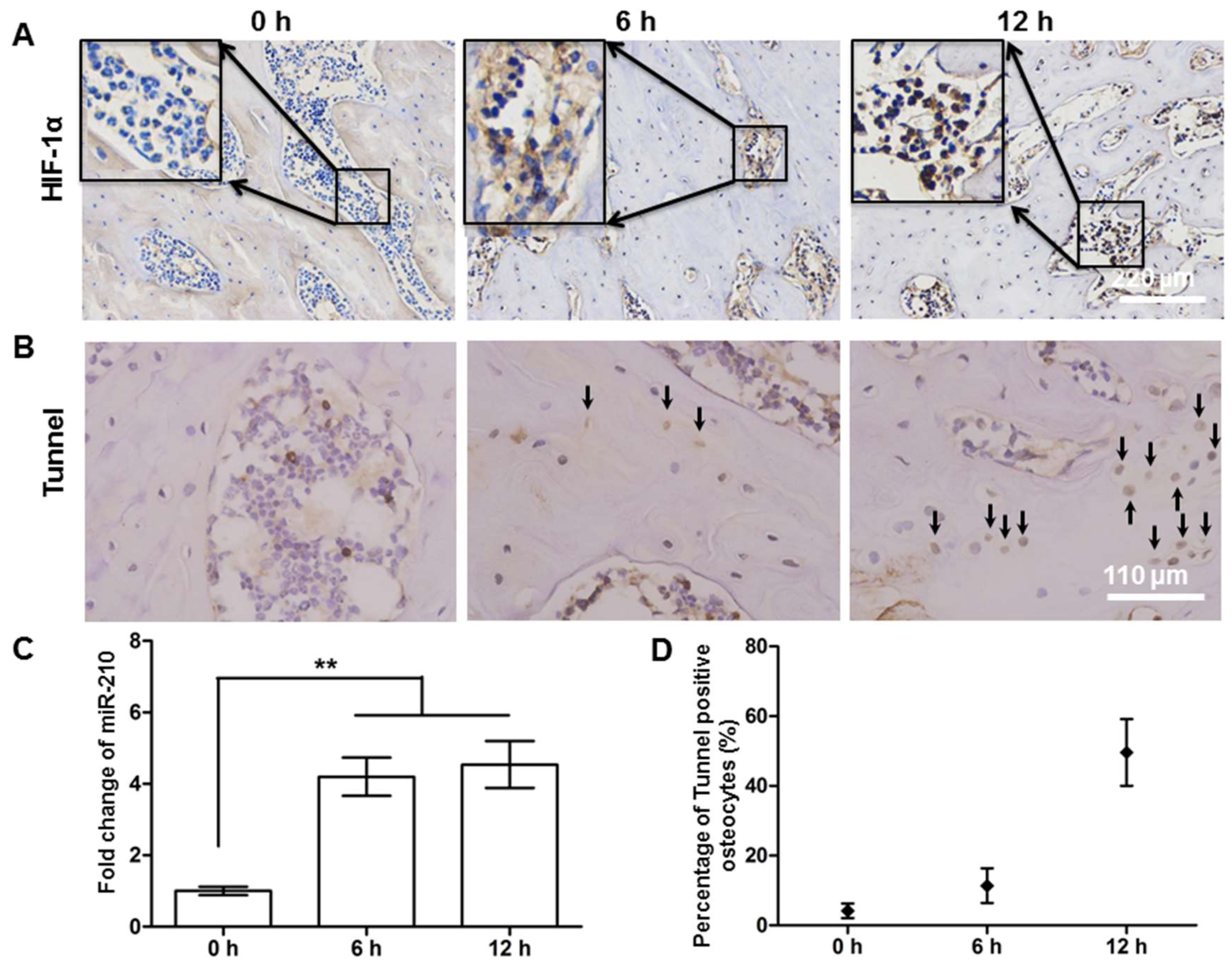
2.4. Hypoxic and Apoptotic Analysis of the Avascular Osteonecrosis Tissue
3. Discussion and Conclusions
4. Materials and Methods
4.1. Animal Preparation
4.2. Bone Cell and Vasculature Labeling
4.3. Multi-Photon Imaging System and Photo-Thrombosis
4.4. NADH and Extracellular Matrix Imaging
4.5. Ischaemic Osteonecrosis Model by Osteotomy Surgery
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mankin, H.J. Nontraumatic necrosis of bone (osteonecrosis). N. Engl. J. Med. 1992, 326, 1473–1479. [Google Scholar] [PubMed]
- Chang, C.C.; Greenspan, A.; Gershwin, M.E. Osteonecrosis: Current perspectives on pathogenesis and treatment. Semin. Arthritis Rheum. 1993, 23, 47–69. [Google Scholar] [CrossRef]
- Jergesen, H.E.; Khan, A.S. The natural history of untreated asymptomatic hips in patients who have non-traumatic osteonecrosis. J. Bone Jt. Surg. Am. 1997, 79A, 359–363. [Google Scholar] [CrossRef]
- Yuan, H.F.; Von Roemeling, C.; Gao, H.D.; Zhang, J.; Guo, C.A.; Yan, Z.Q. Analysis of altered microRNA expression profile in the reparative interface of the femoral head with osteonecrosis. Exp. Mol. Pathol. 2015, 98, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Ragab, Y.; Emad, Y.; Abou-Zeid, A. Bone marrow edema syndromes of the hip: MRI features in different hip disorders. Clin. Rheumatol. 2008, 27, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Luk, W.H.; Au-Yeung, A.W.S.; Yang, M.K.W. Diagnostic value of SPECT versus SPECT/CT in femoral avascular necrosis: Preliminary results. Nucl. Med. Commun. 2010, 31, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Fujioka, M.; Takahashi, K.A.; Ueshima, K.; Arai, Y.; Imahori, Y.; Itani, K.; Nishimura, T.; Kubo, T. Evaluation of the hemodynamics of the femoral head compared with the ilium, femoral neck and femoral intertrochanteric region in healthy adults: Measurement with positron emission tomography (PET). Ann. Nucl. Med. 2005, 19, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.F.; Shen, F.; Zhang, J.; Shi, H.C.; Gu, Y.S.; Yan, Z.Q. Predictive value of single photon emission computerized tomography and computerized tomography in osteonecrosis after femoral neck fracture: A prospective study. Int. Orthop. 2015, 39, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Cohen, P.; Jobsis, F.; Schoener, B. Intracellular oxidation-reduction states in vivo. Science 1962, 137, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Heikal, A.A. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies. Biomark. Med. 2010, 4, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Obitabot, E.T.; Hanrahan, L.M.; Cachecho, R.; Beer, E.R.; Hopkins, S.R.; Chan, J.C.K.; Shapiro, J.M.; Lamorte, W.W. Changes in Hepatocyte Nadh Fluorescence during Prolonged Hypoxia. J. Surg. Res. 1993, 55, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.R.; Davis, K.N.; Medinaramirez, G. Quantitative-Analysis of Heterogenous Nadh Fluorescence in Perfused Rat Hearts during Hypoxia and Ischemia. J. Mol. Cell Cardiol. 1982, 14, 29–35. [Google Scholar] [CrossRef]
- Beilke, D.; Weiss, R.; Lohr, F.; Pristovsek, P.; Hannemann, F.; Bernhardt, R.; Ruterjans, H. A new electron transport mechanism in mitochondrial steroid hydroxylase systems based on structural changes upon the reduction of adrenodoxin. Biochemistry (US) 2002, 41, 7969–7978. [Google Scholar] [CrossRef]
- Mayevsky, A. Brain NADH redox state monitored in vivo by fiber optic surface fluorometry. Brain Res. 1984, 319, 49–68. [Google Scholar] [CrossRef]
- Balaban, R.S.; Heineman, F.W. Control of mitochondrial respiration in the heart in vivo. Mol. Cell Biochem. 1989, 89, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Skala, M.C.; Riching, K.M.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; White, J.G.; Ramanujam, N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc. Natl. Acad. Sci. USA 2007, 104, 19494–19499. [Google Scholar] [CrossRef] [PubMed]
- Frikha-Benayed, D.; Basta-Pljakic, J.; Majeska, R.; Schaffler, M. Regional differences in oxidative metabolism and mitochondrial activity among cortical bone osteocytes. Bone 2016, 90, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Ishii, M. Intravital two-photon imaging: A versatile tool for dissecting the immune system. Ann. Rheum. Dis. 2011, 70 (Suppl. 1), i113–i115. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.G.; Konig, K.; Halbhuber, K.J. Two-photon microscopy of deep intravital tissues and its merits in clinical research. J. Microsc. 2010, 238, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Campagnola, P.J.; Loew, L.M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 2003, 21, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Assouline-Dayan, Y.; Chang, C.; Greenspan, A.; Shoenfeld, Y.; Gershwin, M.E. Pathogenesis and natural history of osteonecrosis. Semin. Arthritis Rheum. 2002, 32, 94–124. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Irisa, T.; Sugioka, Y.; Sueishi, K. Effects of pulse methylprednisolone on bone and marrow tissues—Corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum. 1997, 40, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Ichiseki, T.; Kaneuji, A.; Ueda, Y.; Nakagawa, S.; Mikami, T.; Fukui, K.; Matsumoto, T. Osteonecrosis Development in a Novel Rat Model Characterized by a Single Application of Oxidative Stress. Arthritis Rheum. 2011, 63, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Kikuta, J.; Furuya, M.; Kondo, N.; Endo, N.; Ishii, M. Intravital bone imaging by two-photon excitation microscopy to identify osteocytic osteolysis in vivo. Bone 2015, 74, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried alive: How osteoblasts become osteocytes. Dev. Dyn. 2006, 235, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S.; Nicholas, R.W.; Manolagas, S.C. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J. Clin. Endocr. Metab. 2000, 85, 2907–2912. [Google Scholar] [CrossRef] [PubMed]
- Mutijima, E.; De Maertelaer, V.; Deprez, M.; Malaise, M.; Hauzeur, J.P. The apoptosis of osteoblasts and osteocytes in femoral head osteonecrosis: Its specificity and its distribution. Clin. Rheumatol. 2014, 33, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.J.; Findlay, D.M. Osteocyte regulation of bone mineral: A little give and take. Osteoporosis Int. 2012, 23, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The Amazing Osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sugano, N.; Ohzono, K.; Nomura, S.; Kitamura, Y.; Tsukamoto, Y.; Ogawa, S. Apoptosis and expression of stress protein (ORP150, HO1) during development of ischaemic osteonecrosis in the rat. J. Bone Jt. Surg. Br. 2001, 83, 751–759. [Google Scholar] [CrossRef]
- Huang, A.J.W.; Watson, B.D.; Hernandez, E.; Tseng, S.C.G. Photothrombosis of Corneal Neovascularization by Intravenous Rose-Bengal and Argon-Laser Irradiation. Arch. Ophthalmol. 1988, 106, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, M.; Jander, S.; Stoll, G. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: Characterization of inflammatory responses. J. Neurosci. Meth. 2002, 117, 43–49. [Google Scholar] [CrossRef]
- Lee, L.J.; Yang, Z.; Rahman, M.; Ma, J.; Kwak, K.J.; McElroy, J.; Shilo, K.; Goparaju, C.; Yu, L.; Rom, W.; et al. Extracellular mRNA detected by tethered lipoplex nanoparticle biochip for lung adenocarcinoma detection. Am. J. Respir. Crit. Care Med. 2016, 193, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.F.; Pan, J.F.; Li, S.; Guo, C.A.; Liu, S.H.; Yan, Z.Q. Protective effects of total saponins of panax notoginseng on steroid-induced avascular necrosis of the femoral head in vivo and in vitro. Evid. Based Complement. Alternat. Med. 2015, 2015, 165679. [Google Scholar] [CrossRef] [PubMed]
- Drew, P.J.; Blinder, P.; Cauwenberghs, G.; Shih, A.Y.; Kleinfeld, D. Rapid determination of particle velocity from space-time images using the Radon transform. J. Comput. Neurosci. 2010, 29, 5–11. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Jiang, W.; Chen, Y.; Kim, B.Y.S. Study of Osteocyte Behavior by High-Resolution Intravital Imaging Following Photo-Induced Ischemia. Molecules 2018, 23, 2874. https://doi.org/10.3390/molecules23112874
Yuan H, Jiang W, Chen Y, Kim BYS. Study of Osteocyte Behavior by High-Resolution Intravital Imaging Following Photo-Induced Ischemia. Molecules. 2018; 23(11):2874. https://doi.org/10.3390/molecules23112874
Chicago/Turabian StyleYuan, Hengfeng, Wen Jiang, Yuanxin Chen, and Betty Y. S. Kim. 2018. "Study of Osteocyte Behavior by High-Resolution Intravital Imaging Following Photo-Induced Ischemia" Molecules 23, no. 11: 2874. https://doi.org/10.3390/molecules23112874
APA StyleYuan, H., Jiang, W., Chen, Y., & Kim, B. Y. S. (2018). Study of Osteocyte Behavior by High-Resolution Intravital Imaging Following Photo-Induced Ischemia. Molecules, 23(11), 2874. https://doi.org/10.3390/molecules23112874




