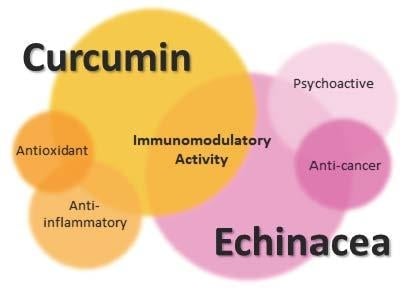
Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea
Abstract
1. Immune System and Immunomodulators
2. Phytochemical Research
3. Echinacea sp.
4. Curcuma Longa
5. Problematics and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yatim, K.M.; Lakkis, F.G. A brief journey through the immune system. Clin. J. Am. Soc. Nephrol. CJASN 2015, 10, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Quintin, J.; van der Meer, J.W.M. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Ueta, M.; Kinoshita, S. Innate immunity of the ocular surface. Brain Res. Bull. 2010, 81, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Pancer, Z.; Cooper, M.D. The evolution of adaptive immunity. Annu. Rev. Immunol. 2006, 24, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Bosma, A. Immune regulatory function of B cells. Annu. Rev. Immunol. 2012, 30, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Josefowicz, S.Z.; Lu, L.-F.; Rudensky, A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 2012, 30, 531–564. [Google Scholar] [CrossRef] [PubMed]
- Oberbarnscheidt, M.H.; Lakkis, F.G. Innate allorecognition. Immunol. Rev. 2014, 258, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Jeremias, P.; Matthias, T. The world incidence and prevalence of autoimmune diseases is increasing. Int. J. Celiac Dis. 2016, 3, 151–155. [Google Scholar] [CrossRef]
- Nilius, B.; Appendino, G. Spices: The savory and beneficial science of pungency. Rev. Physiol. Biochem. Pharmacol. 2013, 164, 1–76. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic phytochemicals in cancer prevention and therapy: Bioavailability versus bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef] [PubMed]
- Annuzzi, G.; Bozzetto, L.; Costabile, G.; Giacco, R.; Mangione, A.; Anniballi, G.; Vitale, M.; Vetrani, C.; Cipriano, P.; Della Corte, G.; et al. Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Safer, A.M.; Menon, M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology 2015, 23, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.; Rahimi, R.; Abdollahi, M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Andreicut, A.-D.; Pârvu, A.E.; Mot, A.C.; Pârvu, M.; Fischer Fodor, E.; Cătoi, A.F.; Feldrihan, V.; Cecan, M.; Irimie, A. Phytochemical analysis of anti-inflammatory and antioxidant effects of Mahonia aquifolium flower and fruit extracts. Oxid. Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Neta, M.T.S.L.; Sathiyabama, R.G.; de Souza Siqueira Quintans, J.; de Oliveira e Silva, A.M.; de Souza Araújo, A.A.; Narain, N.; Júnior, L.J.Q.; Gurgel, R.Q. Flavonoids as Th1/Th2 cytokines immunomodulators: A systematic review of studies on animal models. Phytomedicine 2018, 44, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Boland, J.W.; Foulds, G.A.; Ahmedzai, S.H.; Pockley, A.G. A preliminary evaluation of the effects of opioids on innate and adaptive human in vitro immune function. BMJ Support. Palliat. Care 2014, 4, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.S. Use of echinacea in medicine. Biochem. Pharmacol. 2000, 60, 155–158. [Google Scholar] [CrossRef]
- Davis, J.; Cupp, M.J. Echinacea. In Toxicology and Clinical Pharmacology of Herbal Products; Cupp, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2000; pp. 85–93. ISBN 978-1-59259-020-9. [Google Scholar]
- Barrett, B. Medicinal properties of Echinacea: A critical review. Phytomedicine 2003, 10, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Karsch-Völk, M.; Barrett, B.; Linde, K. Echinacea for preventing and treating the common cold. JAMA 2015, 313, 618. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Miccono, A.; Lamburghini, S.; Avanzato, I.; Riva, A.; Allegrini, P.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Perna, S. Self-care for common colds: The pivotal role of vitamin D, vitamin C, zinc and Echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds—Practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid. Based Complement. Alternat. Med. 2018, 2018, 1–36. [Google Scholar] [CrossRef]
- Bałan, B.J.; Sokolnicka, I.; Skopińska-Różewska, E.; Skopiński, P. The modulatory influence of some Echinacea-based remedies on antibody production and cellular immunity in mice. Cent. Eur. J. Immunol. 2016, 1, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Buttxs, M.S.; Qayyum, M.M.N.; Suleria, H.A.R. Immunity: Plants as effective mediators. Crit. Rev. Food Sci. Nutr. 2014, 54, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Melchart, D.; Walther, E.; Linde, K.; Brandmaier, R.; Lersch, C. Echinacea root extracts for the prevention of upper respiratory tract infections: A double-blind, placebo-controlled randomized trial. Arch. Fam. Med. 1998, 7, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Rapacioli, G.; Ferrara, T.; Togni, S. Use of a standardized extract from Echinacea angustifolia (Polinacea) for the prevention of respiratory tract infections. Altern. Med. Rev. J. Clin. Ther. 2012, 17, 36–41. [Google Scholar]
- Luettig, B.; Steinmüller, C.; Gifford, G.E.; Wagner, H.; Lohmann-Matthes, M.L. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J. Natl. Cancer Inst. 1989, 81, 669–675. [Google Scholar] [CrossRef] [PubMed]
- See, D.M.; Broumand, N.; Sahl, L.; Tilles, J.G. In vitro effects of echinacea and ginseng on natural killer and antibody-dependent cell cytotoxicity in healthy subjects and chronic fatigue syndrome or acquired immunodeficiency syndrome patients. Immunopharmacology 1997, 35, 229–235. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Natural immunomodulators and their stimulation of immune reaction: True or false? Anticancer Res. 2014, 34, 2275–2282. [Google Scholar] [PubMed]
- Li, Y.; Wang, Y.; Wu, Y.; Wang, B.; Chen, X.; Xu, X.; Chen, H.; Li, W.; Xu, X. Echinacea purpurea extracts promote murine dendritic cell maturation by activation of JNK, p38 MAPK and NF-κB pathways. Dev. Comp. Immunol. 2017, 73, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Chiao, M.-T.; Yen, P.-J.; Huang, W.-C.; Hou, C.-C.; Chien, S.-C.; Yeh, K.-C.; Yang, W.-C.; Shyur, L.-F.; Yang, N.-S. Modulatory effects of Echinacea purpurea extracts on human dendritic cells: A cell- and gene-based study. Genomics 2006, 88, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Wang, Y.; Wu, Y.; Chen, H.; Zheng, S.; Li, Y.; Xu, X.; Li, W. Echinacea purpurea extract polarizes M1 macrophages in murine bone marrow-derived macrophages through the activation of JNK: E CHINACEA P URPUREA E XTRACT P OLARIZES M1 M ACROPHAGES. J. Cell. Biochem. 2017, 118, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Steinmüller, C.; Roesler, J.; Gröttrup, E.; Franke, G.; Wagner, H.; Lohmann-Matthes, M.L. Polysaccharides isolated from plant cell cultures of Echinacea purpurea enhance the resistance of immunosuppressed mice against systemic infections with Candida albicans and Listeria monocytogenes. Int. J. Immunopharmacol. 1993, 15, 605–614. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Schoop, R.; Suter, A.; Hudson, J. Prevention of influenza virus induced bacterial superinfection by standardized Echinacea purpurea, via regulation of surface receptor expression in human bronchial epithelial cells. Virus Res. 2017, 233, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Huntley, A.L.; Thompson Coon, J.; Ernst, E. The safety of herbal medicinal products derived from Echinacea species: A systematic review. Drug Saf. 2005, 28, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Raduner, S.; Majewska, A.; Chen, J.-Z.; Xie, X.-Q.; Hamon, J.; Faller, B.; Altmann, K.-H.; Gertsch, J. Alkylamides from Echinacea are a new class of cannabinomimetics: CANNABINOID TYPE 2 RECEPTOR-DEPENDENT AND -INDEPENDENT IMMUNOMODULATORY EFFECTS. J. Biol. Chem. 2006, 281, 14192–14206. [Google Scholar] [CrossRef] [PubMed]
- Chicca, A.; Raduner, S.; Pellati, F.; Strompen, T.; Altmann, K.-H.; Schoop, R.; Gertsch, J. Synergistic immunomopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int. Immunopharmacol. 2009, 9, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Spelman, K.; Iiams-Hauser, K.; Cech, N.B.; Taylor, E.W.; Smirnoff, N.; Wenner, C.A. Role for PPARγ in IL-2 inhibition in T cells by Echinacea-derived undeca-2E-ene-8,10-diynoic acid isobutylamide. Int. Immunopharmacol. 2009, 9, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Tragni, E.; Galli, C.L.; Tubaro, A.; Del Negro, P.; Della Loggia, R. Anti-inflammatory activity of Echinacea angustifolia fractions separated on the basis of molecular weight. Pharmacol. Res. Commun. 1988, 20 (Suppl. 5), 87–90. [Google Scholar] [CrossRef]
- Müller-Jakic, B.; Breu, W.; Pröbstle, A.; Redl, K.; Greger, H.; Bauer, R. In vitro inhibition of cyclooxygenase and 5-lipoxygenase by alkamides from Echinacea and Achillea species. Planta Med. 1994, 60, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Facino, R.M.; Carini, M.; Aldini, G.; Marinello, C.; Arlandini, E.; Franzoi, L.; Colombo, M.; Pietta, P.; Mauri, P. Direct characterization of caffeoyl esters with antihyaluronidase activity in crude extracts from Echinacea angustifolia roots by fast atom bombardment tandem mass spectrometry. Farm. Soc. Chim. Ital. 1989 1993, 48, 1447–1461. [Google Scholar]
- Fonseca, F.N.; Papanicolaou, G.; Lin, H.; Lau, C.B.S.; Kennelly, E.J.; Cassileth, B.R.; Cunningham-Rundles, S. Echinacea purpurea (L.) moench modulates human T-cell cytokine response. Int. Immunopharmacol. 2014, 19, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Voaden, D.J.; Jacobson, M. Tumor inhibitors. 3. Identification and synthesis of an oncolytic hydrocarbon from American coneflower roots. J. Med. Chem. 1972, 15, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Ardjomand-Woelkart, K.; Bauer, R. Review and assessment of medicinal safety data of orally used Echinacea preparations. Planta Med. 2016, 82, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.N.; Werth, V.P. Activation of autoimmunity following use of immunostimulatory herbal supplements. Arch. Dermatol. 2004, 140, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Neri, P.G.; Stagni, E.; Filippello, M.; Camillieri, G.; Giovannini, A.; Leggio, G.M.; Drago, F. Oral Echinacea purpurea extract in low-grade, steroid-dependent, autoimmune idiopathic uveitis: A pilot study. J. Ocul. Pharmacol. Ther. 2006, 22, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.A.; Pelletier, J. Curcumin-biological and medicinal properties. J. Pharma 1815, 2, 50. [Google Scholar]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Mol. Basel Switz. 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Lateef, E.; Mahmoud, F.; Hammam, O.; El-Ahwany, E.; El-Wakil, E.; Kandil, S.; Abu Taleb, H.; El-Sayed, M.; Hassenein, H. Bioactive chemical constituents of Curcuma longa L. rhizomes extract inhibit the growth of human hepatoma cell line (HepG2). Acta Pharm. 2016, 66, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Salvador, J.; Robles, A.; López-Lázaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.M.; Catanzaro, M.; Rosini, M.; Racchi, M.; Lanni, C. Curcumin in Alzheimer’s disease: Can we think to new strategies and perspectives for this molecule? Pharmacol. Res. 2017, 124, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Haftcheshmeh, S.M.; Esmaeili, S.-A.; Johnston, T.P.; Abdollahi, E.; Sahebkar, A. Curcumin: A natural modulator of immune cells in systemic lupus erythematosus. Autoimmun. Rev. 2018, 17, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-Y.; Kim, K.-H.; Lee, S.-H.; Yoon, M.-S.; Lee, H.-J.; Moon, D.-O.; Lee, C.-M.; Ahn, S.-C.; Park, Y.C.; Park, Y.-M. Curcumin inhibits immunostimulatory function of dendritic cells: MAPKs and translocation of NF-kappa B as potential targets. J. Immunol. Baltim. Md 1950 2005, 174, 8116–8124. [Google Scholar] [CrossRef]
- Alagawany, M.; Ashour, E.A.; Reda, F.M. Effect of dietary supplementation of garlic (Allium Sativum) and turmeric (Curcuma Longa) on growth performance, carcass traits, blood profile and oxidative status in growing rabbits. Ann. Anim. Sci. 2016, 16, 489–505. [Google Scholar] [CrossRef]
- Rawlings, J.S. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Bill, M.A.; Nicholas, C.; Mace, T.A.; Etter, J.P.; Li, C.; Schwartz, E.B.; Fuchs, J.R.; Young, G.S.; Lin, L.; Lin, J.; et al. Structurally modified vurcumin analogs inhibit STAT3 phosphorylation and promote apoptosis of human renal cell carcinoma and melanoma cell lines. PLoS ONE 2012, 7, e40724. [Google Scholar] [CrossRef] [PubMed]
- Weissenberger, J.; Priester, M.; Bernreuther, C.; Rakel, S.; Glatzel, M.; Seifert, V.; Kögel, D. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 5781–5795. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.L.; Liu, G.X.; Chen, X.; Yang, K.; Yang, Y.X.; Xie, Q.; Gan, H.K.; Huang, X.L.; Gan, H.T. Curcumin ameliorates dextran sulfate sodium-induced experimental colitis by blocking STAT3 signaling pathway. Int. Immunopharmacol. 2013, 17, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Brück, J.; Holstein, J.; Glocova, I.; Seidel, U.; Geisel, J.; Kanno, T.; Kumagai, J.; Mato, N.; Sudowe, S.; Widmaier, K.; et al. Nutritional control of IL-23/Th17-mediated autoimmune disease through HO-1/STAT3 activation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 17, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.A.; Chen, J.H.; Lee, Y.W.; Lin, C.S.; Hsieh, M.H.; Chang, C.C.; Wong, C.S.; Chen, J.J.; Yeh, G.C.; Lin, F.Y.; et al. Biphasic effect of curcumin on morphine tolerance: A preliminary evidence from cytokine/chemokine protein array analysis. Evid. Based Complement. Altern. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.A.; Baganizi, D.R.; Sahu, R.; Singh, S.R.; Dennis, V.A. SOCS Proteins as Regulators of Inflammatory Responses Induced by Bacterial Infections: A Review. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.R.; Leite, F.R.M.; Spolidorio, L.C.; Kirkwood, K.L.; Rossa, C. Curcumin abrogates LPS-induced pro-inflammatory cytokines in RAW 264.7 macrophages. Evidence for novel mechanisms involving SOCS-1, -3 and p38 MAPK. Arch. Oral Biol. 2013, 58, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, K.; Yan, Q.; Xing, C.; Chen, Y.; Yan, Z.; Shi, Y.; Zhao, K.-W.; Gao, S. Pure curcumin increases the expression of SOCS1 and SOCS3 in myeloproliferative neoplasms through suppressing class Ι histone deacetylases. Carcinogenesis 2013, 34, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation and spices: How are they linked? J. Transl. Med. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, L.; Shen, Y.; Tan, T.; Xie, N.; Luo, M.; Li, Z.; Xie, X. Curcumin ameliorates ischemia-induced limb injury through immunomodulation. Med. Sci. Monit. 2016, 22, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.N.; Barcala Tabarrozzi, A.E.; Winnewisser, J.; Gimeno, M.L.; Antunica Noguerol, M.; Liberman, A.C.; Paz, D.A.; Dewey, R.A.; Perone, M.J. Curcumin ameliorates autoimmune diabetes. Evidence in accelerated murine models of type 1 diabetes: Curcumin ameliorates autoimmunity in NOD. Clin. Exp. Immunol. 2014, 177, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, A.; Calvello, R.; Porro, C.; Trotta, T.; Salvatore, R.; Panaro, M.A. PI3k/Akt signalling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int. Immunopharmacol. 2016, 36, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory effects of curcumin in microglial cells. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ireton, G.C.; Reed, S.G. Adjuvants containing natural and synthetic Toll-like receptor 4 ligands. Expert Rev. Vaccines 2013, 12, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Vaure, C.; Liu, Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front. Immunol. 2014, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bian, C.; Yuan, J.; Chu, W.; Xiang, X.; Chen, F.; Wang, C.; Feng, H.; Lin, J. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury. J. Neuroinflamm. 2014, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Jin, W.; Zhu, T.; Wang, J.; Yuan, B.; Jiang, J.; Liang, W.; Ma, Z. Curcumin modulates TLR4/NF-κB inflammatory signaling pathway following traumatic spinal cord injury in rats. J. Spinal Cord Med. 2015, 38, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Urdzikova, L.M.; Karova, K.; Ruzicka, J.; Kloudova, A.; Shannon, C.; Dubisova, J.; Murali, R.; Kubinova, S.; Sykova, E.; Jhanwar-Uniyal, M.; et al. The anti-inflammatory compound curcumin enhances locomotor and sensory recovery after spinal cord injury in rats by immunomodulation. Int. J. Mol. Sci. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Hashimoto, S.; Horie, T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol. Res. 1999, 39, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-S.; Sun, Y.-Y.; Chiu, W.-T.; Hung, C.-C.; Chang, C.-Y.; Shie, F.-S.; Tsai, S.-H.; Lin, J.-W.; Hung, K.-S.; Lee, Y.-H. Curcumin attenuates the expression and secretion of RANTES after spinal cord injury in vivo and lipopolysaccharide-induced astrocyte reactivation in vitro. J. Neurotrauma 2011, 28, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Lubbad, A.; Oriowo, M.A.; Khan, I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol. Cell. Biochem. 2009, 322, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Gao, R.; Cao, Y.; Guo, M.; Wei, Z.; Zhou, E.; Li, Y.; Yao, M.; Yang, Z.; Zhang, N. Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathway in lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2014, 20, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.; Lopes, T.; Oleastro, M.; Gato, I.V.; Floch, P.; Benejat, L.; Chaves, P.; Pereira, T.; Seixas, E.; Machado, J.; et al. Curcumin inhibits gastric inflammation induced by Helicobacter pylori infection in a mouse model. Nutrients 2015, 7, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Chhibber, S. Curcumin alone and in combination with augmentin protects against pulmonary inflammation and acute lung injury generated during Klebsiella pneumoniae B5055-induced lung infection in BALB/c mice. J. Med. Microbiol. 2010, 59, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Chhibber, S. Phytochemical-induced reduction of pulmonary inflammation during Klebsiella pneumoniae lung infection in mice. J. Infect. Dev. Ctries. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Jang, S.I.; Lillehoj, E.P.; Bravo, D. Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poult. Sci. 2013, 92, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Oberley-Deegan, R.E.; Bai, A.; Ovrutsky, A.R.; Kinney, W.H.; Weaver, M.; Zhang, G.; Honda, J.R.; Chan, E.D. Curcumin enhances human macrophage control of Mycobacterium tuberculosis infection: Curcumin and tuberculosis in macrophages. Respirology 2016, 21, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.L.; Chan, B.C.L.; Hon, P.-M.; Lee, M.Y.H.; Fung, K.-P.; Leung, P.-C.; Lau, C.B.S. Evaluation of in vitro anti-proliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food Chem. Toxicol. 2010, 48, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.G.L.; Chan, B.C.L.; Hon, P.-M.; Kennelly, E.J.; Yeung, S.K.; Cassileth, B.R.; Fung, K.-P.; Leung, P.-C.; Lau, C.B.S. Immunostimulatory activities of polysaccharide extract isolated from Curcuma longa. Int. J. Biol. Macromol. 2010, 47, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.M.W.; Szeto, T.H.; Paul, M.J.; Teh, A.Y.-H.; Ma, J.K.-C. Recombinant biologic products versus nutraceuticals from plants–A regulatory choice? Br. J. Clin. Pharmacol. 2017, 83, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem. Toxicol. 2017, 107, 449–471. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Nekhai, S.; Kulkarni, A.; Lin, X.; McLean, C.; Ammosova, T.; Ivanov, A.; Hipolito, M.; Nwulia, E. Inhibition of HIV-1 by curcumin A, a novel curcumin analog. Drug Des. Dev. Ther. 2015, 5051. [Google Scholar] [CrossRef]
- Jantan, I.; Bukhari, S.N.A.; Lajis, N.H.; Abas, F.; Wai, L.K.; Jasamai, M. Effects of diarylpentanoid analogues of curcumin on chemiluminescence and chemotactic activities of phagocytes: Immunomodulatory effects of diarylpentanoids. J. Pharm. Pharmacol. 2012, 64, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, I.; Ravi, A.; Kumar, D.; Kuttan, R.; Maliakel, B. An enhanced bioavailable formulation of curcumin using fenugreek-derived soluble dietary fibre. J. Funct. Foods 2012, 4, 348–357. [Google Scholar] [CrossRef]
- Krishnakumar, I.; Maliakel, A.; Gopakumar, G.; Kumar, D.; Maliakel, B.; Kuttan, R. Improved blood–brain-barrier permeability and tissue distribution following the oral administration of a food-grade formulation of curcumin with fenugreek fibre. J. Funct. Foods 2015, 14, 215–225. [Google Scholar] [CrossRef]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef] [PubMed]
- Afolayan, F.I.D.; Erinwusi, B.; Oyeyemi, O.T. Immunomodulatory activity of curcumin-entrapped poly d,l-lactic-co-glycolic acid nanoparticles in mice. Integr. Med. Res. 2018, 7, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Cwiklinski, K.; Aalinkeel, R.; Reynolds, J.L.; Sykes, D.E.; Quaye, E.; Oh, J.; Mahajan, S.D.; Schwartz, S.A. Immunomodulatory activities of curcumin-stabilized silver nanoparticles: Efficacy as an antiretroviral therapeutic. Immunol. Investig. 2017, 46, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Palange, A.L.; Di Mascolo, D.; Carallo, C.; Gnasso, A.; Decuzzi, P. Lipid-polymer nanoparticles encapsulating curcumin for modulating the vascular deposition of breast cancer cells. Nanomedicine 2014, 10, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Bottegoni, G.; Favia, A.D.; Recanatini, M.; Cavalli, A. The role of fragment-based and computational methods in polypharmacology. Drug Discov. Today 2012, 17, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Simoni, E.; Serafini, M.M.; Bartolini, M.; Caporaso, R.; Pinto, A.; Necchi, D.; Fiori, J.; Andrisano, V.; Minarini, A.; Lanni, C.; et al. Nature-inspired multifunctional ligands: Focusing on amyloid-based molecular mechanisms of Alzheimer’s disease. ChemMedChem 2016, 11, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Simoni, E.; Serafini, M.M.; Caporaso, R.; Marchetti, C.; Racchi, M.; Minarini, A.; Bartolini, M.; Lanni, C.; Rosini, M. Targeting the Nrf2/amyloid-beta liaison in Alzheimer’s disease: A rational approach. ACS Chem. Neurosci. 2017, 8, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Dudics, S.; Langan, D.; Meka, R.R.; Venkatesha, S.H.; Berman, B.M.; Che, C.T.; Moudgil, K.D. Natural products for the treatment of autoimmune arthritis: Their mechanisms of action, targeted delivery and interplay with the host microbiome. Int. J. Mol. Sci. 2018, 19, 2508. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A novel highly bioavailable curcumin formulation improves symptoms and diagnostic indicators in rheumatoid arthritis patients: A randomized, double-blind, placebo-controlled, two-dose, three-arm and parallel-group study. J. Med. Food. 2017, 20, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
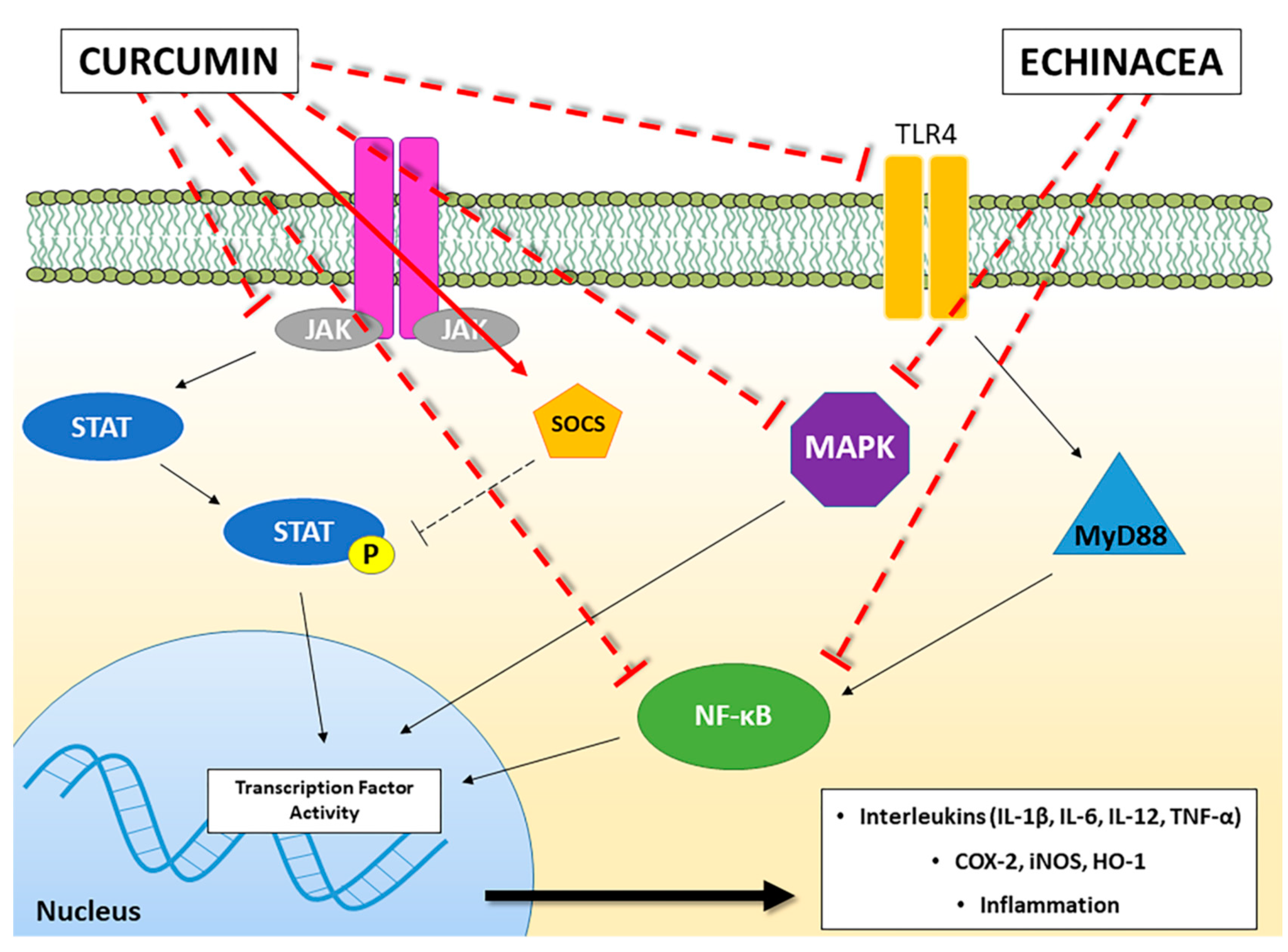
| Source | Model & Concentration | Effects | Ref. |
|---|---|---|---|
| In vitro studies | |||
| Arabinogalactan | Isolated mice macrophages; 3.7–500 μg/mL | ↑ Macrophages activation ↑ IL-1, TNF-α, IFN- | [28] |
| E. purpurea extracts | Human Peripheral Blood Mononuclear Cells; ≥0.1 μg/mL | ↑ NK function | [29] |
| E. purpurea extracts | Bone Marrow-derived Dendritic Cells; 400 mg/mL | ↑ JNK ↑ p38 MAPK, NF-κB | [31] |
| E. purpurea extracts | Human Peripheral Blood Mononuclear Cells; ≥10 μg/mL | ↑ DCs differentiation ↓ HLA-DR, CD32 | [32] |
| E. Purpurea polysaccharide enriched extract | Bone Marrow-derived Dendritic Cells; 100 μg/mL | ↑ Macrophages activation, CCR7 ↑ CD80, CD86, MHCII ↑ IL-1β, IL-6, IL-12p70, TNF-α, NO ↑ Phagocytosis and intracellular bactericidal activity | [33] |
| Alkylamides from E. purpurea | Human whole blood, 5 nM–5 μM | ↑ Cannabinoid receptor type 2 ↓ TNF-α, | [38] |
| Alkylamides from E. purpurea | Human Peripheral Blood Mononuclear Cells; 10 μg/mL | ↑ Cannabinoid receptor type 2 ↓ TNF-α, ↑ IL-10 | [39] |
| Alkylamides from E. purpurea | Jurkat T cells, 330 ng/mL | ↑ PPARγ | [40] |
| E. Angustifolia extract | Porcine leukocytes; 50 μM (for its major constituent) | ↓ Cyclooxygenase, 5-lipoxygenase | [42] |
| E. purpurea extracts | Jurkat T cells, 10–250 μg/mL | ↑ IL-2, IFNγ | [44] |
| Source | Model & Concentration | Effects | Ref. |
|---|---|---|---|
| In vitro studies | |||
| Curcumin | Bone Marrow-derived Dendric Cells; 25 μM | ↓ DC maturation ↓ CD80, CD86 ↓ IL-12, MAPK, NF-κB | [57] |
| Curcumin | Bone Marrow-derived Dendritic Cells; 7.5 μM | ↑ STAT3 | [63] |
| Curcumin | Murine macrophage; 10 μM | ↓ IL-6, TNF-α, PTGS-2 ↓ p38MAPK ↑ SOCS1, SOCS3 | [67] |
| Curcumin | Myelogenous leukemia cells and human erythroleukemia cells; 20 μM | ↑ SOCS1, SOCS3 ↓ HDAC8 | [68] |
| Curcumin | BV-2 microglia cells; ≥10 μM | ↓ NF-κB, iNOS ↓ IL-6, TNF-α, IL-1β | [72] |
| Curcumin | BV-2 microglia cells; ≥10 μM | ↓ iNOS, COX-2, HO-1 ↓ MAPK, NF-κB ↓ TNF-α, NO, PGE-2 | [73] |
| Curcumin | Microglial and cortical neurons co-cultures; 2 μM | ↓ TLR4, MyD88, NF-κB | [76] |
| Curcumin | Human promonocytic cells; 30 μM | ↓ NF-κB, caspase 3 | [88] |
| α-Turmerone ar-Turmerone | Human Peripheral Blood Mononuclear Cells; 5–10 μg/mL | ↑ PBMC proliferation ↑ IL-2, TNF-α | [89] |
| Polar fraction of turmeric hot water extracts | Human Peripheral Blood Mononuclear Cells; 400 μg/μL | ↑ PBMC proliferation ↑ IL-6, TNF-α | [90] |
| In vivo studies | |||
| Curcumin | Healthy rabbits; 2, 4 and 6 g/kg orally | ↑ serum IgG, IgM | [58] |
| Curcumin | Mice with experimental colitis induced by dextran sulfate sodium (DSS); 50 mg/kg orally | ↓ MPO, STAT3 ↓ IL-1β, TNF-α | [62] |
| Curcumin | Mice with cyclophosphamide (CYP)-induced diabetes; 25 mg/kg intraperitoneally | ↓ leucocyte infiltration ↓ NF-κB, NO | [71] |
| Curcumin | Mice with traumatic brain injury; 100 mg/kg intraperitoneally | ↑ activation of microglia/macrophages ↓ TLR4, MyD88, NF-κB | [76] |
| Curcumin | Rats with traumatic spinal cord injury; 100 mg/kg intraperitoneally | ↓ TNF-α, IL-1β, IL-6 ↓ TLR4, NF-κB | [77] |
| Curcumin | Rats with spinal cord injury; 6 mg/kg intraperitoneally | ↓ MIP1α, IL-2, RANTES ↓ NF-κB | [78] |
| Curcumin | Mice with K. pneumoniae induced lung infection; 150 mg/kg orally | ↓ leucocyte infiltration ↓ NO, MPO, TNF-α | [85,86] |
| Curcumin | Broilers with induced Eimeria maxima and Eimeria tenella infections; 35 mg/kg orally | ↑ concanavalin A | [87] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules 2018, 23, 2778. https://doi.org/10.3390/molecules23112778
Catanzaro M, Corsini E, Rosini M, Racchi M, Lanni C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules. 2018; 23(11):2778. https://doi.org/10.3390/molecules23112778
Chicago/Turabian StyleCatanzaro, Michele, Emanuela Corsini, Michela Rosini, Marco Racchi, and Cristina Lanni. 2018. "Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea" Molecules 23, no. 11: 2778. https://doi.org/10.3390/molecules23112778
APA StyleCatanzaro, M., Corsini, E., Rosini, M., Racchi, M., & Lanni, C. (2018). Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules, 23(11), 2778. https://doi.org/10.3390/molecules23112778