Chemical and Biological Evaluation of Essential Oils from Cardamom Species
Abstract
1. Introduction
2. Results
2.1. Essential Oil Composition
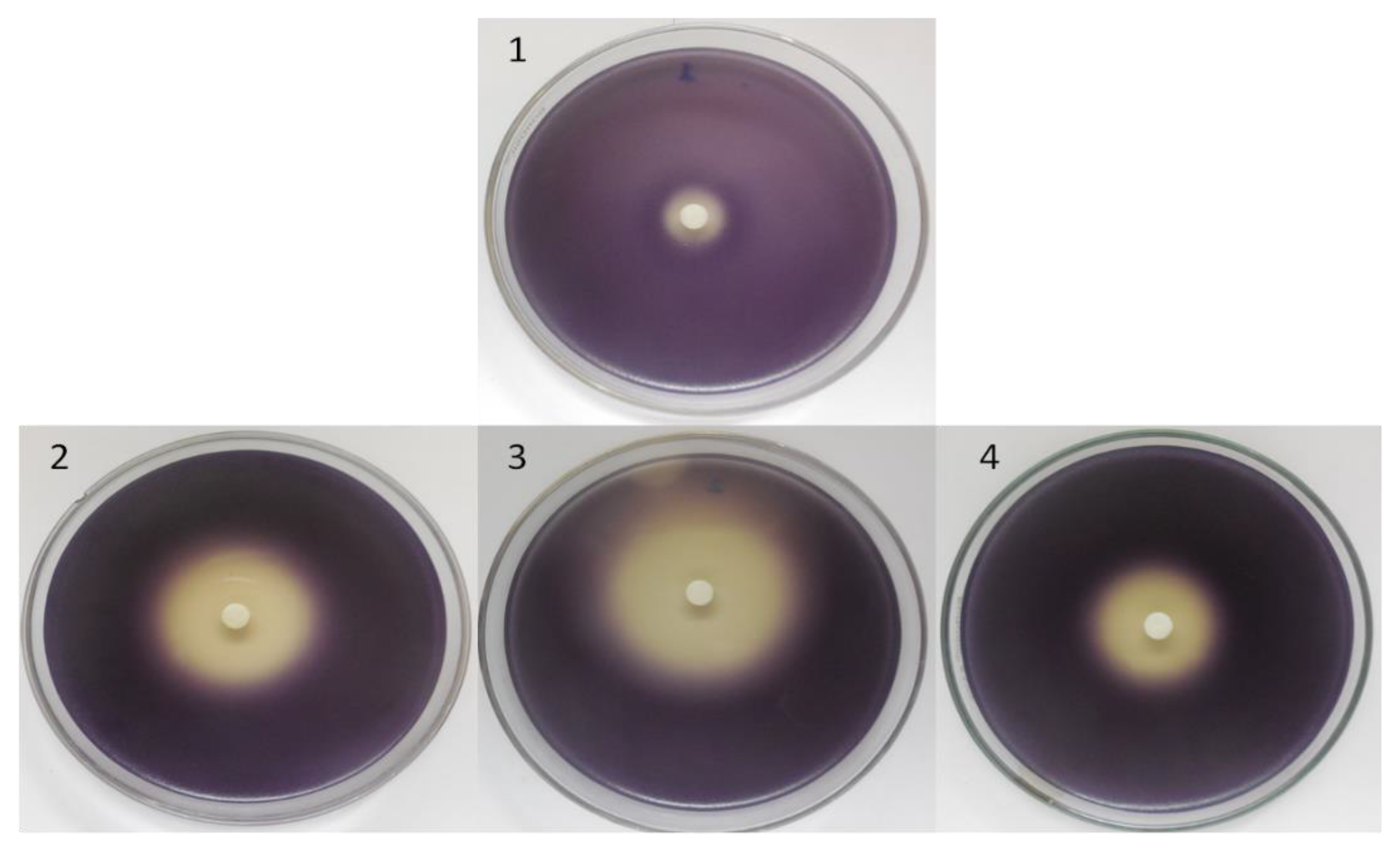
2.2. Antimicrobial Activities
2.3. Phytotoxic Activity
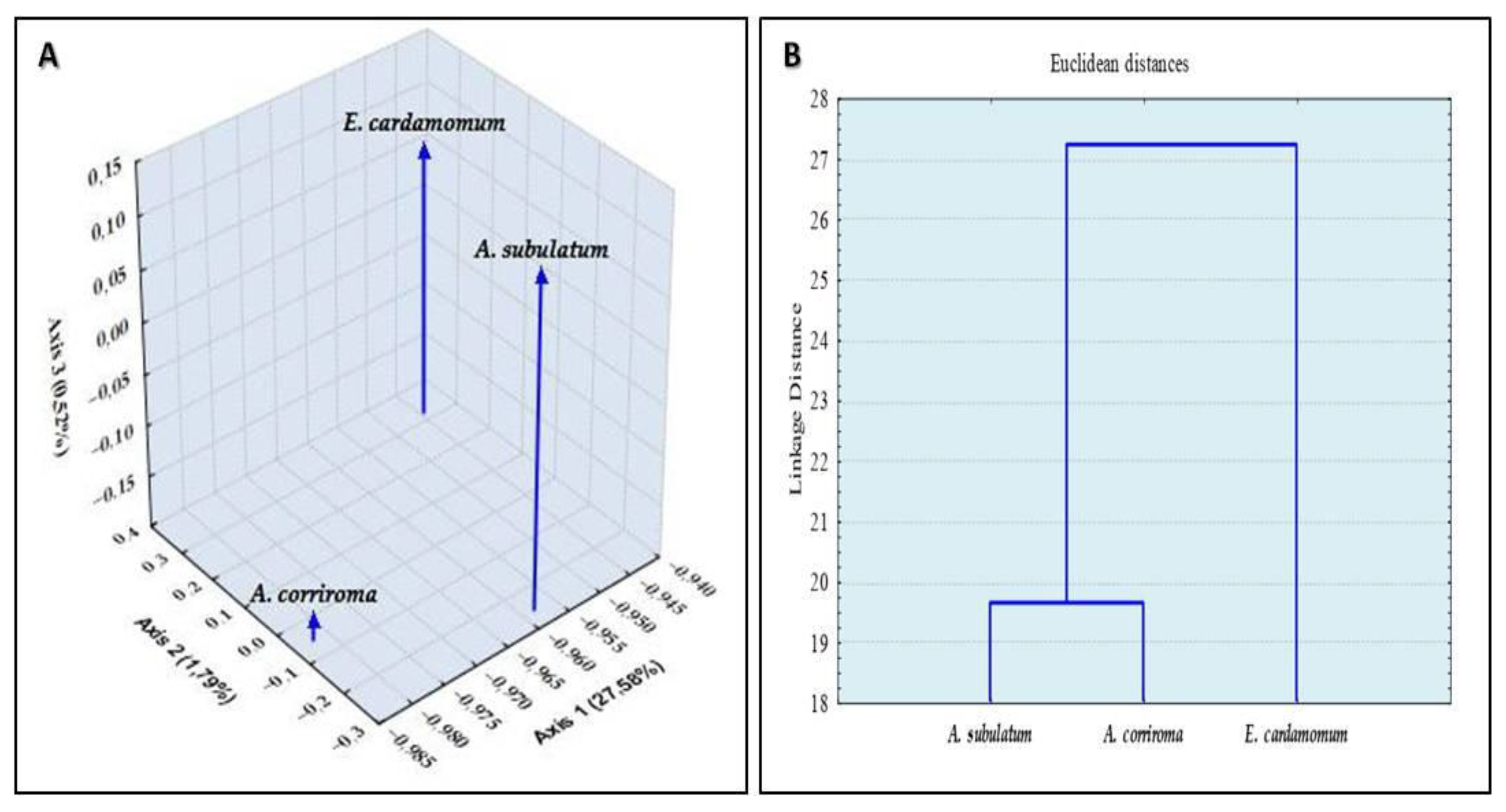
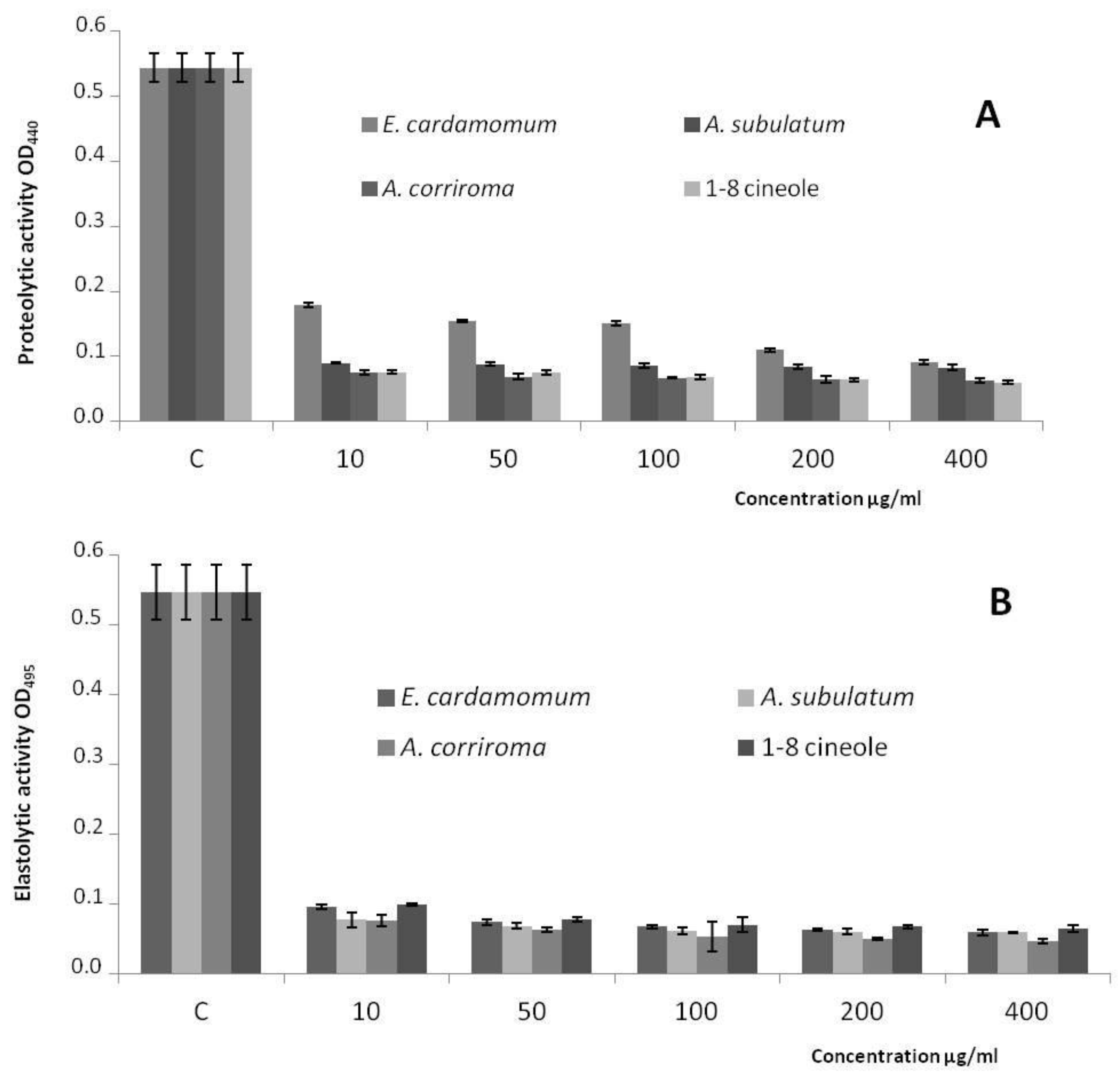
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. GC–EIMS Analysis and Identification of the Components
4.3. Biological Assays
4.3.1. Antimicrobial Activities
4.3.2. Microdilution Method for the Determination of the MIC, MBC/MFC
4.4. Anti-Quorum Sensing Activities of the Essential Oils
4.4.1. Effect on P. aeruginosa PAO1 Virulence Factors
4.4.2. Effect on Violacein Production
4.5. Phytotoxic Activity of the Essential Oils
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 210, 86. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Nazzaro, F.; Mancini, E.; De Feo, V. Essential Oils from Mediterranean aromatic Plants. In The Mediterranean Diet: An Evidence-Based Approach; Preedy, V.R., Watson, R.R., Eds.; Elsevier: London, UK, 2015; pp. 649–661. [Google Scholar]
- Tsigarida, E.; Skandamis, P.; Nychas, G.J.E. Behaviour of Listeria monocytogenes and autochthonous flora on meat stored under aerobic, vacuum and modified atmosphere packaging conditions with or without the presence of oregano essential oil at 5 °C. J. Appl. Microbiol. 2000, 89, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.Z.; Hoiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral. Sci. 2014, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, A.; da Silva, W.M.; Santos, A.V.; de Castro Pimenta, A.M.; Carepo, M.S.; Schneider, M.P.; Azevedo, V.; Silva, A. Chromobacterium violaceum: Important insights for virulence and biotechnological potential by exoproteomic studies. Curr. Microbiol. 2013, 67, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Noumi, E.; Snoussi, M.; Merghni, A.; Nazzaro, F.; Quindós, G.; Akdamar, G.; Mastouri, M.; Al-Sieni, A.; Ceylan, O. Phytochemical composition, anti-biofilm and anti-quorum sensing potential of fruit, stem and leaves of Salvadora persica L. methanolic extracts. Microb. Pathog. 2017, 109, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ruimy, R.; Andremont, A. Quorum sensing in Pseudomonas aeruginosa: Molecular mechanism, clinical impact, and inhibition. Réanimation 2004, 13, 176–184. [Google Scholar] [CrossRef]
- Bacha, K.; Tariku, Y.; Gebreyesus, F.; Zerihun, S.; Mohammed, A.; Weiland-Bräuer, N.; Schmitz, R.A.; Mulat, M. Antimicrobial and antiquorum Sensing activities of selected medicinal plants of Ethiopia: Implication for development of potent antimicrobial agents. BMC Microbiol. 2016, 16, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum Sensing and Phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef] [PubMed]
- Ta, C.A.; Arnason, J.T. Mini Review of Phytochemicals and Plant Taxa with Activity as Microbial Biofilm and Quorum Sensing Inhibitors. Molecules 2016, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, P.N.; Shylaja, M.; Babu, K.N. False cardamoms. Med. Aromat. Plants 2002, 30, 330–340. [Google Scholar]
- Husain, S.S.; Ali, M. Analysis of volatile oil of the fruits of Elettaria cardamomum (L.) Maton and its antimicrobial activity. World J. Pharm. Pharm. Sci. 2014, 3, 1798–1808. [Google Scholar]
- Savan, E.K.; Kucukbay, F.Z. Essential oil composition of Elettaria cardamomum Maton. J. Appl. Biol. Sci. 2013, 7, 42–45. [Google Scholar]
- Gilani, S.R.; Shahid, I.; Javed, M.; Mehmud, S.; Ahme, R. Antimicrobial activities and physico-chemical properties of the essential oil from Amomum subulatum. Int. J. Appl. Chem. 2006, 2, 81–86. [Google Scholar]
- Eyob, S.; Appelgren, M.; Rohloff, J.; Tsegaye, A.; Messele, G. Chemical composition of essential oils from fresh plant parts of Korarima (Aframomum corrorima) cultivated in the highland of southern Ethiopia. J. Essent. Oil Res. 2007, 19, 372–375. [Google Scholar] [CrossRef]
- Eyob, S.; Appelgren, M.; Rohloff, J.; Tsegaye, A.; Messele, G. Traditional medicinal uses and essential oil composition of leaves and rhizomes of korarima (Aframomum corrorima (Braun) P.C.M. Jansen) from southern Ethiopia. S. Afr. J. Bot. 2008, 74, 181–185. [Google Scholar] [CrossRef]
- Kumar, G.; Chauhan, B.; Ali, M. Essential oil Composition of the Fruits of Amomum subulatum Roxb. Am. J. PharmTech Res. 2012, 2, 626–632. [Google Scholar]
- Kaskoos, R.A.; Ali, M.; Kapoor, R.; Akhtar, M.M.S.; Mir, S.R. Essential oil composition of the fruits of Eletteria cardamomum. J. Essent. Oil Bear. Plants 2006, 9, 81–84. [Google Scholar] [CrossRef]
- Kumar, A.; Tandon, S.; Ahmad, J.; Yadav, A.; Kahol, A.P. Essential oil composition of seed and fruit coat of Elettaria cardamomum from South India. J. Essent. Oil Bear. Plants 2005, 8, 204–207. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Gonzalez-Cervera, T.; Guette-Fernandez, J.; Jaramillo-Colorado, B.; Stashenko, E. Chemical composition and antioxidant activity of essential oils isolated from Colombian plants. Rev. Bras. Farmacog. 2010, 20, 568–574. [Google Scholar] [CrossRef]
- Kaskoos, R.A.; Mir, S.R.; Kapoor, R.; Ali, M. Essential oil composition of the fruits of Amomum subulatum Roxb. J. Essent. Oil Bear. Plants 2008, 11, 184–187. [Google Scholar] [CrossRef]
- Kaushik, P.; Goyal, P.; Chauhan, A.; Chauhan, G. In Vitro evaluation of antimicrobial potential of dry fruit extracts of Elettaria cardamomum Maton (Chhot Elaichi). Iran J. Pharm. Res. 2010, 9, 287–292. [Google Scholar] [PubMed]
- Snoussi, M.; Noumi, E.; Dehmani, A.; Flamini, G.; Aouni, M.; Al-sieni, M.; Al-sieni, A. Chemical Composition and Antimicrobial Activities of Elettaria cardamomum L. (Manton) Essential Oil: A High Activity against a Wide Range of Food Borne and Medically Important Bacteria and Fungi. J. Chem. Biol. Phys. Sci. 2015, 6, 248–259. [Google Scholar]
- Jaramillo-Colorado, B.; Olivero-Verbel, J.; Stashenko, E.E.; Wagner-Döbler, I.; Kunze, B. Anti-quorum sensing activity of essential oils from Colombian plants. Nat. Prod. Res. 2012, 26, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.T.; Lou, Z.X.; Yu, F.H.; Wang, P.; Wang, H.X. Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko (Amommum tsao-ko Crevost et Lemarie) on foodborne pathogens. Saudi J. Biol. Sci. 2017, 24, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Leela, N.K.; Prasath, D.; Venugopal, M.N. Essential oil composition of selected cardamom genotypes at different maturity levels. Indian J. Hortic. 2008, 65, 366–369. [Google Scholar]
- Hymete, A.; Rohloff, J.; Iversen, T.-H. Essential oil from seeds and husks of Aframomum corrorima from Ethiopia. Flavour Fragr. J. 2006, 21, 642–644. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kürkçüoglu, M. The Essential Oils of Aframomum corrorima (Braun) Jansen and A. angustifolium K. Schum. from Africa. J. Essent. Oil Res. 2001, 13, 208–209. [Google Scholar] [CrossRef]
- Joshi, R.; Sharma, P.; Sharma, V.; Prasad, R.; Sud, R.K.; Gulati, A. Analysis of the essential oil of large cardamom (Amomum subulatum Roxb.) growing in different agro-climatic zones of Himachal Pradesh, India. J. Sci. Food Agric. 2013, 93, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Grădinaru, A.C.; Aprotosoaie, A.C.; Trifan, A.; Șpac, A.; Brebu, M.; Miron, A. Interactions between cardamom essential oil and conventional antibiotics against Staphylococcus aureus clinical isolates. Farmacia 2014, 62, 1214–1222. [Google Scholar]
- Teneva, D.; Denkova, Z.; Goranov, B.; Denkova, R.; Kostov, G.; Atanasova, T.; Merdzhanov, P. Chemical composition and antimicrobial activity of essential oils from black pepper, cumin, coriander and cardamom against some pathogenic microorganisms. Acta Univ. Cibiniensis Ser. E 2016, 2, 39–52. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Karbancioglu-Guler, F. Cardamom, cumin, and dill weed essential oils: Chemical compositions, antimicrobial activities, and mechanisms of action against Campylobacter spp. Molecules 2017, 22, 1191. [Google Scholar] [CrossRef] [PubMed]
- Satyal, P.; Dosoky, N.S.; Kincer, B.L.; Setzer, W.N. Chemical compositions and biological activities of Amomum subulatum essential oils from Nepal. Nat. Prod. Commun. 2012, 7, 1233–1236. [Google Scholar] [PubMed]
- Naveed, R.; Hussain, I.; Tawab, A.; Tariq, M.; Rahman, M.; Hameed, S.; Mahmood, M.S.; Siddique, A.B.; Iqbal, M. Antimicrobial activity of the bioactive components of essential oils from Pakistani spices against Salmonella and other multi-drug resistant bacteria. BMC Complement. Altern. Med. 2013, 13, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Wakode, S. Antimicrobial activity of essential oil and various extracts of fruits of greater cardamom. Indian J. Pharm. Sci. 2010, 72, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Karaman, I.; Sahin, F.; Gulluce, M.; Ogutcu, H.; Sengul, M.; Adiguzel, A. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J. Ethnopharmacol. 2003, 85, 231–235. [Google Scholar] [CrossRef]
- Al-Haidari, R.A.; Shaaban, M.I.; Ibrahim, S.R.M.; Mohamed, G.A. Anti-quorum sensing activity of some medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 67–71. [Google Scholar] [PubMed]
- Buchbauer, G. Biological activities of essential oils. In Handbook of Essential Oils: Science, Technology, and Applications; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 235–280. [Google Scholar]
- Choi, H.; Sowndhararajan, K.; Cho, N.; Hwang, K.; Koo, S.; Kim, S. Evaluation of Herbicidal Potential of Essential Oils and their components under in vitro and green house experiments. Weed Turf. Sci. 2015, 4, 321–329. [Google Scholar] [CrossRef]
- De Martino, L.; Mancini, E.; Rolim de Almeida, L.F.; De Feo, V. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2004; Volume 1, pp. 217–218.
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicone and Carbowax 20M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Goodner, K.L. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavor and fragrance compounds. LWT Food Sci. Technol. 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Wiley Registry of Mass Spectral Data, with NIST Spectral Data CD Rom, 7th ed.; John Wiley & Sons: New York, NY, USA, 1998.
- Snoussi, M.; Hajlaoui, H.; Noumi, E.; Usai, D.; Sechi, L.A.; Zanetti, S.; Bakhrouf, A. In vitro anti-Vibrio spp. activity and chemical composition of some Tunisian aromatic plants. World J. Microbiol. Biotechnol. 2008, 24, 3071–3076. [Google Scholar] [CrossRef]
- Snoussi, M.; Noumi, E.; Trabelsi, N.; Flamini, G.; Papetti, A.; de Feo, V. Mentha spicata essential oil: Chemical composition, antioxidant and antibacterial activities against planktonic and biofilm cultures of Vibrio spp. strains. Molecules 2015, 20, 14402–14424. [Google Scholar] [CrossRef] [PubMed]
- Gulluce, M.; Sahin, F.; Sokmen, M.; Ozer, H.; Daferera, D.; Sokmen, A.; Polissiou, M.; Adiguzel, A.; Ozkan, H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem. 2007, 103, 1449–1456. [Google Scholar] [CrossRef]
- Snuossi, M.; Trabelsi, N.; Taleb, S.-B.; Dehmeni, A.; Flamini, G.; De Feo, V. Laurus nobilis, Zingiber officinale and Anethum graveolens Essential Oils: Composition, Antioxidant and Antibacterial Activities against Bacteria Isolated from Fish and Shellfish. Molecules 2016, 21, 1414. [Google Scholar] [CrossRef] [PubMed]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.D. Anti-quorum sensing potential of Adenanthera pavonina. Pharmacogn. Res. 2015, 7, 105–109. [Google Scholar]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.D. Anti-quorum sensing activity of flavonoid-rich fraction from Centella asiatica L. against Pseudomonas aeruginosa PAO1. J. Microbiol. Immunol. Infect. 2016, 49, 8–15. [Google Scholar] [CrossRef] [PubMed]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.R.; Rohlf, F.J. Biometry, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 2012. [Google Scholar]
- Bewley, D.; Black, M. Seeds: Physiology of Development and Germination; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
Sample Availability: Samples of the essential oils are available from the authors. |
| Constituents | Ki * | Ki ** | Essential Oils (%) | ||
|---|---|---|---|---|---|
| E. cardamomum | A. corriroma | A. subulatum | |||
| α-thujene | 933 | 1035 | 0.1 ± 0.00 | 0.5 ± 0.1 | - |
| α-pinene | 941 | 1032 | 0.7 ± 0.1 | 1.4 ± 0.2 | 1.3 ± 0.2 |
| sabinene | 977 | 1132 | 1.5 ± 0.2 | 1.2 ± 0.2 | - |
| β-pinene | 982 | 1118 | 0.2 ± 0.1 | 4.6 ± 0.3 | 0.7 ± 0.1 |
| 6-methyl-5-hepten-2-one | 987 | 1319 | - | - | 0.2 ± 0.1 |
| myrcene | 993 | 1174 | 1.2 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.1 |
| α-phellandrene | 1006 | 1176 | - | 0.1 ± 0.0 | 1.6 ± 0.1 |
| α-terpinene | 1020 | 1188 | - | 0.4 ± 0.1 | - |
| p-cymene | 1028 | 1280 | 1.1 ± 0.2 | 4.5 ± 0.3 | 2.6 ± 0.3 |
| limonene | 1032 | 1203 | 2.5 ± 0.2 | 5.4 ± 0.4 | 2.4 ± 0.5 |
| 1.8-cineole | 1034 | 1213 | 55.4 ± 1.5 | 51.8 ± 1.3 | 41.7 ± 1.6 |
| γ-terpinene | 1063 | 1255 | 0.1 ± 0.1 | 0.9 ± 0.1 | 0.3 ± 0.1 |
| cis-sabinene hydrate | 1070 | 1556 | - | 0.1 ± 0.1 | - |
| cis-linalool oxide (furanoid) | 1076 | 1450 | 0.1 ± 0.0 | - | - |
| terpinolene | 1090 | 1265 | 0.2 ± 0.1 | 0.4 ± 0.1 | - |
| 2-hexyl furan | 1094 | - | - | 0.3 ± 0.1 | |
| linalool | 1101 | 1553 | 0.9 ± 0.1 | 0.6 ± 0.1 | 3.0 ± 0.2 |
| cis-p-menth-2-en-1-ol | 1123 | 1230 | 0.1 ± 0.0 | 0.3 ± 0.1 | - |
| trans-p-menth-2-en-1-ol | 1142 | - | 0.4 ± 0.1 | - | |
| sabina ketone | 1158 | 1652 | - | 0.1 ± 0. | - |
| δ-terpineol | 1172 | - | 0.2±0.0 | 0.6 ± 0.1 | |
| 4-terpineol | 1179 | 1611 | 3.3 ± 0.3 | 10.4 ± 0.8 | 1.7 ± 0.2 |
| p-cymen-8-ol | 1185 | 1864 | - | 0.1 ± 0.1 | - |
| α-terpineol | 1191 | 1706 | 3.1 ± 0.3 | 4.9 ± 0.5 | 5.4 ± 0.4 |
| trans-piperitol | 1206 | - | 0.1 ± 0.1 | - | |
| neral | 1242 | 1656 | - | - | 0.9 ± 0.2 |
| carvone | 1244 | 1752 | - | 0.2 ± 0.1 | - |
| geraniol | 1256 | 1857 | - | 1.9 ± 0.3 | 12.5 ± 0.8 |
| linalyl acetate | 1259 | 1565 | 0.4 ± 0.1 | - | - |
| (E)-2-decenal | 1263 | - | - | 0.7 ± 0.1 | |
| geranial | 1271 | 1267 | - | 0.2 ± 0.0 | 3.1 ± 0.1 |
| 2-phenyl-2-butenal | 1285 | - | - | 2.9 ± 0.1 | |
| Thymol | 1292 | 2198 | - | - | 0.3 ± 0.1 |
| cis-2.3-pinanediol | 1315 | - | - | 0.7 ± 0.1 | |
| methyl geranate | 1325 | 0.2 ± 0.0 | - | - | |
| myrtenyl acetate | 1327 | 1698 | - | 0.1 ± 0.0 | - |
| exo-2-hydroxycineol acetate | 1345 | - | 0.3 ± 0.1 | - | |
| α-terpinyl acetate | 1352 | 1709 | 28.6 ± 1.1 | 3.6 ± 0.5 | - |
| α-copaene | 1377 | 1497 | - | 0.1 ± 0.0 | - |
| geranyl acetate | 1385 | 1765 | 0.2 ± 0.1 | 1.2 ± 0.3 | 6.0 ± 0.7 |
| (E)-2-decenyl acetate | 1407 | - | - | 1.1 ± 0.1 | |
| β-caryophyllene | 1419 | 1612 | - | 0.4±0.1 | |
| γ-muurolene | 1478 | 1704 | - | - | 0.3 ± 0.1 |
| δ-cadinene | 1524 | 1773 | - | - | 0.3 ± 0.1 |
| elemol | 1550 | - | - | 0.2 ± 0.0 | |
| (E)-nerolidol | 1565 | 2050 | - | 1.3±0.2 | 1.1 ± 0.1 |
| caryophyllene oxide | 1582 | 2008 | - | 0.3±0.1 | - |
| Oxygenated monoterpenes | 63.0 ± 0.3 | 71.4 ± 0.3 | 51.0 ± 0.4 | ||
| Monoterpene hydrocarbons | 36.9 ± 0.2 | 24.7 ± 0.2 | 34.2 ± 0.3 | ||
| Sesquiterpene hydrocarbons | 0.0 | 0.5 ± 0.1 | 0.6 ± 0.1 | ||
| Oxygenated sesquiterpenes | 0.0 | 1.6 ± 0.2 | 1.3 ± 0.1 | ||
| Others | 0.0 | 0.0 | 5.2 ± 0.1 | ||
| Total identified | 99.9 | 98.2 | 92.3 | ||
| Microorganisms | Green cardamom | Ethiopian cardamom | Black cardamom | Amphotericin B (10 mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ZI * ± SD ** | MIC *** | MFC **** | ZI * ±SD ** | MIC *** | MFC **** | ZI ± SD | MIC | MFC | ||
| Candida tropicalis 06–085 | 15.33 ± 0.57 i | 0.048 | 6.25 | 34.33 ± 0.57 a | 0.048 | 0.19 | 6.00 ± 0.00 l | 0.048 | 3.125 | - |
| Candida parapsilosis ATCC 22019 | 21.67 ± 0.57 cd | 0.048 | 6.25 | 24.33 ± 0.57 f | 0.19 | 0.78 | 6.00 ± 0.00 l | 0.097 | 6.25 | 10.33 ± 0.57 |
| Candida krusei ATCC 6258 | 14.33 ± 0.57 ij | 0.048 | 12.5 | 15.33 ± 0.57 jkl | 0.097 | 1.75 | 6.00 ± 0.00 l | 0.048 | 6.25 | 12.00 ± 0.00 |
| Candida glabrata ATCC 900030 | 16.33 ± 0.57 h | 0.097 | 12.5 | 31.00 ± 1.00 bc | 0.097 | 078 | 40.33 ± 0.57 b | 0.048 | 6.25 | 14.33 ± 0.57 |
| Candida guilliermondi 06–018 | 16.67 ± 0.57 gh | 0.048 | 6.25 | 30.33 ± 0.57 cd | 0.097 | 0.78 | 41.00 ± 0.00b | 0.097 | 3.125 | - |
| Candida albicans ATCC 2019 | 15.33 ± 0.57 i | 0.097 | 6.25 | 12.67 ± 0.57 nop | 0.097 | 0.39 | 12.00 ± 0.00 h | 0.048 | 3.125 | 14.66 ± 0.57 |
| Saccharomyces cerevisiae 11–161 | 18.67 ± 0.57 f | 0.097 | 6.25 | 21.67 ± 0.57 g | 0.097 | 0.39 | 40.33 ± 0.57 b | 0.048 | 6.25 | - |
| Microorganisms | Green cardamom | Ethiopian cardamom | Black cardamom | Ampicillin (10 mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ZI * ± SD ** | MIC *** | MBC **** | ZI * ± SD ** | MIC *** | MBC **** | ZI * ± SD ** | MIC *** | MBC **** | ||
| Aerococcus viridans | 20.33 ± 0.57 e | 0.048 | 6.25 | 25.67 ± 0.57 e | 0.048 | 0.39 | 35.00 ± 0.00 c | 0.048 | 3.125 | 14.67 ± 0.57 |
| Bacillus cereus | 11.67 ± 0.57 n | 0.048 | 6.25 | 16.33 ± 0.57 ij | 0.048 | 3.125 | 35.67 ± 0.57 c | 0.048 | 1.56 | - |
| Bacillus subtilis ATCC 6633 | 12.67 ± 0.57 klmn | 0.048 | 12.5 | 14.67 ± 0.57 klm | 0.048 | 3.125 | 7.00 ± 0.00 k | 0.048 | 0.78 | 11.33 ± 0.57 |
| Enterococcus faecalis ATCC 29212 | 21.00 ± 1.00 de | 0.048 | 6.25 | 12.00 ± 0.00 opq | 0.048 | 12.5 | 13.67 ± 0.57 g | 0.048 | 6.25 | 13.67 ± 0.57 |
| Listeria monocytogenes ATCC 19115 | 17.33 ± 0.57 g | 0.048 | 12.5 | 14.33 ± 0.57 lm | 0.19 | 6.25 | 6.00 ± 0.00 l | 0.048 | 1.56 | - |
| Micrococcus luteus NCIMB 8166 | 41.33 ± 1.15 a | 0.048 | 6.25 | 29.33 ± 0.57 d | 0.048 | 6.25 | 7.67 ± 0.57 jk | 0.048 | 3.125 | 30.33 ± 0.57 |
| Staphylococcus aureus MR (B2) | 13.67 ± 0.57 jk | 0.048 | 12.5 | 17.00 ± 1.00 i | 0.048 | 12.5 | 6.00 ± 0.00 l | 0.048 | 6.25 | 16.33 ± 0.57 |
| Staphylococcus aureus ATCC 6816 | 32.67 ± 0.57 b | 0.048 | 6.25 | 32.00 ± 0.00 b | 0.097 | 12.5 | 19.67 ± 0.57 f | 0.048 | 3.125 | 24.33 ± 0.57 |
| Staphylococcus epidermidis ATCC 12228 | 11.67 ± 0.57 n | 0.048 | 6.25 | 13.67 ± 0.57 mn | 0.097 | 6.25 | 43.00 ± 1.00 a | 0.097 | 1.56 | 12.33 ± 0.57 |
| Escherichia coli | 12.00 ± 0.00 mn | 0.097 | 6.25 | 13.00 ± 1.00 no | 0.048 | 3.125 | 6.00 ± 0.00 l | 0.048 | 1.56 | 27.33 ± 0.57 |
| Escherichia coli ATCC 25922 | 15.33 ± 0.57 i | 0.048 | 12.5 | 15.33 ± 0.57 jkl | 0.048 | 12.5 | 6.00 ± 0.00 l | 0.048 | 0.78 | 11.67 ± 0.57 |
| Klebsiella pneumoniae | 10.33 ± 0.57 o | 0.048 | 12.5 | 12.00 ± 0.00 opq | 0.097 | 3.125 | 8.33 ± 0.57 j | 0.097 | 6.25 | 17.33 ± 0.57 |
| Pseudomonas aeruginosa ATCC 27853 | 6.00 ± 0.00 r | 0.048 | 12.5 | 11.33 ± 0.57 q | 0.048 | 3.125 | 10.33 ± 0.57 i | 0.048 | 3.125 | 22.67 ± 0.57 |
| Proteus mirabils | 8.00 ± 0.00 p | 0.048 | 25 | 11.67 ± 0.57 pq | 0.048 | 6.25 | 13.67 ± 0.57 g | 0.048 | 12.5 | 25.67 ± 0.57 |
| Shewanella putrefaciens | 13.00 ± 0.00 klm | 0.097 | 12.5 | 15.33 ± 0.57 jkl | 0.19 | 6.25 | 30.33 ± 0.57 d | 0.048 | 3.125 | 7.00 ± 0.00 |
| Salmonella typhimirium ATCC 14028 | 10.00 ± 0.00 o | 0.048 | 12.5 | 9.33 ± 0.57 r | 0.048 | 3.125 | 7.67 ± 0.57 jk | 0.048 | 0.78 | 17.67 ± 1.15 |
| Shigella flexenerii ATCC 12022 | 22.33 ± 0.57 c | 0.048 | 6.25 | 19.67 ± 0.57 h | 0.048 | 6.25 | 14.33 ± 0.57 g | 0.048 | 0.39 | 10.67 ± 0.57 |
| Vibrio alginolyticus ATCC 17749 | 20.67 ± 0.57 de | 0.048 | 3.125 | 16.33 ± 0.57 ij | 0.097 | 25 | 7.00 ± 0.00 k | 0.048 | 1.56 | - |
| Vibrio alginolyticus ATCC 33787 | 12.33 ± 0.57 lmn | 0.048 | 12.5 | 15.33 ± 0.57 jkl | 0.097 | 25 | 26.67 ± 0.57 e | 0.048 | 6.25 | 13.33 ± 0.57 |
| Vibrio cholerae ATCC 9459 | 7.00 ± 0.00 q | 0.097 | 12.5 | 14.33 ± 0.57 lm | 0.048 | 25 | 6.00 ± 0.00 l | 0.048 | 12.5 | 7.00 ± 0.00 |
| Vibrio parahaemolyticus ATCC 17802 | 20.67 ± 0.57 de | 0.097 | 12.5 | 14.33 ± 0.57 lm | 0.048 | 12.5 | 7.00 ± 0.00 k | 0.048 | 1.56 | 13.33 ± 0.57 |
| Vibrio parahaemolyticus ATCC 43996 | 21.67 ± 0.57 cd | 0.048 | 12.5 | 14.33 ± 0.57 lm | 0.048 | 25 | 6.00 ± 0.00 l | 0.048 | 6.25 | 12.00 ± 0.00 |
| Vibrio vulnificus ATCC 27562 | 13.00 ± 0.00 klm | 0.048 | 12.5 | 15.67 ± 0.57 jk | 0.097 | 12.5 | 29.67 ± 0.57 d | 0.097 | 6.25 | 30.33 ± 0.57 |
| Vibrio vulnificus ATCC 33149 | 13.33 ± 0.57 o | 0.048 | 12.5 | 13.00 ± 1.00 no | 0.048 | 6.25 | 6.00 ± 0.00 l | 0.048 | 6.25 | 12.33 ± 0.57 |
| Serratia marcescens | 14.33 ± 0.57 ij | 0.048 | 12.5 | 12.33 ± 0.57 opq | 0.048 | 3.125 | 8.33 ± 0.57 j | 0.048 | 3.125 | 13.67 ± 0.57 |
| Concentration (µg/mL) | Essential Oils | Main Component 1,8-Cineole | ||
|---|---|---|---|---|
| Green cardamom | Black cardamom | Ethiopian cardamom | ||
| M ± SD * | M ± SD * | M ± SD * | M ± SD * | |
| Control | 54.00 ± 1.00 | 54.00 ± 1.00 | 54.00 ± 1.00 | 54.00 ± 1.00 |
| 10 | 22.00 ± 1.00 | 52.33 ± 0.58 | 53.76 ± 0.58 | 17.33 ± 1.15 |
| 50 | 17.67 ± 0.58 | 50.67 ± 1.15 | 53.00 ± 0.00 | 15.00 ± 0.00 |
| 100 | 13.67 ± 0.58 | 46.76 ± 1.15 | 32.67 ± 0.58 | 13.67 ± 0.58 |
| 200 | 11.13 ± 1.00 | 35.76 ± 0.58 | 13.33 ± 1.15 | 12.33 ± 0.58 |
| 400 | 8.33 ± 0.58 | 33.00 ± 2.65 | 8.33 ± 0.58 | 10.33 ± 0.58 |
| Raphanus sativus Germinated Seeds | |||
| Doses (µg/mL) | A. corriroma | A. subulatum | E. cardamomum |
| Control | 5.70 ± 1.50 | 5.00 ± 1.70 | 7.30 ± 1.20 |
| 0.062 | 7.30 ± 1.20 | 4.70 ± 1.20 | 4.00 ± 0.00 |
| 0.125 | 5.30 ± 2.30 | 7.00 ± 1.00 | 4.70 ± 2.50 |
| 0.250 | 4.33 ± 2.08 | 6.00 ± 1.00 | 5.67 ± 1.53 |
| 0.625 | 5.00 ± 1.00 * | 6.33 ± 2.08 | 5.33 ± 0.58 |
| 1.25 | 5.00 ± 1.00 * | 5.67 ± 1.53 | 5.67 ± 2.89 |
| 2.5 | 3.67 ± 0.58 ** | 5.00 ± 1.73 | 6.67 ± 2.52 |
| Lactuca sativa Germinated Seeds | |||
| Doses (µg/mL) | A. corriroma | A. subulatum | E. cardamomum |
| Control | 8.70 ± 0.60 | 9.30 ± 1.20 | 6.70 ± 0.60 |
| 0.062 | 8.30 ± 0.60 | 8.70 ± 1.50 | 8.00 ± 1.00 |
| 0.125 | 8.70 ± 0.60 | 8.70 ± 0.60 | 9.70 ± 0.60 |
| 0.250 | 8.00 ± 1.00 | 8.70 ± 1.50 | 9.00 ± 1.00 |
| 0.625 | 9.00 ± 1.00 | 8.70 ± 1.20 | 8.30 ± 2.10 |
| 1.25 | 9.00 ± 0.00 | 8.70 ± 1.50 | 8.30 ± 0.60 |
| 2.5 | 8.00 ± 1.00 | 8.30 ± 1.50 | 8.70 ± 0.60 |
| Lepidium sativum Germinated Seeds | |||
| Doses (µg/mL) | A. corriroma | A. subulatum | E. cardamomum |
| Control | 3.30 ± 0.60 | 6.3 ± 1.50 | 4.30 ± 1.20 |
| 0.062 | 4.70 ± 2.50 | 6.00 ± 1.70 | 5.30 ± 1.20 |
| 0.125 | 2.30 ± 1.50 | 5.30 ± 1.50 | 6.00 ± 2.60 |
| 0.250 | 3.67 ± 2.50 | 4.67 ± 0.60 | 6.00 ± 2.00 |
| 0.625 | 2.30 ± 0.60 | 6.70 ± 1.20 | 4.00 ± 3.50 |
| 1.25 | 4.00 ± 2.00 | 3.70 ± 2.10 | 3.30 ± 2.10 |
| 2.5 | 3.30 ± 1.50 | 4.70 ± 2.30 | 4.00 ± 1.70 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noumi, E.; Snoussi, M.; Alreshidi, M.M.; Rekha, P.-D.; Saptami, K.; Caputo, L.; De Martino, L.; Souza, L.F.; Msaada, K.; Mancini, E.; et al. Chemical and Biological Evaluation of Essential Oils from Cardamom Species. Molecules 2018, 23, 2818. https://doi.org/10.3390/molecules23112818
Noumi E, Snoussi M, Alreshidi MM, Rekha P-D, Saptami K, Caputo L, De Martino L, Souza LF, Msaada K, Mancini E, et al. Chemical and Biological Evaluation of Essential Oils from Cardamom Species. Molecules. 2018; 23(11):2818. https://doi.org/10.3390/molecules23112818
Chicago/Turabian StyleNoumi, Emira, Mejdi Snoussi, Mousa M. Alreshidi, Punchappady-Devasya Rekha, Kanekar Saptami, Lucia Caputo, Laura De Martino, Lucéia Fatima Souza, Kamel Msaada, Emilia Mancini, and et al. 2018. "Chemical and Biological Evaluation of Essential Oils from Cardamom Species" Molecules 23, no. 11: 2818. https://doi.org/10.3390/molecules23112818
APA StyleNoumi, E., Snoussi, M., Alreshidi, M. M., Rekha, P.-D., Saptami, K., Caputo, L., De Martino, L., Souza, L. F., Msaada, K., Mancini, E., Flamini, G., Al-sieni, A., & De Feo, V. (2018). Chemical and Biological Evaluation of Essential Oils from Cardamom Species. Molecules, 23(11), 2818. https://doi.org/10.3390/molecules23112818









