The Influence of Vacuum Impregnation on Nutritional Properties of Fluidized Bed Dried Kale (Brassica oleracea L. Var. Acephala) Leaves
Abstract
1. Introduction
2. Results
2.1. Nutritional Properties of Dried Kale
2.2. Dry Matter Content, Water Activity and Colour of Dried Kale
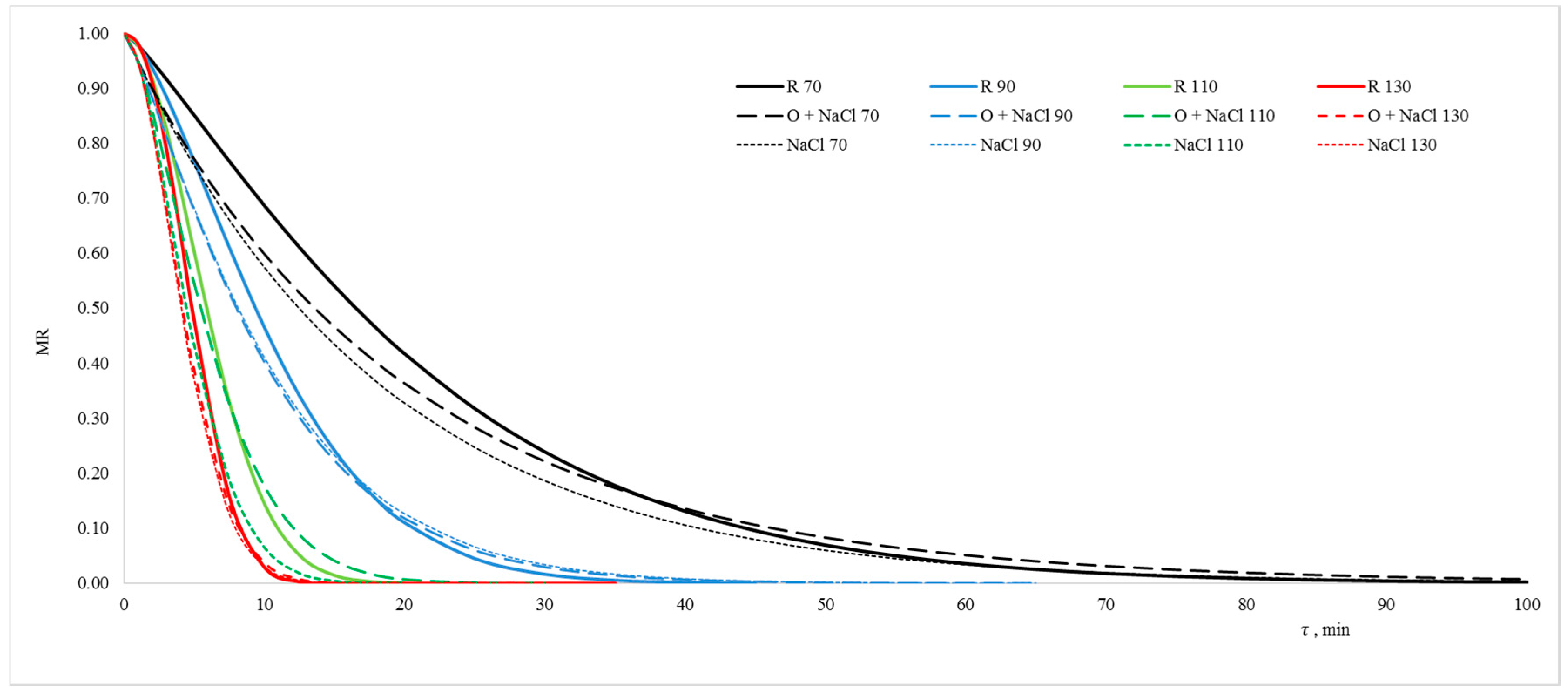
2.3. Drying Kinetics
3. Discussion
- ΔE = 0–2—colour difference is undetectable;
- ΔE = 2–3.5—colour difference is detectable by a human eye;
- ΔE ≥ 3.5—clear colour difference.
4. Materials and Methods
4.1. Material
4.2. Vacuum Impregnation
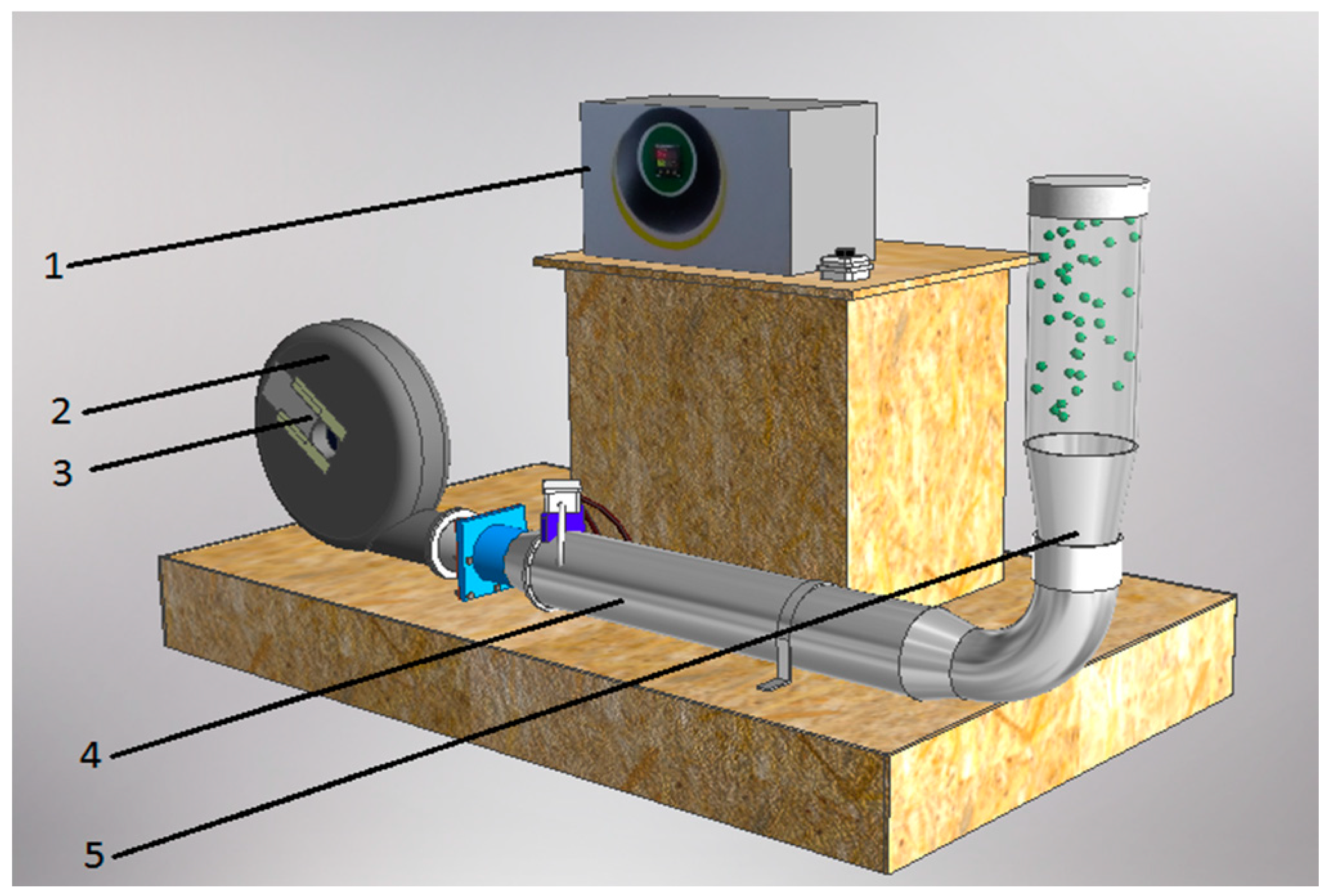
4.3. Drying
4.4. Chemical Analysis
4.5. Statistical Analyses and Software
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeon, J.; Kim, J.K.; Kim, H.; Kim, Y.; Park, Y.J.; Kim, S.J.; Kim, C.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red kale (Brassica oleracea var. acephala) sedlings. Food Chem. 2018, 241, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Record, I.R.; Dreosti, I.E.; Mcinerney, J.K. Changes in plasma antioxidant status following consumption of diets high or low in fruit and vegetables or following dietary supplementation with an antioxidant mixture. Br. J. Nutr. 2001, 85, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant capacity of tea and common vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Glew, R.H.; Millson, M.; Huang, H.S.; Chuang, L.T.; Sanz, C.; Hayirhoglu-Ayaz, S. Nutrient content of kale (Brassica oleracea L. var. acephala DC.). Food Chem. 2006, 96, 572–579. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Šamec, D.; Pavlović, I.; Radojčić Redovniković, I.; Salopek-Sondi, B. Comparative analysis of phytochemicals and activity of endogenous enzymes associated with their stability, bioavailability and food quality in five Brassicaceae sprouts. Food Chem. 2018, 269, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Herz, C.; Schlotz, N.; Kupke, F.; Bartolomé Rodríguez, M.M.; Schreiner, M.; Lamy, E. The Brassica epithionitrile 1-cyano-2,3-epithiopropane triggers cell death in human liver cancer cells in vitro. Mol. Nutr. Food Res. 2015, 59, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Maeda, Y.; Mizutani, K.; Wakatsuki, N.; Hagiwara, S.; Nabetani, H. Impact of blanching and freeze-thaw pretreatment on drying rate of carrot roots in relation to changes in cell membrane function and cell wall structure. LWT-Food Sci. Technol. 2016, 71, 40–46. [Google Scholar] [CrossRef]
- Belay, A.Z.; Caleb, O.J.; Opara, U.L. Modelling approaches for designing and evaluating the performance of modified atmosphere packaging (MAP) systems for fresh produce: A review. Food Packag. Shelf 2016, 10, 1–15. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Mujumdar, A.S.; Thang, J.; Miao, S.; Wang, Y. Recent developments in high-quality drying of vegetables, fruits and aquatic products. Crit. Rev. Food Sci. Nutr. 2015, 57, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.G. Fluidized bed drier. In Food Processing Handbook; James, G.B., Alistair, S.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 90–93. ISBN 978-3-527-30719-7. [Google Scholar]
- Kudra, T.; Strumiłło, C. Thermal Processing of Biomaterials; Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Mujumdar, A.S. Fluidized bed dryers. In Handbook of Industrial Drying; CRC Press: New York, NY, USA, 2015; pp. 173–202. ISBN 978-1-4665-9665-8. [Google Scholar]
- Ciurzyńska, A.; Kowalska, H.; Czajkowska, K.; Lenart, A. Osmotic dehydration in production of sustainable and healthy food. Trends Food Sci. Technol. 2016, 50, 186–192. [Google Scholar] [CrossRef]
- Lazarides, H.; Katsanidis, E.; Nickolaidis, A. Mass transfer kinetics during osmotic preconcentration aiming at minimal solid uptake. J. Food Eng. 1995, 25, 151–166. [Google Scholar] [CrossRef]
- Seguí, L.; Fito, P.J.; Fito, P. Understanding osmotic dehydration of tissue structured foods by means of a cellular approach. J. Food Eng. 2012, 110, 240–247. [Google Scholar] [CrossRef]
- De Lima, M.M.; Tribuzi, G.; De Souza, J.A.R.; De Souza, I.G.; Laurindo, J.B.; Carciofi, B.A.M. Vacuum impregnation and drying of calcium-fortified pineapple snacks. LWT-Food Sci. Technol. 2016, 72, 501–509. [Google Scholar] [CrossRef]
- Nowacka, M.; Fijałkowska, A.; Dadan, M.; Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D. Effect of ultrasound treatment during osmotic dehydration on bioactive compounds of cranberries. Ultrasonics 2018, 83, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhao, Y. Practical applications of vacuum impregnation in fruit and vegetable processing. Trends Food Sci. Technol. 2004, 15, 434–451. [Google Scholar]
- Panarese, V.; Rocculi, P.; Baldi, E.; Wadsö, L.; Rasmusson, A.; Galindo, F. Vacuum impregnation modulates the metabolic activity of spinach leaves. Innov. Food Sci. Emerg. 2014, 26, 286–293. [Google Scholar] [CrossRef]
- Fito, P. Modelling of vacuum osmotic dehydration of food. J. Food Eng. 1994, 22, 313–328. [Google Scholar] [CrossRef]
- Laurindo, J.B.; Stringari, G.B.; Carciofi, B.A.M. Experimental Determination of the Dynamics of Vacuum Impregnation of Apples. J. Food Sci. 2007, 72, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Pasławska, M.; Stępień, B.; Nawirska-Olszańska, A.; Radosław, M.; Leszek, R. Effect of Vacuum Impregnation on Drying Kinetics and Selected Quality Factors of Apple Cubes. Int. J. Food Eng. 2017, 13. [Google Scholar] [CrossRef]
- Correa, J.L.G.; Ernesto, D.B.; de Mendoca, K.S. Pulsed vacuum osmotic dehydration of tomatoes: Sodium incorporation reduction and kinetics modeling. LWT-Food Sci. Technol. 2016, 71, 17–24. [Google Scholar] [CrossRef]
- Occhino, E.; Hernando, I.; Llorca, E.; Neri, L.; Pittia, P. Effect of vacuum impregnation treatments to improve quality and texture of zucchini (Cucurbita pepo, L.). Procedia Food Sci. 2011, 1, 829–835. [Google Scholar] [CrossRef]
- Babu, A.K.; Kumaresan, G.; Raj, A.A.; Velraj, R. Review of leaf drying: Mechanism and influencing parameters, drying methods, nutrient preservation, and mathematical models. Renew. Sust. Energ. Rev. 2018, 90, 536–556. [Google Scholar] [CrossRef]
- Mwithiga, G.; Olwal, J. The drying kinetics of kale (Brassica oleracea) in a convective hot air dryer. J. Food Eng. 2005, 71, 373–378. [Google Scholar] [CrossRef]
- El-Sebail, A.A.; Shalaby, S.M. Experimental investigation of an indirect-mode forced convection solar dryer for drying thymus and mint. Energ. Convers. Manag. 2013, 74, 109–116. [Google Scholar] [CrossRef]
- Costa, A.B.S.; Freire, F.B.; Ferreira, M.C.; Freire, J.T. Convective drying of regular mint leaves: Analysis based on fitting empirical correlations, response surface methodology and neural networks. Acta. Sci. Technol. 2014, 36, 271–278. [Google Scholar] [CrossRef]
- Bensebia, O.; Allia, K. Drying and extraction kinetics of rosemary leaves: Experiments and modeling. J. Essent. Oil Bear Plants 2015, 18, 99–111. [Google Scholar] [CrossRef]
- Kavak, A.E.; Bicer, Y.; Cetinkaya, F. Modelling of thin layer drying of parsley leaves in a convective dryer and under open sun. J. Food Eng. 2006, 75, 308–315. [Google Scholar] [CrossRef]
- Wiktor, A.; Łuczywek, K.; Witrowa-Rajchert, D. Mathematical modeling of microwave assisted convective drying of basil leaves. Adv. Agric. Sci. Probl. Issues 2012, 570, 127–141. (In Polish) [Google Scholar]
- Premi, M.; Harish, S.; Ashutosh, U. Effect of air velocity and temperature on the drying kinetics of drumstick leaves (Moringa oleifera). Int. J. Food Eng. 2012, 8. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Mulet, A.; García-Pérez, V.J. Influence of freezing and dehydration of olive leaves (var. Serrana) on extract composition and antioxidant potential. Food Res. Int. 2013, 50, 189–196. [Google Scholar] [CrossRef]
- Shaw, M.; Meda, V.; Tabil, J.L.; Opoku, A. Drying and color characteristics of coriander foliage using convective thin layer and microwave drying. J. Microw. Power Electromagn. Energy 2007, 41, 56–65. [Google Scholar] [CrossRef]
- Rocha, R.P.; Melo, E.C.; Radünz, L.L. Influence of drying process on the quality of medicinal plants: A review. J. Med. Plants Res. 2011, 5, 7076–7084. [Google Scholar] [CrossRef]
- Kusturee, J.; Mudtapha, Y.; Phadungsak, R. Design and analysis of the commercialized drier processing using a combined unsymmetrical double-feed microwave and vacuum system (case study: Tea leaves). Chem. Eng. Process 2010, 49, 89–95. [Google Scholar]
- McGuire, R. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar]
- Di Cesare, L.F.; Forni, E.; Viscardi, D.; Nani, R.C. Changes in the chemical composition of basil caused by different drying procedures. J. Agric. Food Chem. 2003, 51, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Armesto, J.; Gomez-Limia, L.; Carballo, J.; Martínez, S. Impact of vacuum cooking and boiling, and refrigerated storage on the quality of galega kale (Brassica oleracea var. acephala cv. Galega). LWT-Food Sci. Technol. 2017, 79, 267–277. [Google Scholar] [CrossRef]
- Turkmen, N.; Poyrazoglu, E.S.; Sari, F.; Velioglu, Y.S. Effects of cooking methods on chlorophylls, pheophytins and colour of selected green vegetables. Int. J. Food Sci. Technol. 2006, 41, 281–288. [Google Scholar] [CrossRef]
- Korus, A. Effect of preliminary processing and technological treatments on the content of chlorophylls and carotenoids in kale (Brassica oleracea L. Var. acephala). J. Food Process Pres. 2013, 37, 335–344. [Google Scholar] [CrossRef]
- Araújo, A.C.; Oliveira, S.M.; Ramos, I.N.; Brandão, T.R.S.; Monteiro, M.J.; Silva, C.L.M. Evaluation of drying and storage conditions on nutritional and sensory properties of dried galega kale (Brassica oleracea L. Var. acephala). J. Food Quality 2017, 2017, 9393482. [Google Scholar] [CrossRef]
- Lafarga, T.; Viñas, I.; Bobo, G.; Simó, J.; Aguiló-Aguayo, I. Effect of steaming and sous vide processing on the total phenolic content, vitamin C and antioxidant potential of the genus Brassica. Innov. Food Sci. Emerg. 2018, 47, 412–420. [Google Scholar] [CrossRef]
- Korus, A. Effect of preliminary processing, method of drying and storage temperature on the level of antioxidants in kale (Brassica oleracea L. var. acephala) leaves. LWT-Food Sci. Technol. 2011, 44, 1711–1716. [Google Scholar] [CrossRef]
- Lafarga, T.; Bobo, G.; Viñas, I.; Zudaire, L.; Simó, J.; Aguiló-Aguayo, I. Steaming and sous-vide: Effects on antioxidant activity, vitamin C, and total phenolic content of Brassica vegetables. Int. J. Gastron. Food Sci. 2018, 13, 134–139. [Google Scholar] [CrossRef]
- Anese, M.; Manzocco, L.; Nicoli, M.; Lerici, C. Antioxidant properties of tomato juice as affected by heating. J. Sci. Food Agric. 1999, 79, e750–e754. [Google Scholar] [CrossRef]
- Harbourne, N.; Marete, E.; Jacquier, J.C.; O’Riordan, D. Effect of drying methods on the phenolic constituents of meadowsweet (Filipendula ulmaria) and willow (Salix alba). LWT-Food Sci. Technol. 2009, 42, 1468–1473. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- ASAE. ASAE Standard; ASAE: Washington, WA, USA, 1986; pp. 1–361. [Google Scholar]
- Nawirska-Olszańska, A.; Stępień, B.; Biesiada, A. Effectiveness of the fountain-microwave drying method in some selected pumpkin cultivars. LWT-Food Sci. Technol. 2017, 77, 276–281. [Google Scholar] [CrossRef]
- Olssen, M.E.; Andersson, S.; Oredsson, S.; Berglund, R.H.; Gustavsson, K.R. Antioxidant levels and inhibition of cancer cell proliferation in vitro by extracts from organically and conventionally cultivated strawberries. J. Agric. Food Chem. 2006, 54, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Wiktor, A.; Łuczywek, K.; Witrowa-Rajchert, D.; Hankus, M.; Królikowski, K. Mathematical modeling of microwave assisted convective drying of oregano leaves. Adv. Agric. Sci. Probl. Issues 2013, 573, 61–73. (In Polish) [Google Scholar]
Sample Availability: Not available. |

| Drying Conditions | Bioactive Components | ||||
|---|---|---|---|---|---|
| Material | T [°C] | Chlorophylls [mg/100 g d.m.] | Carotenoids [mg/100 g d.m.] | Polyphenols [mgGA/100 g d.m.] | ABTS+ [µMol/100 g d.m.] |
| R | - | 832.64 ± 12.45 a | 140.06 ± 10.23 a | 626.17 ± 54.32 a | 57.17 ± 2.15 a |
| 70 | 103.77 ± 9.35 g | 104.21 ± 11.36 c,d | 170.06 ± 15.21 e | 21.37 ± 1.21 g | |
| 90 | 203.88 ± 17.36 e | 102.86 ± 8.14 d | 233.45 ± 12.23 c | 28.89 ± 1.36 e | |
| 110 | 453.33 ± 28.37 d | 134.66 ± 9.74 b | 268.88 ± 20.54 b,c | 34.81 ± 2.25 d | |
| 130 | 141.44 ± 12.45 f | 62.31 ± 4.21 f | 240.34 ± 21.25 c | 30.84 ± 2.18 d,e | |
| O + NaCl | - | 739.21 ± 13.51 b | 138.52 ± 11.53 a | 658.65 ± 25.41 a | 50.12 ± 1.27 a |
| 70 | 141.75 ± 11.01 f | 133.53 ± 12.65 b | 206.04 ± 17.25 d | 23.31 ± 1.98 f | |
| 90 | 723.89 ± 35.01 b | 131.47 ± 11.58 b | 271.25 ± 21.36 b,c | 29.86 ± 1.14 e | |
| 110 | 729.09 ± 48.14 b | 138.21 ± 14.24 a | 337.85 ± 29.24 b | 47.99 ± 3.25 b | |
| 130 | 169.79 ± 12.12 f | 93.07 ± 6.32 e | 306.20 ± 24.12 b | 38.11 ± 2.15 c | |
| NaCl | - | 732.78 ± 12.54 b | 108.08 ± 14.52 c | 534.25 ± 17.21 a | 45.87 ± 2.54 b |
| 70 | 132.83 ± 11.32 f | 103.94 ± 13.65 d | 183.57 ± 15.87 e | 21.60 ± 1.96 g | |
| 90 | 639.53 ± 35.21 c | 102.44 ± 10.29 d | 227.50 ± 19.23 c | 27.27 ± 1.36 e | |
| 110 | 723.83 ± 42.79 b | 103.44 ± 12.06 d | 298.28 ± 21.59 b | 40.49 ± 2.34 c | |
| 130 | 164.96 ± 14.25 f | 66.19 ± 2.13 f | 285.27 ± 24.24 b | 34.42 ± 2.54 d | |
| Drying Conditions | Drying Results | ||
|---|---|---|---|
| Material | T [°C] | d.m. [%] | Aw [−] |
| R | - | 17.51 ± 1.12 b | 0.9888 ± 0.0300 a |
| 70 | 94.13 ± 1.74 d | 0.3831 ± 0.0126 b | |
| 90 | 95.20 ± 1.56 e | 0.3814 ± 0.0026 b | |
| 110 | 95.55 ± 2.25 e | 0.3953 ± 0.0466 b | |
| 130 | 96.62 ± 1.98 f | 0.3620 ± 0.0066 c | |
| O + NaCl | - | 17.15 ± 2.00 b | 0.9826 ± 0.0010 a |
| 70 | 92.48 ± 0.18 c | 0.4011 ± 0.0025 b | |
| 90 | 93.24 ± 0.37 c | 0.3704 ± 0.0134 c | |
| 110 | 94.28 ± 0.69 d | 0.3416 ± 0.0609 d | |
| 130 | 94.74 ± 0.54 d | 0.3445 ± 0.0276 d | |
| NaCl | - | 13.42 ± 1.07 a | 0.9859 ± 0.0033 a |
| 70 | 93.79 ± 0.30 c | 0.2941 ± 0.0339 e | |
| 90 | 94.93 ± 0.22 d | 0.2401 ± 0.0037 e | |
| 110 | 94.91 ± 1.37 d | 0.1930 ± 0.0030 f | |
| 130 | 95.76 ± 0.10 e | 0.1837 ± 0.0104 f | |
| Drying Conditions | Colour Factors | ||||||
|---|---|---|---|---|---|---|---|
| Material | T [°C] | L* | a* | b* | h* | C* | ΔE |
| R | - | 44.26 | −13.09 | 22.30 | −58.8 a | 29.11 | - |
| 70 | 35.13 | −6.23 | 8.79 | −60.70 c | 10.19 | 22.14 | |
| 90 | 37.32 | −6.27 | 11.31 | −62.96 c | 12.92 | 39.49 | |
| 110 | 37.74 | −6.62 | 10.98 | −58.73 a | 12.82 | 39.85 | |
| 130 | 39.35 | −7.31 | 10.97 | −62.65 c | 15.24 | 42.20 | |
| O + NaCl | - | 36.72 | −0.63 | 17.23 | −87.90 f | 17.24 | 20.08 |
| 70 | 38.50 | −5.11 | 10.70 | −64.44 d | 11.86 | 8.88 | |
| 90 | 37.75 | −6.67 | 11.29 | −58.98 a | 13.21 | 39.95 | |
| 110 | 38.01 | −7.37 | 12.42 | −59.29 a | 14.45 | 40.67 | |
| 130 | 38.39 | −7.02 | 13.03 | −61.73 c | 14.83 | 41.16 | |
| NaCl | - | 27.67 | −0.96 | 3.57 | −74.91 e | 3.69 | 31.82 |
| 70 | 31.37 | −4.27 | 7.30 | −59.68 a | 8.47 | 6.21 | |
| 90 | 34.79 | −6.31 | 10.80 | −59.72 a | 12.52 | 36.98 | |
| 110 | 37.16 | −8.85 | 13.58 | −56.90 b | 16.22 | 40.55 | |
| 130 | 32.91 | −5.99 | 9.45 | −57.61 a,b | 11.20 | 34.76 | |
| Drying Conditions | Page Model Coefficients | Statistical Coefficients | ||||
|---|---|---|---|---|---|---|
| Material | T [°C] | k | a | RMSE | Ve [%] | X2 |
| R | 70 | 0.023 | 1.2168 | 0.01325 | 4.5 | 0.00070 |
| 90 | 1.5256 | 0.01401 | 4.8 | 0.00078 | ||
| 110 | 1.9278 | 0.01383 | 2.1 | 0.00077 | ||
| 130 | 2.1500 | 0.00311 | 1.4 | 0.00002 | ||
| O + NaCl | 70 | 0.054 | 0.9800 | 0.02220 | 9.3 | 0.00197 |
| 90 | 1.2292 | 0.01728 | 6.9 | 0.00119 | ||
| 110 | 1.5083 | 0.01025 | 3.6 | 0.00042 | ||
| 130 | 1.7855 | 0.00404 | 2.5 | 0.00007 | ||
| NaCl | 70 | 0.054 | 1.0087 | 0.02410 | 11.2 | 0.00232 |
| 90 | 1.2145 | 0.01658 | 6.2 | 0.00082 | ||
| 110 | 1.7704 | 0.00178 | 1.3 | 0.00110 | ||
| 130 | 1.8077 | 0.00230 | 1.1 | 0.00002 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasławska, M.; Nawirska-Olszańska, A.; Stępień, B.; Klim, A. The Influence of Vacuum Impregnation on Nutritional Properties of Fluidized Bed Dried Kale (Brassica oleracea L. Var. Acephala) Leaves. Molecules 2018, 23, 2764. https://doi.org/10.3390/molecules23112764
Pasławska M, Nawirska-Olszańska A, Stępień B, Klim A. The Influence of Vacuum Impregnation on Nutritional Properties of Fluidized Bed Dried Kale (Brassica oleracea L. Var. Acephala) Leaves. Molecules. 2018; 23(11):2764. https://doi.org/10.3390/molecules23112764
Chicago/Turabian StylePasławska, Marta, Agnieszka Nawirska-Olszańska, Bogdan Stępień, and Angelika Klim. 2018. "The Influence of Vacuum Impregnation on Nutritional Properties of Fluidized Bed Dried Kale (Brassica oleracea L. Var. Acephala) Leaves" Molecules 23, no. 11: 2764. https://doi.org/10.3390/molecules23112764
APA StylePasławska, M., Nawirska-Olszańska, A., Stępień, B., & Klim, A. (2018). The Influence of Vacuum Impregnation on Nutritional Properties of Fluidized Bed Dried Kale (Brassica oleracea L. Var. Acephala) Leaves. Molecules, 23(11), 2764. https://doi.org/10.3390/molecules23112764






