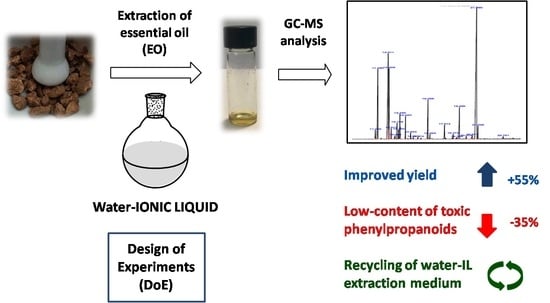
A Design of Experiment Approach for Ionic Liquid-Based Extraction of Toxic Components-Minimized Essential Oil from Myristica fragrans Houtt. Fruits †
Abstract
1. Introduction
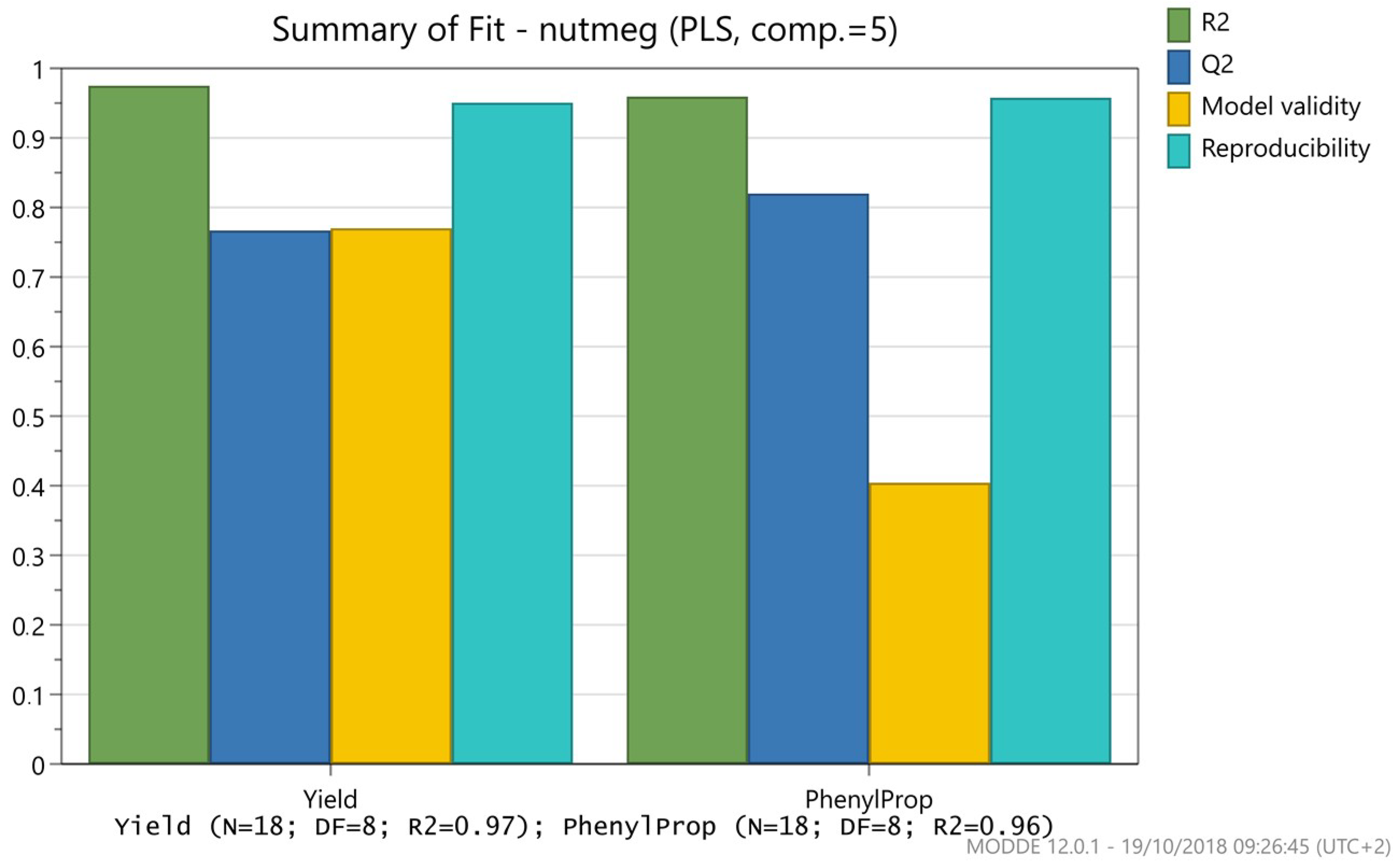
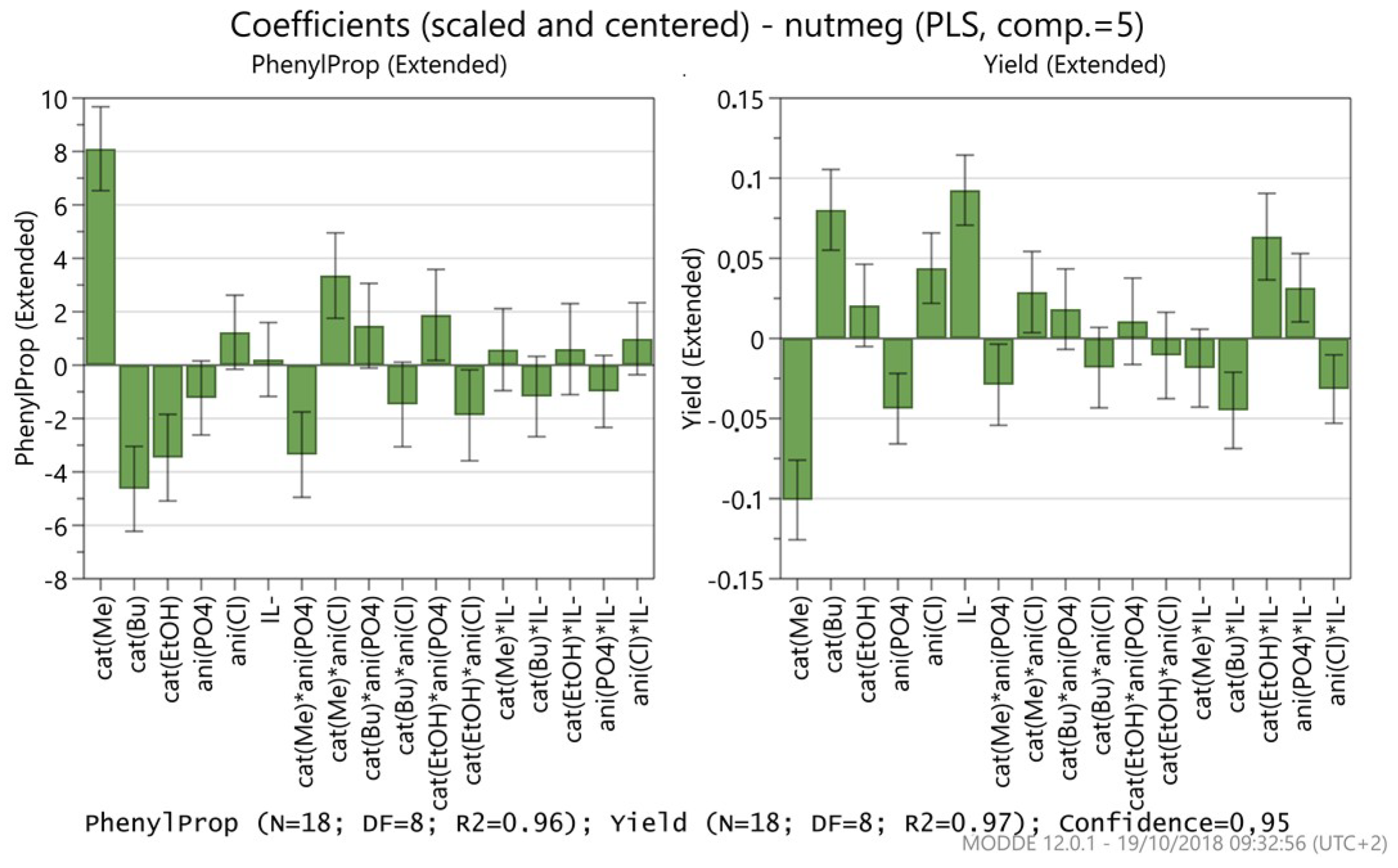
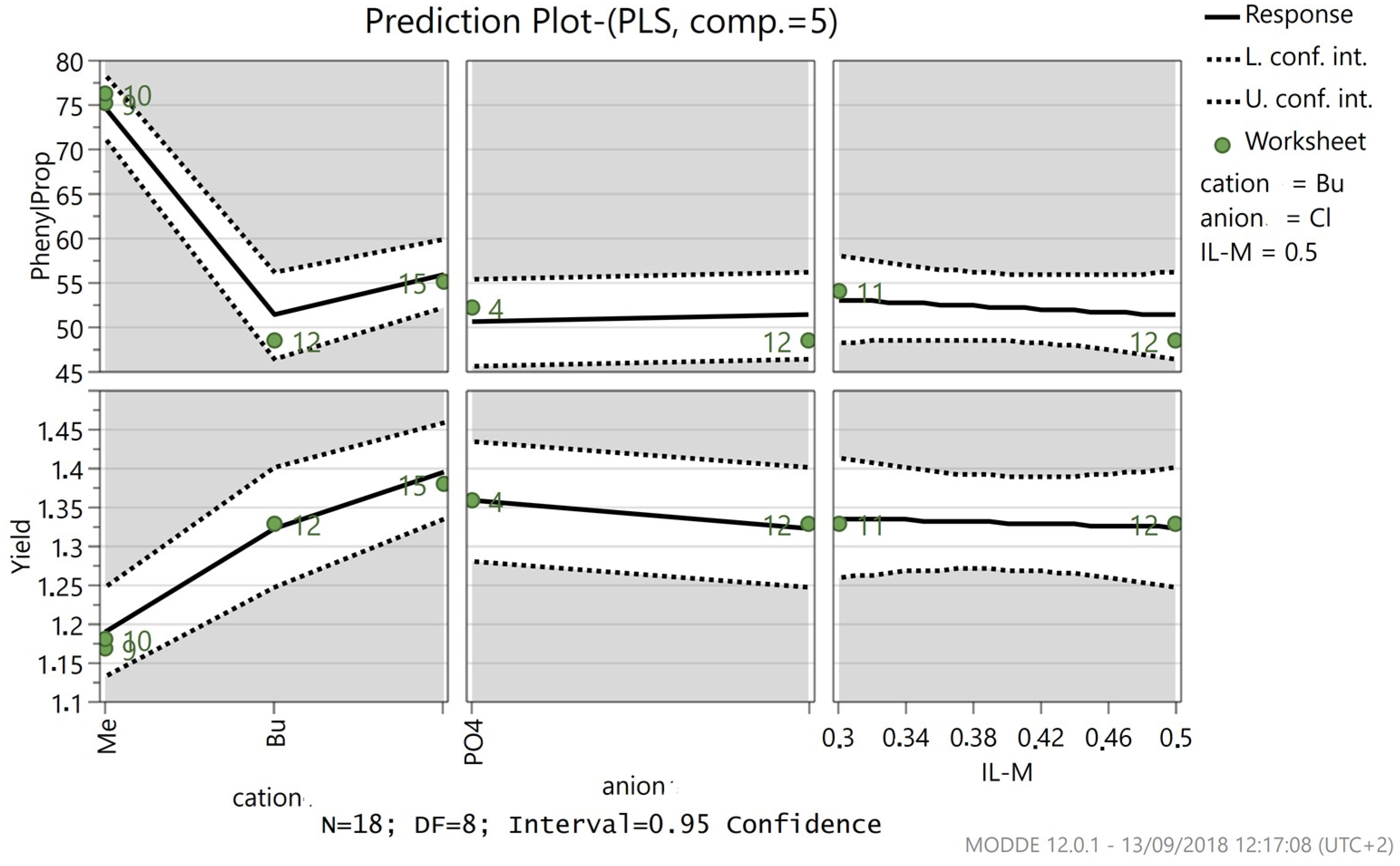
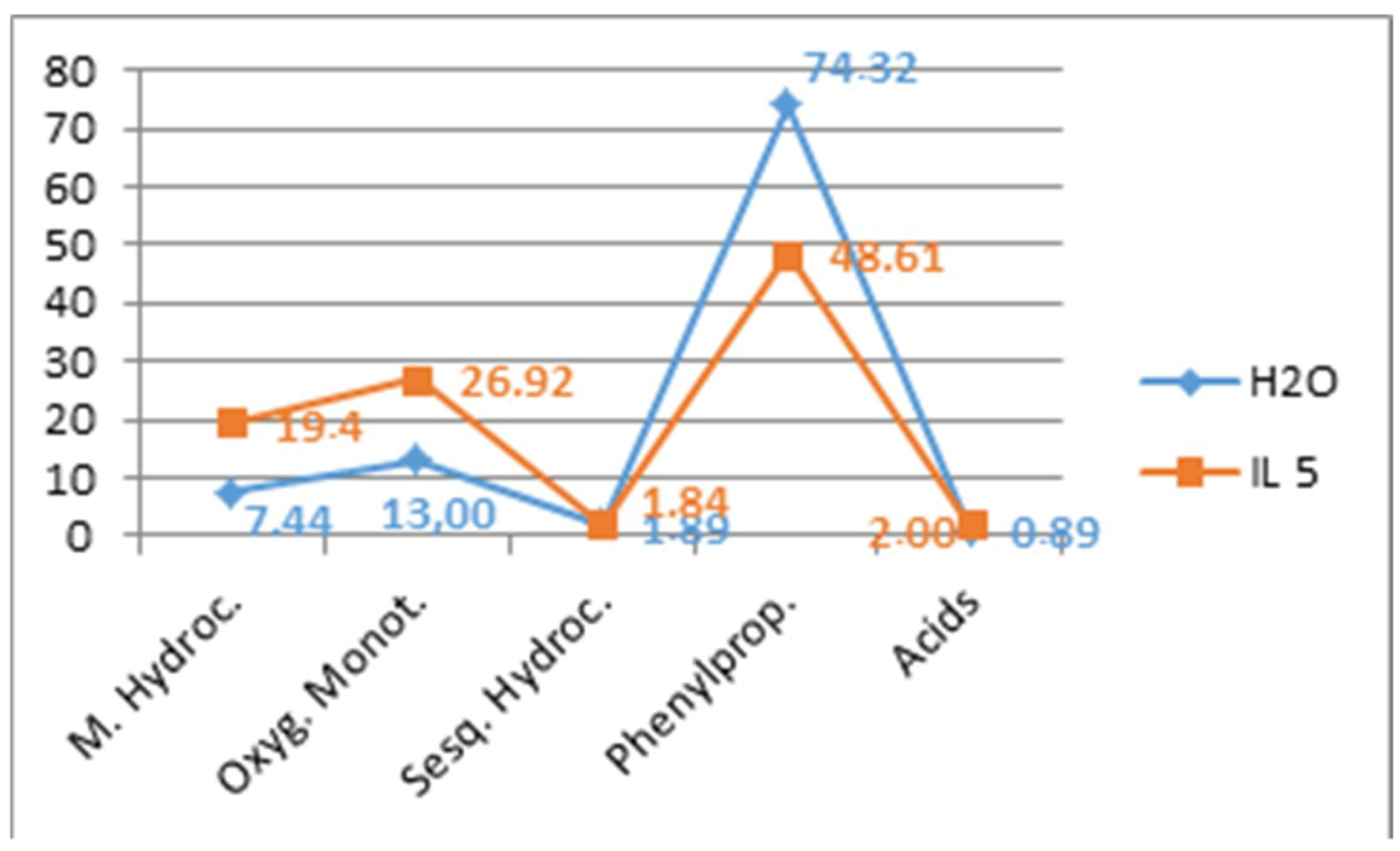
2. Results and Discussion
Optimization of Hydrodistillation Conditions
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Hydrodistillation
- -
- 250 mL of deionized water,
- -
- 250 mL of a 0.5 M NaCl solution,
- -
- 250 mL of a 0.3 M solution of selected ILs in water,
- -
- 250 mL of a 0.5 M solution of selected IL in water, and
- -
- 250 mL of a 0.4 M solution of selected IL in water.
3.4. Design of Experiment
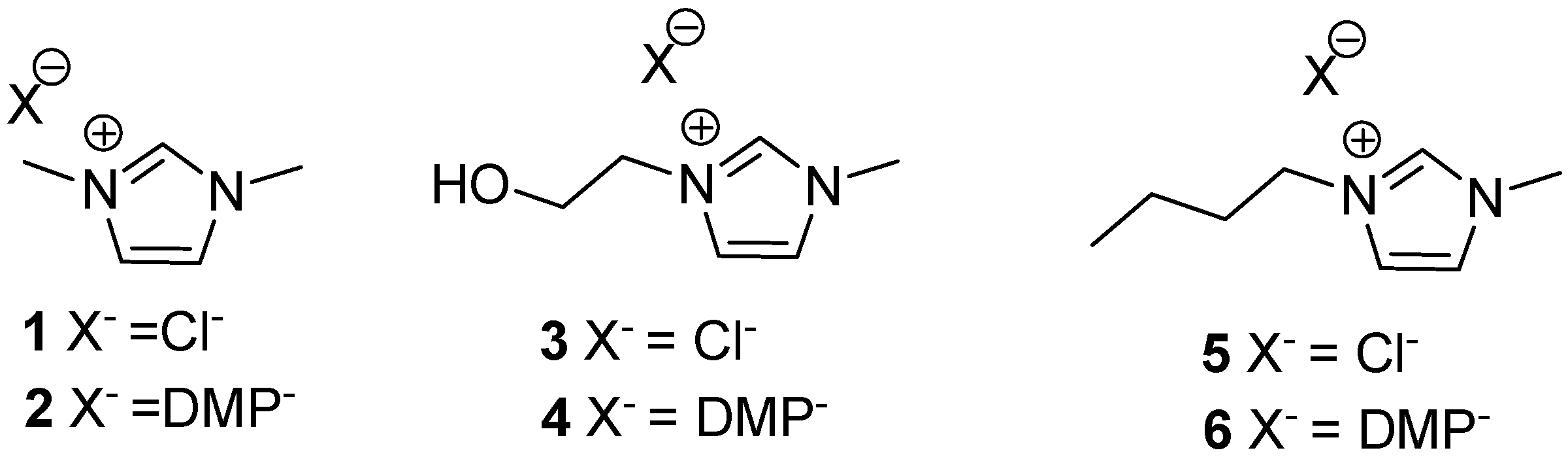
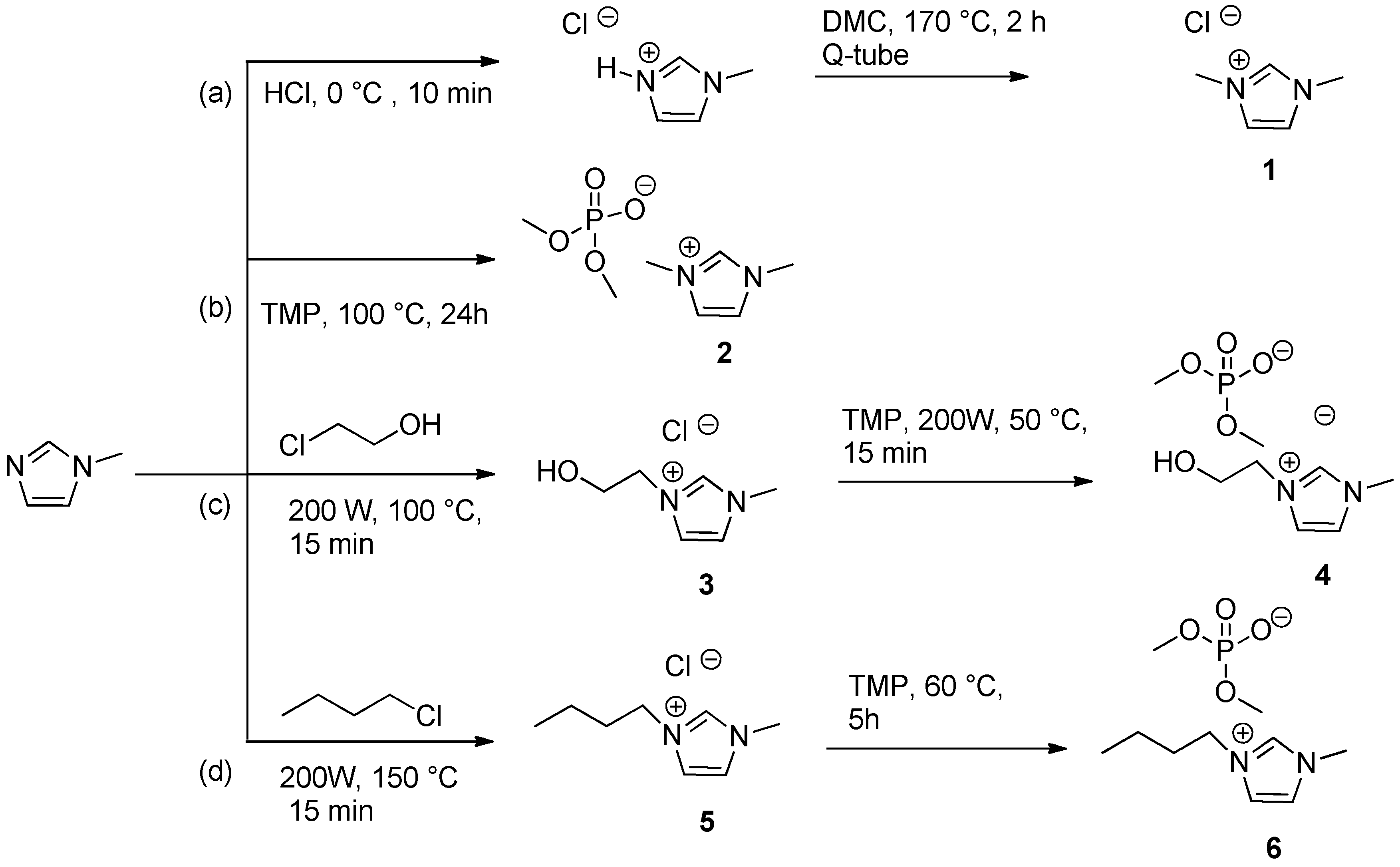
3.5. Synthesis of Ionic Liquids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parthasarathy, V.A.; Chempakam, B.; Zachariah, T.J. Chemistry of Spices; CABI: Wallingford, UK, 2008. [Google Scholar]
- Khalsa, K.P.S.; Tierra, M. The Way of Ayurvedic Herbs: The Most Complete Guide to Natural Healing and Health with Traditional Ayurvedic Herbalism; Lotus Press: Detroit, MI, USA, 2008. [Google Scholar]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef]
- Du, S.S.; Yang, K.; Wang, C.F.; You, C.X.; Geng, Z.F.; Guo, S.S.; Deng, Z.W.; Liu, Z.L. Chemical constituents and activities of the essential oil from Myristica fragrans against cigarette beetle Lasioderma serricorne. Chem. Biodivers. 2014, 11, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Piaru, S.P.; Mahmud, R.; Abdul Majid, A.M.; Ismail, S.; Man, C.N. Chemical composition, antioxidant and cytotoxicity activities of the essential oils of Myristica fragrans and Morinda citrifolia. J. Sci. Food Agric. 2012, 92, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Haq, R.U.; Ahmed, A.; Khan, R.A.; Raza, M. Anticonvulsant activities of nutmeg oil of Myristica fragrans. Phytother. Res. 2009, 23, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Piaru, S.P.; Mahmud, R.; Ismail, S. Studies on the Phytochemical Properties and Brine Shrimp Toxicity of Essential Oil Extracted from Myristica fragrans Houtt. (Nutmeg). J. Essent. Oil Bear. Plants 2012, 15, 53–57. [Google Scholar] [CrossRef]
- Nurjanah, S.; Putri, I.L.; Sugiarti, D.P. Antibacterial Activity of Nutmeg Oil. KnE Life Sci. 2017, 2, 563–569. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Dybowski, M.P. Determination of myristicin in commonly spices applying SPE/GC. Food Chem. Toxicol. 2012, 50, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Rosa, A.; Marongiu, B.; Atzeri, A.; Dessi, M.A.; Falconieri, D.; Porcedda, S. Extraction and separation of volatile and fixed oils from seeds of Myristica fragrans by supercritical CO2: Chemical composition and cytotoxic activity on Caco-2 cancer cells. J. Food Sci. 2012, 77, C448–C453. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, S.S.; Ibrahim, A.H.; Rahman, N.N.N.A.; Nama, M.M.B.; Majid, A.M.S.A.; Kadir, M.O.A. The Effect of Supercritical Fluid Extraction Parameters on the Nutmeg Oil Extraction and Its Cytotoxic and Antiangiogenic Properties. Proc. Food. Sci. 2011, 1, 1946–1952. [Google Scholar] [CrossRef]
- Machmudah, S.; Sulaswatty, A.; Sasaki, M.; Goto, M.; Hirose, T. Supercritical CO2 extraction of nutmeg oil: Experiments and modeling. J. Supercrit. Fluids 2006, 39, 30–39. [Google Scholar] [CrossRef]
- Abourashed, E.A.; El-Alfy, A.T. Chemical diversity and pharmacological significance of the secondary metabolites of nutmeg (Myristica fragrans Houtt.). Phytochem. Rev. 2016, 15, 1035–1056. [Google Scholar] [CrossRef] [PubMed]
- Morsy, N.F.S. A comparative study of nutmeg (Myristica fragrans Houtt.) oleoresins obtained by conventional and green extraction techniques. J. Food Sci. Technol. 2016, 53, 3770–3777. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Melai, B.; Pistelli, L.; Chiappe, C. How to make a green product greener: Use of ionic liquids as additives during essential oil hydrodistillation. RSC Adv. 2015, 5, 69894–69898. [Google Scholar] [CrossRef]
- Lago, S.; Rodríguez, H.; Arce, A.; Soto, A. Improved concentration of citrus essential oil by solvent extraction with acetate ionic liquids. Fluid Phase Equilib. 2014, 361, 37–44. [Google Scholar] [CrossRef]
- Bica, K.; Gaertner, P.; Rogers, R.D. Ionic liquids and fragrances–direct isolation of orange essential oil. Green Chem. 2011, 13, 1997–1999. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar] [CrossRef]
- Pistelli, L.; Giovanelli, S.; Margari, P.; Chiappe, C. Considerable effect of dimethylimidazolium dimethylphosphate in cinnamon essential oil extraction by hydrodistillation. RSC Adv. 2016, 6, 52421–52426. [Google Scholar] [CrossRef]
- Ma, C.-H.; Liu, T.-T.; Yang, L.; Zu, Y.-G.; Chen, X.; Zhang, L.; Zhang, Y.; Zhao, C. Ionic liquid-based microwave-assisted extraction of essential oil and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J. Chromatogr. A 2011, 1218, 8573–8580. [Google Scholar] [CrossRef] [PubMed]
- Froschauer, C.; Hummel, M.; Laus, G.; Schottenberger, H.; Sixta, H.; Weber, H.K.; Zuckerstatter, G. Dialkyl phosphate-related ionic liquids as selective solvents for xylan. Biomacromolecules 2012, 13, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, G.; Li, N. Ionic Liquid Solutions as a Green Tool for the Extraction and Isolation of Natural Products. Molecules 2018, 23, 1765. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.M.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Ranke, J.; Stolte, S.; Störmann, R.; Arning, J.; Jastorff, B. Design of Sustainable Chemical Products the Example of Ionic Liquids. Chem. Rev. 2007, 107, 2183–2206. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.; Gathergood, N. Biodegradation studies of ionic liquids. Chem. Soc. Rev. 2010, 39, 600–637. [Google Scholar] [CrossRef] [PubMed]
- Pflaum, T.; Hausler, T.; Baumung, C.; Ackermann, S.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Carcinogenic compounds in alcoholic beverages: An update. Arch. Toxicol. 2016, 90, 2349–2367. [Google Scholar] [CrossRef] [PubMed]
- Opinion of the Scientific Committee on Food on the Safety of the Presence of Safrole (1-allyl-3,4-methylene dioxy benzene) in Flavourings and Other Food Ingredients with Flavouring Properties; European Commission, Scientific Committee on Food: Brussel, Belgium, 2001.
- Al-Malahmeh Amer, J.; Alajlouni Abdalmajeed, M.; Ning, J.; Wesseling, S.; Vervoort, J.; Rietjens Ivonne, M.C.M. Determination and risk assessment of naturally occurring genotoxic and carcinogenic alkenylbenzenes in nutmeg-based plant food supplements. J. Appl. Toxicol. 2017, 37, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.A.; Andrade, L.N.; Batista Vieira de Sousa, E.; de Sousa, D.P. Antitumor Phenylpropanoids Found in Essential Oils. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Draman, S.F.S.; Salleh, M.S.N.; Yusof, N.B. Dissolution of cellulose in ionic liquid: A review. AIP Conf. Proc. 2017, 1809. [Google Scholar] [CrossRef]
- Al-Maaieh, A.; Flanagan, D.R. Salt effects on caffeine solubility, distribution, and self-association. J. Pharm. Sci. 2002, 91, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Holbrey, J.D.; Reichert, W.M.; Nieuwenhuyzen, M.; Sheppard, O.; Hardacre, C.; Rogers, R.D. Liquid clathrate formation in ionic liquid–aromatic mixtures. Chem. Commun. 2003, 4, 476–477. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 0-931710-42-1. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 1st ed.; Council of Europe: Strasbourg, France, 1997. [Google Scholar]
- Wenjun, X.; Xiaoxing, W.; Qin, C.; Tinghua, W.; Ying, W.; Lizong, D.; Chunshan, S. A Novel and Green Method for the Synthesis of Ionic Liquids Using the Corresponding Acidic Ionic Liquid Precursors and Dialkyl Carbonate. Chem. Lett. 2010, 39, 1112–1113. [Google Scholar]
- Gong, X.; Yan, X.; Li, T.; Wu, X.; Chen, W.; Huang, S.; Wu, Y.; Zhen, D.; He, G. Design of pendent imidazolium side chain with flexible ether-containing spacer for alkaline anion exchange membrane. J. Membr. Sci. 2017, 523, 216–224. [Google Scholar] [CrossRef]
- Erdmenger, T.; Vitz, J.; Wiesbrock, F.; Schubert, U.S. Influence of different branched alkyl side chains on the properties of imidazolium-based ionic liquids. J. Mater. Chem. 2008, 18, 5267–5273. [Google Scholar] [CrossRef]
- Brica, S.; Freimane, L.; Kulikovska, L.; Zicmanis, A. N,N′-Dialkylimidazolium Dimethyl Phosphates–Promising Media and Catalysts at the Same Time for Condensation Reactions. Chem. Sci. Int. J. 2017, 19, 2456-706X. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Exp No. | Run Order | Cation a | Anion b | IL c | IL-[M] d | Yield % e | Phenylpropanoids % f |
|---|---|---|---|---|---|---|---|
| 1 | 11 | [1,3-diMIM] | DMP | 2 | 0.3 | 0.87 | 63.57 |
| 2 | 18 | [1,3-diMIM] | DMP | 2 | 0.5 | 1.13 | 60.68 |
| 3 | 15 | [1-But-3-MIM] | DMP | 6 | 0.5 | 1.36 | 52.14 |
| 4 | 16 | [1-But-3-MIM] | DMP | 6 | 0.3 | 1.26 | 54.74 |
| 5 | 12 | [1,3-diMIM] | DMP | 2 | 0.3 | 0.87 | 64.07 |
| 6 | 3 | [1-EtOH-3-MIM] | DMP | 4 | 0.3 | 1.02 | 55.65 |
| 7 | 8 | [1-EtOH-3-MIM] | DMP | 4 | 0.5 | 1.40 | 56.38 |
| 8 | 7 | [1,3-diMIM] | Cl | 1 | 0.3 | 1.13 | 68.95 |
| 9 | 14 | [1,3-diMIM] | Cl | 1 | 0.5 | 1.17 | 75.23 |
| 10 | 5 | [1,3-diMIM] | Cl | 1 | 0.5 | 1.18 | 76.41 |
| 11 | 17 | [1-But-3-MIM] | Cl | 5 | 0.3 | 1.33 | 54.01 |
| 12 | 10 | [1-But-3-MIM] | Cl | 5 | 0.5 | 1.33 | 48.61 |
| 13 | 13 | [1-EtOH-3-MIM] | Cl | 3 | 0.3 | 1.12 | 50.90 |
| 14 | 9 | [1-EtOH-3-MIM] | Cl | 3 | 0.3 | 1.10 | 55.88 |
| 15 | 6 | [1-EtOH-3-MIM] | Cl | 3 | 0.5 | 1.38 | 55.16 |
| 16 | 4 | [1-EtOH-3-MIM] | Cl | 3 | 0.4 | 1.33 | 55.21 |
| 17 | 1 | [1-EtOH-3-MIM] | Cl | 3 | 0.4 | 1.27 | 55.18 |
| 18 | 2 | [1-EtOH-3-MIM] | Cl | 3 | 0.4 | 1.22 | 55.24 |
| Phenylpropanoids | DF | SS | MS (Variance) | F | p | SD |
| Total corrected | 17 | 1063.38 | 62.5519 | 7.90898 | ||
| Regression | 9 | 1020.05 | 113.339 | 20.9267 | 0.000 | 10.6461 |
| Residual | 8 | 43.3281 | 5.41601 | 2.32723 | ||
| Lack of Fit (Model error) | 3 | 30.1049 | 10.035 | 3.79445 | 0.093 | 3.1678 |
| Pure error (Replicate error) | 5 | 13.2232 | 2.64464 | 1.62623 | ||
| Yield | DF | SS | MS (Variance) | F | p | SD |
| Total corrected | 17 | 0.434362 | 0.0255507 | 0.159846 | ||
| Regression | 9 | 0.423497 | 0.0470552 | 34.6453 | 0.000 | 0.216922 |
| Residual | 8 | 0.0108656 | 0.0013582 | 0.0368538 | ||
| Lack of Fit (Model error) | 3 | 0.00454893 | 0.00151631 | 1.20025 | 0.399 | 0.0389398 |
| Pure error (Replicate error) | 5 | 0.00631667 | 0.00126333 | 0.0355434 |
| Exp No | Yield % | Phenylpropanoids % |
|---|---|---|
| 1 | 1.48 | 48.46 |
| 2 | 1.52 | 48.68 |
| 3 | 1.47 | 49.02 |
| 4 | 1.47 | 48.60 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanari, D.; Marcotullio, M.C.; Neri, A. A Design of Experiment Approach for Ionic Liquid-Based Extraction of Toxic Components-Minimized Essential Oil from Myristica fragrans Houtt. Fruits †. Molecules 2018, 23, 2817. https://doi.org/10.3390/molecules23112817
Lanari D, Marcotullio MC, Neri A. A Design of Experiment Approach for Ionic Liquid-Based Extraction of Toxic Components-Minimized Essential Oil from Myristica fragrans Houtt. Fruits †. Molecules. 2018; 23(11):2817. https://doi.org/10.3390/molecules23112817
Chicago/Turabian StyleLanari, Daniela, Maria Carla Marcotullio, and Andrea Neri. 2018. "A Design of Experiment Approach for Ionic Liquid-Based Extraction of Toxic Components-Minimized Essential Oil from Myristica fragrans Houtt. Fruits †" Molecules 23, no. 11: 2817. https://doi.org/10.3390/molecules23112817
APA StyleLanari, D., Marcotullio, M. C., & Neri, A. (2018). A Design of Experiment Approach for Ionic Liquid-Based Extraction of Toxic Components-Minimized Essential Oil from Myristica fragrans Houtt. Fruits †. Molecules, 23(11), 2817. https://doi.org/10.3390/molecules23112817