Quantitative Assessment of Tetrel Bonding Utilizing Vibrational Spectroscopy
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Tetrel Bonds (TB) in Neutral Complexes
3.2. Charge-Assisted Tetrel Bonds
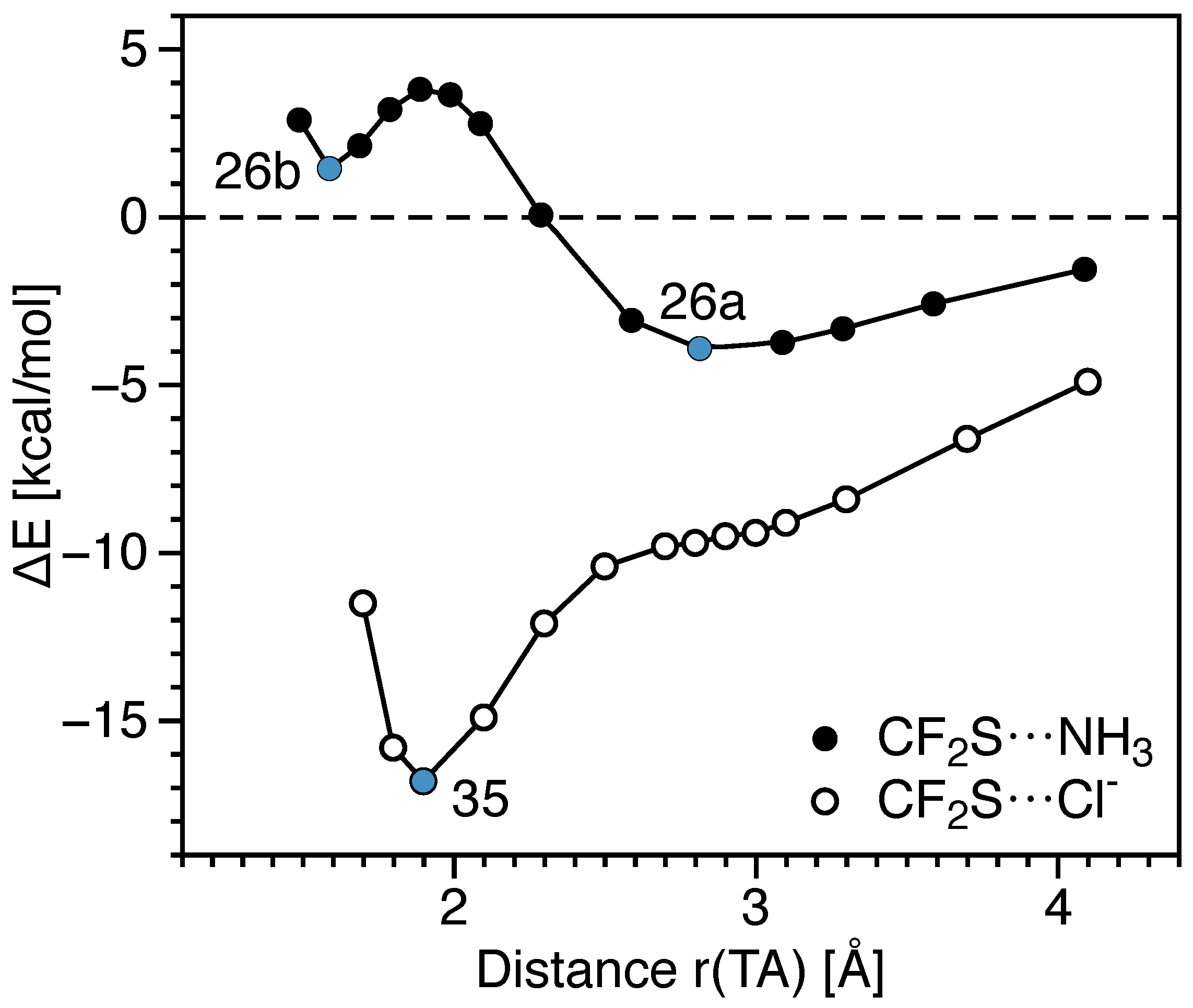
3.3. Tetrel Bonds vs. Other Noncovalent Interactions
4. Conclusions
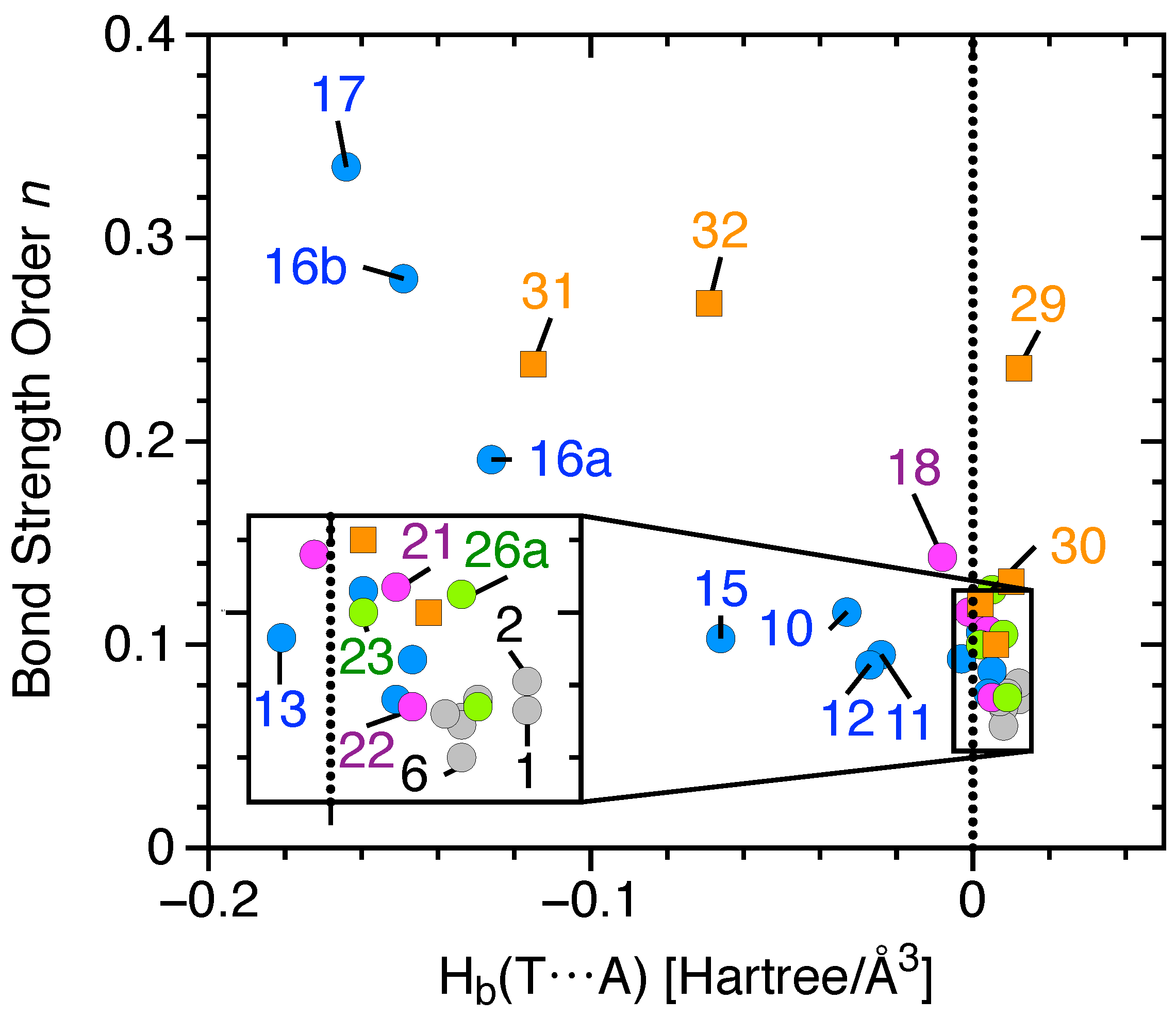
- Tetrel bonding becomes stronger as the atomic mass of the tetrel center increases as a consequence of increasing the polarizability.
- For X−TH3···NH3 complexes, the tetrel bond strength weakens in the order (X = F) > (X = Cl) > (X = Br) ≥ (X = OH) as the magnitude of the -hole decreases in the order of F−TH3>Cl−TH3>Br−TH3≥ OH−TH3.
- Successive fluorination of SiH4 impacts both the strength and the nature of the tetrel bond. The successive fluorinations result in stronger tetrel bonding as a consequence of (i) higher at the -hole region; (ii) the partial covalent character of the interaction; (iii) higher electron delocalization that occurs from the highest occupied molecular orbital (HOMO) of the T acceptor to the lowest unoccupied molecular orbital (LUMO) of the T donor. In this series, the binding energy trend deviates from BSO n values due to the high energetic cost associated with the geometric deformation of the monomers upon complexation () which is a consequence of the exchange-repulsion between the lone pair orbitals of the peripheral atoms of the T donor.
- Tetrel bonds in double bonded C donors, e.g., CO2 with NH3, are weak and electrostatic in nature. Substituting a C=O double bond with an electron withdrawing group (F atoms) strengthens the tetrel bond.
- A positively-charged Tdonor or negatively-charged T-acceptor strengthens the tetrel bond. It creates higher at the -hole, resulting in a stronger electrostatic interaction.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Adibatic connection scheme |
| BSO n | Bond strength order |
| CCSD(T) | Coupled cluster theory with singles, doubles, and perturbative triples |
| CT | Intermonomer charge transfer |
| EDG | Electron donating group |
| EWG | Electron withdrawing group |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest unoccupied molecular orbital |
| NBO | Natural bond orbital |
| NCI | Noncovalent interaction |
| NPA | Natural population analysis |
| TB | Tetrel bond |
References
- Schneider, H.J. Binding Mechanisms in Supramolecular Complexes. Angew. Chem. Int. Ed. 2009, 48, 3924–3977. [Google Scholar] [CrossRef] [PubMed]
- Bene, J.E.D.; Alkorta, I.; Elguero, J. Exploring the (H2C=PH2)+:N-Base Potential Surfaces: Complexes Stabilized by Pnicogen, Hydrogen, and Tetrel Bonds. J. Phys. Chem. A 2015, 119, 11701–11710. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. Analysis of Halogen and Other σ-Hole Bonds in Crystals. Crystals 2018, 8, 42. [Google Scholar] [CrossRef]
- Dubecký, M.; Mitas, L.; Jurečka, P. Noncovalent Interactions by Quantum Monte Carlo. Chem. Rev. 2016, 116, 5188–5215. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Hydrogen Bonding: A Theoretical Perspective; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.E.; Hobza, P. Noncovalent interactions in biochemistry. WIREs: Comput. Mol. Sci. 2011, 1, 3–17. [Google Scholar] [CrossRef]
- Alkorta, I.; Legon, A. Nucleophilicities of Lewis Bases B and Electrophilicities of Lewis Acids A Determined from the Dissociation Energies of Complexes B···A Involving Hydrogen Bonds, Tetrel Bonds, Pnictogen Bonds, Chalcogen Bonds and Halogen Bonds. Molecules 2017, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Gholipour, A. Mutual interplay between pnicogen-π and tetrel bond in PF3⊥X-Pyr···SiH3CN complexes: NMR, SAPT, AIM, NBO, and MEP analysis. Struct. Chem. 2018. [Google Scholar] [CrossRef]
- Christensen, A.S.; Kubař, T.; Cui, Q.; Elstner, M. Semiempirical Quantum Mechanical Methods for Noncovalent Interactions for Chemical and Biochemical Applications. Chem. Rev. 2016, 116, 5301–5337. [Google Scholar] [CrossRef] [PubMed]
- Sessions, R.B.; Gibbs, N.; Dempsey, C.E. Hydrogen Bonding in Helical Polypeptides from Molecular Dynamics Simulations and Amide Hydrogen Exchange Analysis: Alamethicin and Melittin in Methanol. Biophys. J. 1998, 74, 138–152. [Google Scholar] [CrossRef]
- Bene, J.E.D.; Alkorta, I.; Elguero, J. Anionic complexes of F− and Cl− with substituted methanes: Hydrogen, halogen, and tetrel bonds. Chem. Phys. Lett. 2016, 655–656, 115–119. [Google Scholar] [CrossRef]
- Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The Halogen Bond in the Design of Functional Supramolecular Materials: Recent Advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.Q.; Li, X.; Xia, Y.; Zhang, L.; Yu, Z.X. DFT Study of the Mechanisms of In Water Au(I)-Catalyzed Tandem [3,3]-Rearrangement/Nazarov Reaction/[1,2]-Hydrogen Shift of Enynyl Acetates: A Proton-Transport Catalysis Strategy in the Water-Catalyzed [1,2]-Hydrogen Shift. J. Am. Chem. Soc. 2007, 129, 15503–15512. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.J.; Jin, W.J. Strong halogen bonding of 1,2-diiodoperfluoroethane and 1,6-diiodoperfluorohexane with halide anions revealed by UV-Vis, FT-IR, NMR spectroscopes and crystallography. Phys. Chem. Chem. Phys. 2011, 13, 13721–13729. [Google Scholar] [CrossRef] [PubMed]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Defining the hydrogen bond: An account (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1619–1636. [Google Scholar] [CrossRef]
- Freindorf, M.; Kraka, E.; Cremer, D. A comprehensive analysis of hydrogen bond interactions based on local vibrational modes. Int. J. Quant. Chem. 2012, 112, 3174–3187. [Google Scholar] [CrossRef]
- Kalescky, R.; Zou, W.; Kraka, E.; Cremer, D. Local vibrational modes of the water dimer—Comparison of theory and experiment. Chem. Phys. Lett. 2012, 554, 243–247. [Google Scholar] [CrossRef]
- Kalescky, R.; Kraka, E.; Cremer, D. Local vibrational modes of the formic acid dimer—The strength of the double hydrogen bond. Mol. Phys. 2013, 111, 1497–1510. [Google Scholar] [CrossRef]
- Kraka, E.; Freindorf, M.; Cremer, D. Chiral Discrimination by Vibrational Spectroscopy Utilizing Local Modes. Chirality 2013, 25, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zou, W.; Jia, J.; Li, W.; Cremer, D. Different Ways of Hydrogen Bonding in Water—Why Does Warm Water Freeze Faster than Cold Water? J. Theory Comp. Chem. 2016, 13, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. Aerogen Bonding Interaction: A New Supramolecular Force? Angew. Chem. Int. Ed. 2015, 54, 7340–7343. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. Theoretical Study on the Dual Behavior of XeO3 and XeF4 toward Aromatic Rings: Lone Pair-π versus Aerogen-π Interactions. ChemPhysChem 2015, 16, 3625–3630. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. π-Hole aerogen bonding interactions. Phys. Chem. Chem. Phys. 2015, 17, 24748–24753. [Google Scholar] [CrossRef] [PubMed]
- Frontera, A.; Bauzá, A. Concurrent aerogen bonding and lone pair/anion-π interactions in the stability of organoxenon derivatives: A combined CSD and ab initio study. Phys. Chem. Chem. Phys. 2017, 19, 30063–30068. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Halogen Bonding: An Interim Discussion. ChemPhysChem 2013, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Kraka, E.; Cremer, D. The intrinsic strength of the halogen bond: Electrostatic and covalent contributions described by coupled cluster theory. Phys. Chem. Chem. Phys. 2016, 18, 33031–33046. [Google Scholar] [CrossRef] [PubMed]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef] [PubMed]
- Wolters, L.P.; Schyman, P.; Pavan, M.J.; Jorgensen, W.L.; Bickelhaupt, F.M.; Kozuch, S. The many faces of halogen bonding: A review of theoretical models and methods. WIREs Comput. Mol. Sci. 2014, 4, 523–540. [Google Scholar] [CrossRef]
- Alikhani, E.; Fuster, F.; Madebene, B.; Grabowski, S.J. Topological reaction sites—Very strong chalcogen bonds. Phys. Chem. Chem. Phys. 2014, 16, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Cremer, D.; Kraka, E. The Many Facets of Chalcogen Bonding: Described by Vibrational Spectroscopy. J. Phys. Chem. A 2017, 121, 6845–6862. [Google Scholar] [CrossRef] [PubMed]
- Gleiter, R.; Haberhauer, G.; Werz, D.B.; Rominger, F.; Bleiholder, C. From Noncovalent Chalcogen-Chalcogen Interactions to Supramolecular Aggregates: Experiments and Calculations. Chem. Rev. 2018, 118, 2010–2041. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Kopylovich, M.N.; da Silva, M.F.C.G.; Pombeiro, A.J.L. Chalcogen bonding in synthesis, catalysis and design of materials. Dalton Trans. 2017, 46, 10121–10138. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Bene, J.E.D. Complexes of O=C=S with Nitrogen Bases: Chalcogen Bonds, Tetrel Bonds, and Other Secondary Interactions. ChemPhysChem 2018, 19, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. The Pnicogen Bond: Its Relation to Hydrogen, Halogen, and Other Noncovalent Bonds. Acc. Chem. Res. 2012, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Pavan, M.S.; Row, T.N.G. Experimental validation of ‘pnicogen bonding’ in nitrogen by charge density analysis. Phys. Chem. Chem. Phys. 2015, 17, 2330–2334. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, D.; Kraka, E.; Cremer, D. Strength of the Pnicogen Bond in Complexes Involving Group 5A Elements N, P, and As. J. Phys. Chem. A 2014, 119, 1642–1656. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, D.; Kraka, E.; Cremer, D. Description of pnicogen bonding with the help of vibrational spectroscopy—The missing link between theory and experiment. Chem. Phys. Lett. 2014, 614, 136–142. [Google Scholar] [CrossRef]
- Setiawan, D.; Cremer, D. Super-pnicogen bonding in the radical anion of the fluorophosphine dimer. Chem. Phys. Lett. 2016, 662, 182–187. [Google Scholar] [CrossRef]
- Thomas, S.P.; Pavan, M.S.; Row, T.N.G. Experimental evidence for ‘carbon bonding’ in the solid state from charge density analysis. Chem. Commun. 2014, 50, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel-Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel Bonding Interactions. Chem. Rec. 2016, 16, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Bene, J.E.D.; Alkorta, I.; Elguero, J. Carbenes as Electron-Pair Donors To CO2 for C···C Tetrel Bonds and C−C Covalent Bonds. J. Phys. Chem. A 2017, 121, 4039–4047. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Bene, J.E.D. Azines as Electron-Pair Donors to CO2 for N···C Tetrel Bonds. J. Phys. Chem. A 2017, 121, 8017–8025. [Google Scholar] [CrossRef] [PubMed]
- Bene, J.E.D.; Alkorta, I.; Elguero, J. Carbon-Carbon Bonding between Nitrogen Heterocyclic Carbenes and CO2. J. Phys. Chem. A 2017, 121, 8136–8146. [Google Scholar] [CrossRef] [PubMed]
- Bene, J.D.; Elguero, J.; Alkorta, I. Complexes of CO2 with the Azoles: Tetrel Bonds, Hydrogen Bonds and Other Secondary Interactions. Molecules 2018, 23, 906. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Triel Bonds, π-Hole-π-Electrons Interactions in Complexes of Boron and Aluminium Trihalides and Trihydrides with Acetylene and Ethylene. Molecules 2015, 20, 11297–11316. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. On the Versatility of BH2X (X=F, Cl, Br, and I) Compounds as Halogen-, Hydrogen-, and Triel-Bond Donors: An Ab Initio Study. ChemPhysChem 2016, 17, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Asadollahi, S.; Mousavian, P. Anionic tetrel bonds: An ab initio study. Chem. Phys. Lett. 2018, 691, 394–400. [Google Scholar] [CrossRef]
- Li, Q.Z.; Zhuo, H.Y.; Li, H.B.; Liu, Z.B.; Li, W.Z.; Cheng, J.B. Tetrel-Hydride Interaction between XH3F (X = C, Si, Ge, Sn) and HM (M = Li, Na, BeH, MgH). J. Phys. Chem. A 2014, 119, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Zhu, W. Nonbonding interactions of organic halogens in biological systems: Implications for drug discovery and biomolecular design. Phys. Chem. Chem. Phys. 2010, 12, 4543–4551. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Arunan, E. The X−C··· (X = F, Cl, Br, CN) Carbon Bond. J. Phys. Chem. A 2014, 118, 10081–10089. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cheng, J.; Yang, X.; Liu, Z.; Li, W.; Li, Q. Comparison of σ-Hole and π-Hole Tetrel Bonds Formed by Pyrazine and 1,4-Dicyanobenzene: The Interplay between Anion-π and Tetrel Bonds. ChemPhysChem 2017, 18, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Tetrel bond- σ-hole bond as a preliminary stage of the SN2 reaction. Phys. Chem. Chem. Phys. 2014, 16, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the σ-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Scilabra, P.; Kumar, V.; Ursini, M.; Resnati, G. Close contacts involving germanium and tin in crystal structures: Experimental evidence of tetrel bonds. J. Mol. Model. 2018, 24, 37. [Google Scholar] [CrossRef] [PubMed]
- Donald, K.J.; Tawfik, M. The Weak Helps the Strong: Sigma-Holes and the Stability of MF4·Base Complexes. J. Phys. Chem. A 2013, 117, 14176–14183. [Google Scholar] [CrossRef] [PubMed]
- Bundhun, A.; Ramasami, P.; Murray, J.S.; Politzer, P. Trends in σ-hole strengths and interactions of F3MX molecules (M = C, Si, Ge and X = F, Cl, Br, I). J. Mol. Model. 2013, 19, 2739–2746. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Comparison of CH···O, SH···O, Chalcogen, and Tetrel Bonds Formed by Neutral and Cationic Sulfur-Containing Compounds. J. Phys. Chem. A 2015, 119, 9189–9199. [Google Scholar] [CrossRef] [PubMed]
- Azofra, L.M.; Scheiner, S. Tetrel, chalcogen, and CH···O hydrogen bonds in complexes pairing carbonyl-containing molecules with 1, 2, and 3 molecules of CO2. J. Chem. Phys. 2015, 142, 034307. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Q.; Scheiner, S. The π-Tetrel Bond and its Influence on Hydrogen Bonding and Proton Transfer. ChemPhysChem 2018, 19, 736–743. [Google Scholar] [CrossRef] [PubMed]
- McDowell, S.A.C.; Joseph, J.A. The effect of atomic ions on model σ-hole bonded complexes of AH3Y (A = C, Si, Ge; Y = F, Cl, Br). Phys. Chem. Chem. Phys. 2014, 16, 10854. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. σ-Hole Interactions: Perspectives and Misconceptions. Crystals 2017, 7, 212. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The σ-hole revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, B.; Karlström, G.; Wennerström, H. Ab initio molecular orbital calculations on the water-carbon dioxide system: Molecular complexes. Chem. Phys. Lett. 1975, 30, 58–59. [Google Scholar] [CrossRef]
- Peterson, K.I.; Klemperer, W. Structure and internal rotation of H2O−CO2, HDO−CO2, and D2O−CO2 van der Waals complexes. J. Chem. Phys. 1984, 80, 2439–2445. [Google Scholar] [CrossRef]
- Mitzel, N.W.; Blake, A.J.; Rankin, D.W.H. β-Donor Bonds in SiON Units: An Inherent Structure- Determining Property Leading to (4+4)-Coordination in Tetrakis-(N,N-dimethylhydroxylamido)silane. J. Am. Chem. Soc. 1997, 119, 4143–4148. [Google Scholar] [CrossRef]
- Mitzel, N.W.; Losehand, U. β-Donorbindungen in Molekülen mit SiON-Einheiten. Angew. Chem. 1997, 109, 2897–2899. [Google Scholar] [CrossRef]
- Southern, S.A.; Bryce, D.L. NMR Investigations of Noncovalent Carbon Tetrel Bonds. Computational Assessment and Initial Experimental Observation. J. Phys. Chem. A 2015, 119, 11891–11899. [Google Scholar] [CrossRef] [PubMed]
- Brammer, L. Halogen bonding, chalcogen bonding, pnictogen bonding, tetrel bonding: Origins, current status and discussion. Faraday Discuss. 2017, 203, 485–507. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Q.; Cheng, J.; Liu, Z.; Li, W. The Prominent Enhancing Effect of the Cation-π Interaction on the Halogen-Hydride Halogen Bond in M1···C6H5X···HM2. ChemPhysChem 2011, 12, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Sun, L.; Liu, X.F.; Li, W.Z.; Cheng, J.B.; Zeng, Y.L. Enhancement of Iodine-Hydride Interaction by Substitution and Cooperative Effects in NCX-NCI-HMY Complexes. ChemPhysChem 2012, 13, 3997–4002. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Dronskowski, R. Tetrel Bonds in Infinite Molecular Chains by Electronic Structure Theory and Their Role for Crystal Stabilization. J. Phys. Chem. A 2017, 121, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, Q. Interplay between tetrel bonding and hydrogen bonding interactions in complexes involving F2XO (X=C and Si) and HCN. Comput. Theor. Chem. 2014, 1050, 51–57. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Li, W.; Cheng, J.; McDowell, S.A.C. Comparison of hydrogen, halogen, and tetrel bonds in the complexes of HArF with YH3X (X = halogen, Y = C and Si). RSC Adv. 2016, 6, 19136–19143. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.W.; Li, Q.Z.; Li, W.Z.; Cheng, J.B. Competition and cooperativity between tetrel bond and chalcogen bond in complexes involving F2CX (X = Se and Te). Chem. Phys. Lett. 2015, 620, 7–12. [Google Scholar] [CrossRef]
- Marín-Luna, M.; Alkorta, I.; Elguero, J. Cooperativity in Tetrel Bonds. J. Phys. Chem. A 2016, 120, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Solimannejad, M.; Orojloo, M.; Amani, S. Effect of cooperativity in lithium bonding on the strength of halogen bonding and tetrel bonding: (LiCN)···ClYF3 and (LiCN)···YF3Cl (Y= C, Si and n=1-5) complexes as a working model. J. Mol. Model. 2015, 21. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Mohammadirad, N.; Solimannejad, M. Tetrel bond cooperativity in open-chain (CH3CN)n and (CH3NC)n clusters (n = 2–7): An ab initio study. Chem. Phys. Lett. 2015, 628, 16–20. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Li, W.; Cheng, J. Tetrel bonds between PySiX3 and some nitrogenated bases: Hybridization, substitution, and cooperativity. J. Mol. Graphics Modell. 2016, 65, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Q.; Yang, X.; McDowell, S.A.C. Intramolecular Si···O Tetrel Bonding: Tuning of Substituents and Cooperativity. ChemistrySelect 2017, 2, 11104–11112. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, J.; Li, W.; Li, Q. Regulation of coin metal substituents and cooperativity on the strength and nature of tetrel bonds. RSC Adv. 2017, 7, 46321–46328. [Google Scholar] [CrossRef]
- Mani, D.; Arunan, E. The X−C···Y Carbon Bond. In Noncovalent Forces; Springer International Publishing: Cham, Switzerland, 2015; pp. 323–356. [Google Scholar]
- Mahadevi, A.S.; Sastry, G.N. Cooperativity in Noncovalent Interactions. Chem. Rev. 2016, 116, 2775–2825. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S. The fundamental nature and role of the electrostatic potential in atoms and molecules. Theor. Chem. Acc. 2002, 108, 134–142. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. WIREs Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Mani, D.; Arunan, E. The X−C···Y (X = O/F, Y = O/S/F/Cl/Br/N/P) ‘carbon bond’ and hydrophobic interactions. Phys. Chem. Chem. Phys. 2013, 15, 14377–14383. [Google Scholar] [CrossRef] [PubMed]
- Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Comparison between Tetrel Bonded Complexes Stabilized by σ and π Hole Interactions. Molecules 2018, 23, 1416. [Google Scholar] [CrossRef] [PubMed]
- Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Implications of monomer deformation for tetrel and pnicogen bonds. Phys. Chem. Chem. Phys. 2018, 20, 8832–8841. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.; Kraka, E. From Molecular Vibrations to Bonding, Chemical Reactions, and Reaction Mechanism. Curr. Org. Chem. 2010, 14, 1524–1560. [Google Scholar] [CrossRef]
- Kraka, E.; Setiawan, D.; Cremer, D. Re-evaluation of the Bond Length-Bond Strength Rule: The Stronger Bond Is not Always the Shorter Bond. J. Comp. Chem. 2016, 37, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, D.; Kraka, E.; Cremer, D. Hidden Bond Anomalies: The Peculiar Case of the Fluorinated Amine Chalcogenides. J. Phys. Chem. A 2015, 119, 9541–9556. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, D.; Sethio, D.; Cremer, D.; Kraka, E. From strong to weak NF bonds: On the design of a new class of fluorinating agents. Phys. Chem. Chem. Phys. 2018, 20, 23913–23927. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Gilbert, A.T.B.; Gill, P.M.W. Calculating molecular vibrational spectra beyond the harmonic approximation. Theor. Chem. Acc. 2007, 120, 23–35. [Google Scholar] [CrossRef]
- Roy, T.K.; Gerber, R.B. Vibrational self-consistent field calculations for spectroscopy of biological molecules: New algorithmic developments and applications. Phys. Chem. Chem. Phys. 2013, 15, 9468–9492. [Google Scholar] [CrossRef] [PubMed]
- Panek, P.T.; Jacob, C.R. Anharmonic Theoretical Vibrational Spectroscopy of Polypeptides. J. Phys. Chem. Lett. 2016, 7, 3084–3090. [Google Scholar] [CrossRef] [PubMed]
- Konkoli, Z.; Cremer, D. A New Way of Analyzing Vibrational Spectra I. Derivation of Adiabatic Internal Modes. Int. J. Quant. Chem. 1998, 67, 1–9. [Google Scholar] [CrossRef]
- Konkoli, Z.; Larsson, J.A.; Cremer, D. A new way of analyzing vibrational spectra. IV. Application and testing of adiabatic modes within the concept of the characterization of normal modes. Int. J. Quant. Chem. 1998, 67, 41–55. [Google Scholar] [CrossRef]
- Zou, W.; Kalescky, R.; Kraka, E.; Cremer, D. Relating Normal Vibrational Modes to Local Vibrational Modes with the Help of an Adiabatic Connection Scheme. J. Chem. Phys. 2012, 137, 084114. [Google Scholar] [CrossRef] [PubMed]
- Kalescky, R.; Kraka, E.; Cremer, D. Description of Aromaticity with the Help of Vibrational Spectroscopy: Anthracene and Phenanthrene. J. Phys. Chem. A 2014, 118, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Freindorf, M.; Tao, Y.; Sethio, D.; Cremer, D.; Kraka, E. New Mechanistic Insights into the Claisen Rearrangement of Chorismate—A Unified Reaction Valley Approach Study. Mol. Phys. 2018, in press. [Google Scholar] [CrossRef]
- Cremer, D.; Larsson, J.A.; Kraka, E. New developments in the analysis of vibrational spectra On the use of adiabatic internal vibrational modes. In Theoretical and Computational Chemistry; Parkanyi, C., Ed.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 259–327. [Google Scholar]
- Zou, W.; Kalescky, R.; Kraka, E.; Cremer, D. Relating normal vibrational modes to local vibrational modes: Benzene and naphthalene. J. Mol. Model. 2012, 19, 2865–2877. [Google Scholar] [CrossRef] [PubMed]
- Humason, A.; Zou, W.; Cremer, D. 11,11-Dimethyl-1,6-methano[10]annulene—An Annulene with an Ultralong CC Bond or a Fluxional Molecule? J. Phys. Chem. A 2015, 119, 1666–1682. [Google Scholar] [CrossRef] [PubMed]
- Kalescky, R.; Kraka, E.; Cremer, D. Identification of the Strongest Bonds in Chemistry. J. Phys. Chem. A 2013, 117, 8981–8995. [Google Scholar] [CrossRef] [PubMed]
- Kalescky, R.; Kraka, E.; Cremer, D. New Approach to Tolman’s Electronic Parameter Based on Local Vibrational Modes. Inorg. Chem. 2014, 53, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Kraka, E.; Cremer, D. Characterization of CF Bonds with Multiple-Bond Character: Bond Lengths, Stretching Force Constants, and Bond Dissociation Energies. ChemPhysChem 2009, 10, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Kalescky, R.; Kraka, E.; Cremer, D. Are carbon-halogen double and triple bonds possible? Int. J. Quantum Chem. 2014, 114, 1060–1072. [Google Scholar] [CrossRef]
- Kalescky, R.; Zou, W.; Kraka, E.; Cremer, D. Quantitative Assessment of the Multiplicity of Carbon-Halogen Bonds: Carbenium and Halonium Ions with F, Cl, Br, and I. J. Phys. Chem. A 2014, 118, 1948–1963. [Google Scholar] [CrossRef] [PubMed]
- Oomens, J.; Kraka, E.; Nguyen, M.K.; Morton, T.H. Structure, Vibrational Spectra, and Unimolecular Dissociation of Gaseous 1-Fluoro-1-phenethyl Cations. J. Phys. Chem. A 2008, 112, 10774–10783. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Kraka, E.; Cremer, D. Quantitative Assessment of Halogen Bonding Utilizing Vibrational Spectroscopy. Inorg. Chem. 2016, 56, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Kraka, E. Systematic Coupled Cluster Study of Noncovalent Interactions Involving Halogens, Chalcogens, and Pnicogens. J. Phys. Chem. A 2017, 121, 9544–9556. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.; Cremer, D. Transition from metal-ligand bonding to halogen bonding involving a metal as halogen acceptor a study of Cu, Ag, Au, Pt, and Hg complexes. Chem. Phys. Lett. 2017, 681, 56–63. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, H.; Yan, H.; Zou, W.; Cremer, D. B−H···π Interaction: A New Type of Nonclassical Hydrogen Bonding. J. Am. Chem. Soc. 2016, 138, 4334–4337. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zhang, X.; Dai, H.; Yan, H.; Cremer, D.; Kraka, E. Description of an unusual hydrogen bond between carborane and a phenyl group. J. Organ. Chem. 2018, 865, 114–127. [Google Scholar] [CrossRef]
- Purvis, G.D.; Bartlett, R.J. A full coupled-cluster singles and doubles model: The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Pople, J.A.; Head-Gordon, M.; Raghavachari, K. Quadratic configuration interaction. A general technique for determining electron correlation energies. J. Chem. Phys. 1987, 87, 5968–5975. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. IV. Calculation of static electrical response properties. J. Chem. Phys. 1994, 100, 2975–2988. [Google Scholar] [CrossRef]
- Wilson, E.B.; Decius, J.C.; Cross, P.C. Molecular Vibrations. The Theory of Infrared and Raman Vibrational Spectra; McGraw-Hill: New York, NY, USA, 1955. [Google Scholar]
- Kraka, E.; Larsson, J.A.; Cremer, D. Generalization of the Badger Rule Based on the Use of Adiabatic Vibrational Modes. In Computational Spectroscopy; Grunenberg, J., Ed.; Wiley: New York, NY, USA, 2010; pp. 105–149. [Google Scholar]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Mentel, ŁM.; Baerends, E.J. Can the Counterpoise Correction for Basis Set Superposition Effect Be Justified? J. Chem. Theory Comput. 2013, 10, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Riplinger, C.; Neese, F. An efficient and near linear scaling pair natural orbital based local coupled cluster method. J. Chem. Phys. 2013, 138, 034106. [Google Scholar] [CrossRef] [PubMed]
- Riplinger, C.; Sandhoefer, B.; Hansen, A.; Neese, F. Natural triple excitations in local coupled cluster calculations with pair natural orbitals. J. Chem. Phys. 2013, 139, 134101. [Google Scholar] [CrossRef] [PubMed]
- Stanton, J.F.; Gauss, J.; Cheng, L.; Harding, M.E.; Matthews, D.A.; Szalay, P.G. CFOUR, Coupled-Cluster techniques for Computational Chemistry, a Quantum-Chemical Program Package. Available online: http://www.cfour.de (accessed on 1 October 2018).
- Harding, M.; Mezroth, T.; Gauss, J.; Auer, A. Parallel Calculation of CCSD and CCSD(T) Analytic First and Second Derivatives. J. Chem. Theory Comput. 2008, 4, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2011, 2, 73–78. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Valency and Bonding: A Natural Bond Orbital Donor-Acceptor Perspective; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Reed, A.; Curtiss, L.; Weinhold, F. Intermolecular Interactions from A Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO6. In Theoretical Chemistry Institute; University of Wisconsin: Madison, WI, USA, 2013. [Google Scholar]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural bond orbital analysis program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Keith, T. TK Gristmill Software. Overland Park, KS, USA. Available online: http//aim.tkgristmill.com (accessed on 1 October 2018).
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Nori-Shargh, D.; Boggs, J.E. On the Covalent Character of Rare Gas Bonding Interactions: A New Kind of Weak Interaction. J. Phys. Chem. A 2012, 117, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Kraka, E.; Zou, W.; Filatov, M.; Gräfenstein, J.; Izotov, D.; Gauss, J.; He, Y.; Wu, A.; Konkoli, Z.; Polo, V.; et al. COLOGNE. 2018. Available online: http://www.smu.edu/catco (accessed on 1 October 2018).
- Li, Y.; Oliveira, V.; Tang, C.; Cremer, D.; Liu, C.; Ma, J. The Peculiar Role of the Au3 Unit in Aum Clusters: σ-Aromaticity of the Au5Zn+ Ion. Inorg. Chem. 2017, 56, 5793–5803. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.; Kraka, E. Chemical Bonds without Bonding Electron Density? Does the Difference Electron- Density Analysis Suffice for a Description of the Chemical Bond? Angew. Chem. Int. Ed. 1984, 23, 627–628. [Google Scholar] [CrossRef]
- Cremer, D.; Kraka, E. A Description of the Chemical Bond in Terms of Local Properties of Electron Density and Energy. Croatica Chem. Acta 1984, 57, 1259–1281. [Google Scholar]
- Grabowski, S. Lewis Acid Properties of Tetrel Tetrafluorides—The Coincidence of the σ-Hole Concept with the QTAIM Approach. Crystals 2017, 7, 43. [Google Scholar] [CrossRef]
- Scheiner, S. Systematic Elucidation of Factors That Influence the Strength of Tetrel Bonds. J. Phys. Chem. A 2017, 121, 5561–5568. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Steric Crowding in Tetrel Bonds. J. Phys. Chem. A 2018, 122, 2550–2562. [Google Scholar] [CrossRef] [PubMed]
- Angarov, V.; Kozuch, S. On the σ, π and δ hole interactions: A molecular orbital overview. New J. Chem. 2018, 42, 1413–1422. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Jin, W.J. σ-Hole Bond vs π-Hole Bond: A Comparison Based on Halogen Bond. Chem. Rev. 2016, 116, 5072–5104. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, Y.; Cheng, J.; Yang, X.; Li, Q. Competition between σ-hole pnicogen bond and π-hole tetrel bond in complexes of CF2=CFZH2 (Z= P, As, and Sb). Mol. Phys. 2018, 1–9. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen bonds, and σ-hole and π-hole bonds—Mechanisms protecting doublet and octet electron structures. Phys. Chem. Chem. Phys. 2017, 19, 29742–29759. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zeng, Y.; Li, X.; Meng, L.; Zhang, X. Insight into the π-holebond···π-electrons tetrel bonds between F2ZO (Z = C, Si, Ge) and unsaturated hydrocarbons. Int. J. Quantum Chem. 2017, 118, e25521. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Li, W.; Cheng, J. Carbene tetrel-bonded complexes. Struct. Chem. 2017, 28, 823–831. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, J.; Yu, X.; Li, Q. Abnormal Tetrel Bonds between Formamidine and TH3F: Substituent Effects. Chem. Sel. 2018, 3, 2842–2849. [Google Scholar]
- Zierkiewicz, W.; Michalczyk, M. On the opposite trends of correlations between interaction energies and electrostatic potentials of chlorinated and methylated amine complexes stabilized by halogen bond. Theor. Chem. Acc. 2017, 136. [Google Scholar] [CrossRef]
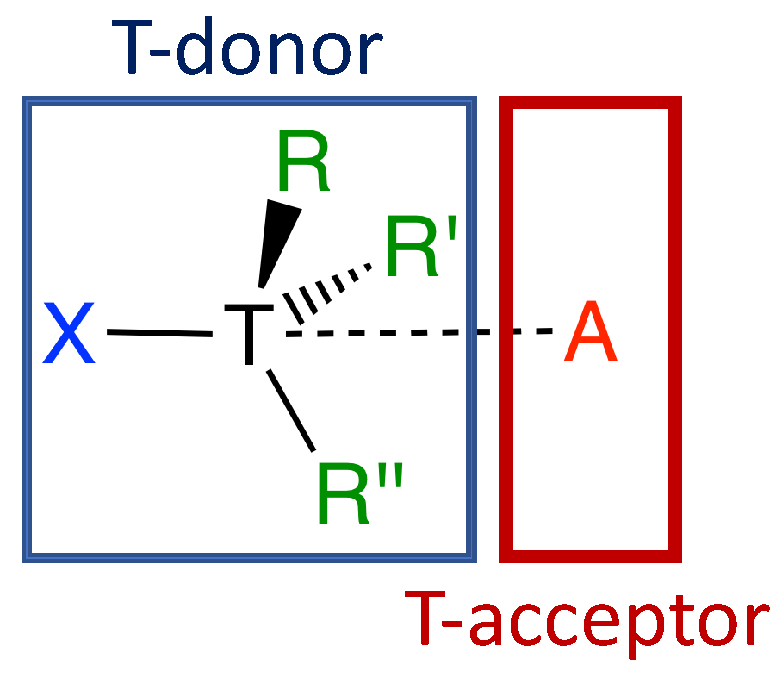
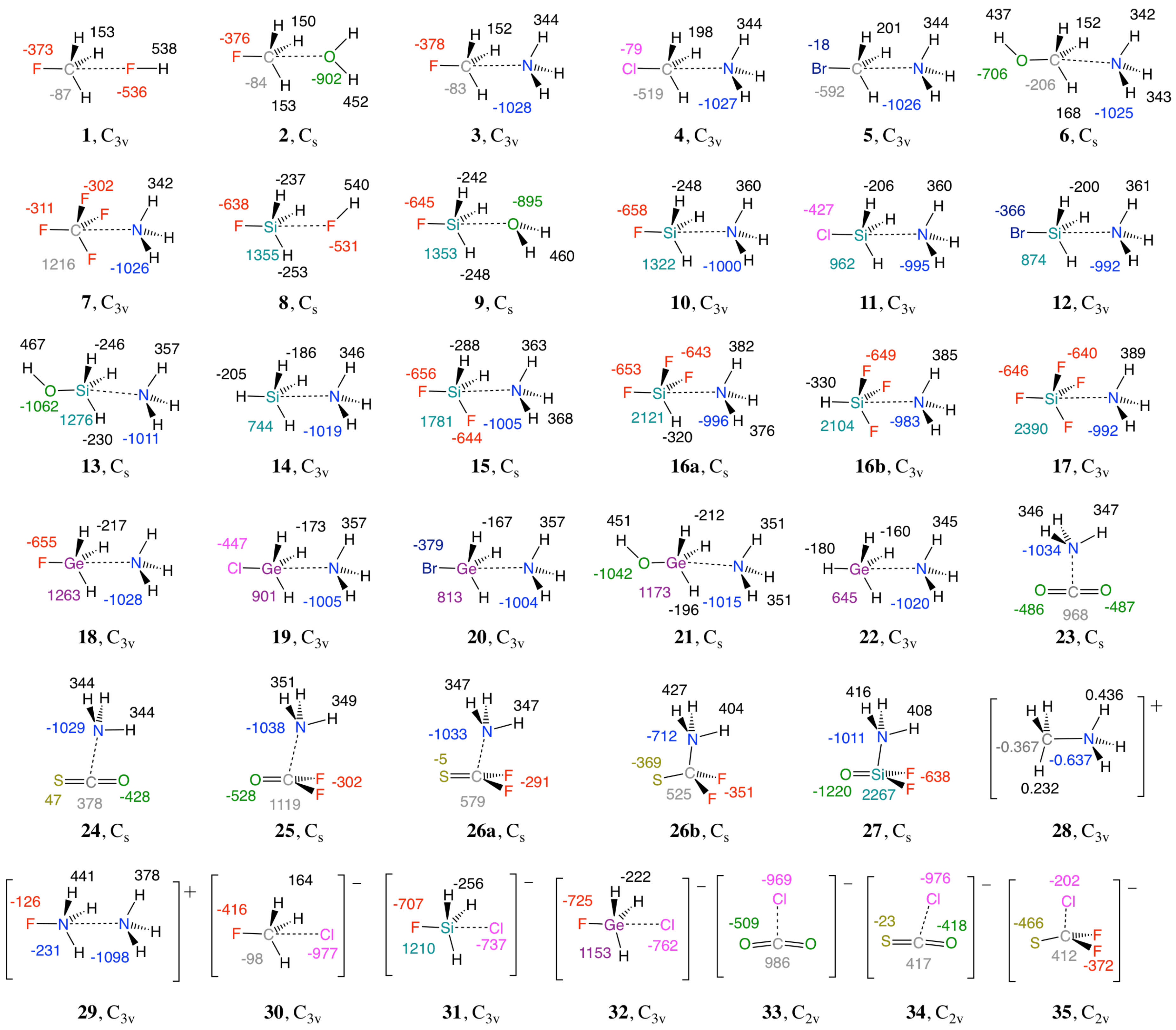
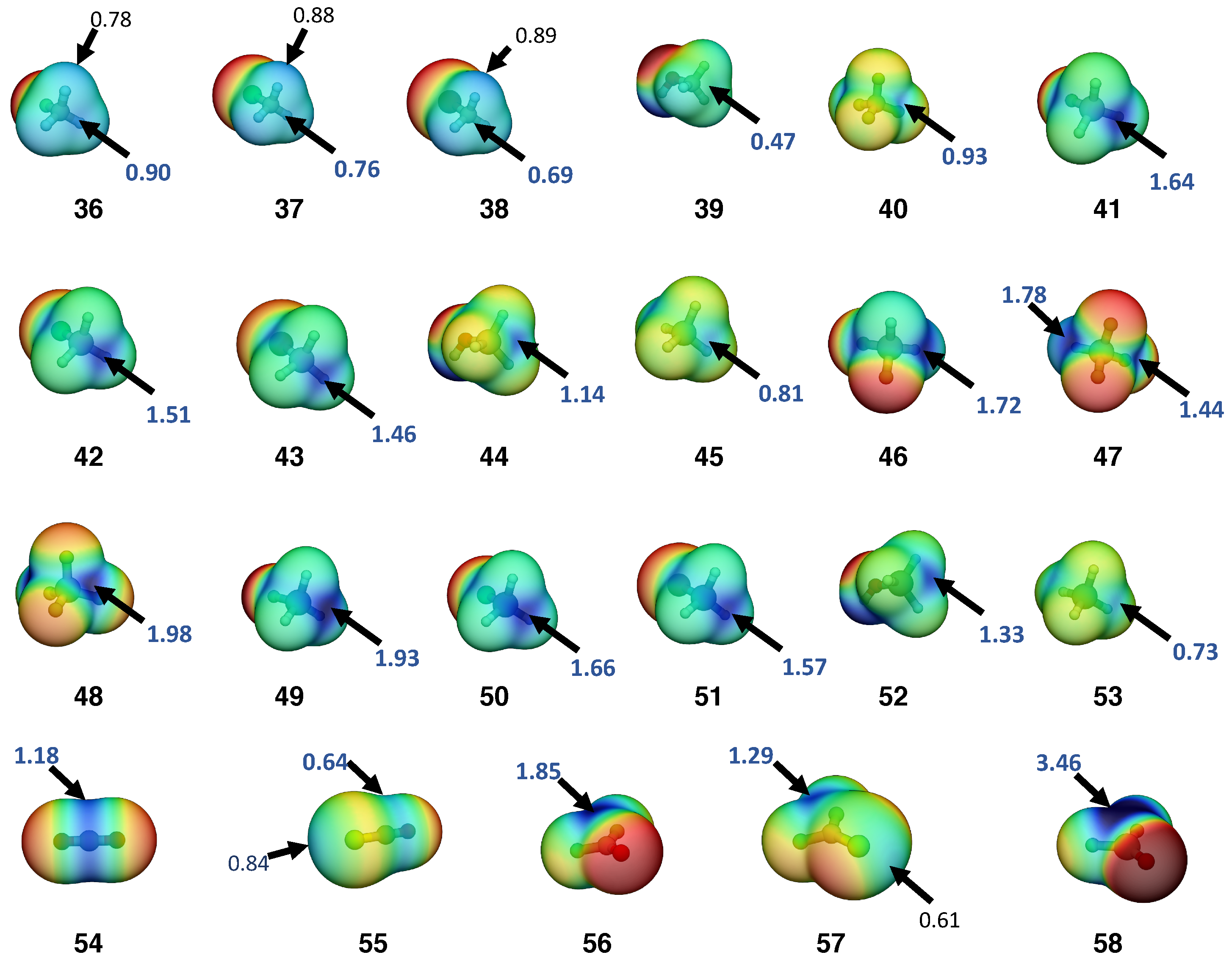

| # | Complex (symm.) | E | r | r | CT | n | n | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TA | XT | TA | TA | TA | TA | XT | XT | ||||||
| Neutral tetrel bonds involving C donors | |||||||||||||
| 1 | FCH3···FH (C3v) | −1.50 | −1.29 | 0.01 | 2.972 | 1.392 | 2 | 0.034 | 0.012 | 0.045 | 0.073 | 5.018 | 1.038 |
| 2 | FCH3···OH2 (Cs) | −2.10 | −1.87 | 0.02 | 3.035 | 1.394 | 3 | 0.041 | 0.012 | 0.055 | 0.081 | 4.956 | 1.030 |
| 3 | FCH3···NH3 (C3v) | −2.25 | −2.05 | 0.02 | 3.218 | 1.395 | 5 | 0.040 | 0.009 | 0.049 | 0.076 | 4.912 | 1.025 |
| 4 | ClCH3···NH3 (C3v) | −2.08 | −1.88 | 0.02 | 3.289 | 1.798 | 6 | 0.037 | 0.008 | 0.043 | 0.071 | 2.943 | 0.768 |
| 5 | BrCH3···NH3 (C3v) | −2.00 | −1.80 | 0.02 | 3.304 | 1.953 | 6 | 0.037 | 0.008 | 0.041 | 0.069 | 2.515 | 0.703 |
| 6 | (HO)CH3···NH3 (Cs) | −1.38 | −1.21 | 0.01 | 3.362 | 1.429 | 3 | 0.031 | 0.008 | 0.032 | 0.060 | 4.652 | 0.994 |
| 7 | CF4···NH3 (C3v) | −1.62 | −1.24 | 0.06 | 3.426 | 1.328 | 1 | 0.030 | 0.007 | 0.044 | 0.072 | 5.926 | 1.140 |
| Neutral tetrel bonds involving Si donors | |||||||||||||
| 8 | FSiH3···FH (Cs) | −2.28 | −1.85 | 0.06 | 2.964 | 1.617 | 9 | 0.055 | 0.005 | 0.062 | 0.087 | 4.970 | 1.032 |
| 9 | FSiH3···OH2 (Cs) | −4.20 | −3.61 | 0.35 | 2.774 | 1.623 | 25 | 0.092 | 0.002 | 0.088 | 0.106 | 4.762 | 1.007 |
| 10 | FSiH3···NH3 (C3v) | −6.80 | −5.94 | 2.11 | 2.523 | 1.637 | 81 | 0.179 | −0.033 | 0.103 | 0.116 | 4.209 | 0.940 |
| 11 | ClSiH3···NH3 (C3v) | −6.13 | −5.41 | 2.02 | 2.580 | 2.117 | 84 | 0.165 | −0.024 | 0.073 | 0.095 | 1.941 | 0.607 |
| 12 | BrSiH3···NH3 (C3v) | −6.11 | −5.35 | 2.23 | 2.566 | 2.290 | 90 | 0.170 | −0.027 | 0.066 | 0.090 | 1.505 | 0.526 |
| 13 | (HO)SiH3···NH3 (Cs) | −4.13 | −3.61 | 0.68 | 2.825 | 1.680 | 42 | 0.108 | −0.003 | 0.070 | 0.093 | 4.065 | 0.921 |
| 14 | SiH4···NH3 (C3v) | −2.27 | −1.97 | 0.15 | 3.202 | 1.490 | 18 | 0.060 | 0.004 | 0.049 | 0.076 | 2.793 | 0.746 |
| 15 | SiF2H2···NH3 (Cs) | −6.99 | −5.73 | 4.74 | 2.400 | 1.613 | 95 | 0.225 | −0.066 | 0.083 | 0.103 | 4.573 | 0.985 |
| 16a | SiF3H···NH3 (Cs) | −7.66 | −5.77 | 11.77 | 2.205 | 1.617 | 139 | 0.320 | −0.126 | 0.249 | 0.191 | 4.698 | 1.000 |
| 16b | HSiF3···NH3 (C3v) | −6.30 | −4.14 | 21.22 | 2.104 | 1.474 | 172 | 0.390 | −0.149 | 0.493 | 0.280 | 2.974 | 0.772 |
| 17 | SiF4···NH3 (C3v) | −11.40 | −8.86 | 21.15 | 2.072 | 1.609 | 176 | 0.419 | −0.164 | 0.678 | 0.335 | 5.046 | 1.041 |
| Neutral tetrel bonds involving Ge donors | |||||||||||||
| 18 | FGeH3···NH3 (C3v) | −7.77 | −7.18 | 1.40 | 2.624 | 1.816 | 44 | 0.169 | −0.008 | 0.149 | 0.143 | 4.125 | 0.929 |
| 19 | ClGeH3···NH3 (C3v) | −6.22 | −5.75 | 1.07 | 2.755 | 2.216 | 64 | 0.134 | −0.001 | 0.103 | 0.116 | 1.921 | 0.604 |
| 20 | BrGeH3···NH3 (C3v) | −6.01 | −5.53 | 1.07 | 2.776 | 2.375 | 66 | 0.132 | 0.000 | 0.097 | 0.112 | 1.591 | 0.543 |
| 21 | (HO)GeH3···NH3 (Cs) | −4.58 | −4.18 | 0.50 | 2.910 | 1.818 | 39 | 0.101 | 0.004 | 0.089 | 0.107 | 3.49 | 0.845 |
| 22 | GeH4···NH3 (C3v) | −1.99 | −1.79 | 0.09 | 3.323 | 1.550 | 15 | 0.052 | 0.005 | 0.047 | 0.074 | 2.580 | 0.713 |
| Neutral tetrel bonds involving double bonded C or Si donors | |||||||||||||
| 23 | CO2···NH3 (Cs) | −3.09 | −2.84 | 0.11 | 2.922 | 1.167 | 5 | 0.107 | 0.002 | 0.079 | 0.100 | 15.183 | 1.938 |
| 24 | SCO···NH3 (Cs) | −1.97 | −1.69 | 0.02 | 3.209 | 1.573 | 3 | 0.046 | 0.009 | 0.047 | 0.074 | 7.081 | 1.260 |
| 25 | CF2O···NH3 (Cs) | −5.55 | −4.82 | 0.27 | 2.687 | 1.178 | 12 | 0.113 | 0.005 | 0.122 | 0.127 | 14.393 | 1.880 |
| 26a | CF2S···NH3 (Cs) | −3.91 | −3.23 | 0.11 | 2.897 | 1.607 | 9 | 0.078 | 0.008 | 0.086 | 0.105 | 6.397 | 1.190 |
| 26b | CF2S···NH3 (Cs) | 1.45 | 4.28 | 24.13 | 1.587 | 1.701 | 545 | 1.388 | −1.339 | 1.414 | 0.508 | 3.828 | 0.891 |
| 27 | SiF2O···NH3 (Cs) | −44.14 | −42.16 | 7.96 | 1.917 | 1.529 | 229 | 0.569 | −0.224 | 1.838 | 0.589 | 8.803 | 1.425 |
| Charge-assisted interactions | |||||||||||||
| 28 | CH3+···NH3 (C3v) | −110.25 | −109.01 | 24.95 | 1.511 | 1.087 | 329 | 1.517 | −1.952 | 3.766 | 0.882 | 5.458 | 1.088 |
| 29 | FNH3+···NH3 (C3v) | −23.14 | −22.77 | 0.43 | 2.619 | 1.374 | 35 | 0.142 | 0.012 | 0.364 | 0.236 | 5.226 | 1.062 |
| 30 | FCH3···Cl− (C3v) | −9.77 | −9.34 | 0.39 | 3.179 | 1.419 | 23 | 0.064 | 0.010 | 0.128 | 0.131 | 4.155 | 0.933 |
| 31 | FSiH3···Cl− (C3v) | −20.73 | −19.49 | 12.03 | 2.504 | 1.703 | 263 | 0.277 | −0.115 | 0.370 | 0.238 | 2.793 | 0.746 |
| 32 | FGeH3···Cl− (C3v) | −26.10 | −25.09 | 10.71 | 2.566 | 1.892 | 238 | 0.290 | −0.069 | 0.455 | 0.268 | 2.451 | 0.693 |
| 33 | CO2···Cl− (Cs) | −7.45 | −6.99 | 1.44 | 2.920 | 1.170 | 31 | 0.107 | 0.002 | 0.109 | 0.120 | 14.879 | 1.916 |
| 34 | SCO···Cl− (Cs) | −5.36 | −4.96 | 0.52 | 3.143 | 1.581 | 24 | 0.073 | 0.006 | 0.079 | 0.100 | 6.568 | 1.208 |
| 35 | CF2S···Cl− (Cs) | −16.81 | −13.83 | 32.63 | 1.898 | 1.725 | 798 | 1.031 | −0.593 | 1.100 | 0.441 | 3.414 | 0.835 |
| # | Monomers | (X) | r(XT) | (XT) | n(XT) | Dipole | |
|---|---|---|---|---|---|---|---|
| 36 | F−CH3 | 0.90 | 1.389 | 5.107 | 1.048 | 1.88 | 2.5 |
| 37 | Cl−CH3 | 0.76 | 1.792 | 3.068 | 0.786 | 1.92 | 4.3 |
| 38 | Br−CH3 | 0.69 | 1.948 | 2.616 | 0.718 | 1.86 | 5.4 |
| 39 | HO−CH3 | 0.47 | 1.426 | 4.749 | 1.006 | 3.1 | |
| 40 | F−CF3 | 0.93 | 1.321 | 6.204 | 1.170 | 0.00 | 2.8 |
| 41 | F−SiH3 | 1.64 | 1.613 | 5.120 | 1.049 | 1.38 | 4.1 |
| 42 | Cl−SiH3 | 1.51 | 2.072 | 2.799 | 0.746 | 1.41 | 6.2 |
| 43 | Br−SiH3 | 1.46 | 2.238 | 2.321 | 0.672 | 1.38 | 7.4 |
| 44 | HO−SiH3 | 1.14 | 1.664 | 4.517 | 0.978 | 4.9 | |
| 45 | H−SiH3 | 0.81 | 1.483 | 2.903 | 0.762 | 0.00 | 4.6 |
| 46 | F−SiH2F | 1.72 | 1.597 | 5.497 | 1.092 | 3.5 | |
| 47a | F−SiF2H | 1.78 | 1.583 | 5.884 | 1.135 | 3.8 | |
| 47b | H−SiF3 | 1.44 | 1.458 | 3.273 | 0.815 | 1.43 | 3.8 |
| 48 | F−SiF3 | 1.98 | 1.571 | 6.281 | 1.178 | 0.00 | 3.3 |
| 49 | F−GeH3 | 1.93 | 1.793 | 4.951 | 1.030 | 2.25 | 4.7 |
| 50 | Cl−GeH3 | 1.66 | 2.175 | 2.491 | 0.699 | 2.04 | 6.9 |
| 51 | Br−GeH3 | 1.57 | 2.330 | 2.091 | 0.633 | 1.93 | 8.1 |
| 52 | HO−GeH3 | 1.33 | 1.802 | 3.872 | 0.896 | 5.5 | |
| 53 | H−GeH3 | 0.73 | 1.542 | 2.693 | 0.730 | 0.00 | 5.2 |
| 54 | O=CO | 1.18 | 1.167 | 15.613 | 1.969 | 0.00 | 2.6 |
| 55 | S=CO | 0.64 | 1.575 | 7.227 | 1.275 | 0.68 | 5.2 |
| 56 | O=CF2 | 1.85 | 1.177 | 14.680 | 1.902 | 1.00 | 2.8 |
| 57 | S=CF2 | 1.29 | 1.603 | 6.626 | 1.214 | 0.16 | 5.2 |
| 58 | O=SiF2 | 3.46 | 1.517 | 9.243 | 1.465 | 2.31 | 4.0 |
| 59 | CH3+ | 10.01 | 0.00 | 1.3 | |||
| 60 | F−NH3+ | 8.58 | 1.368 | 5.642 | 1.109 | 4.78 | 1.7 |
| # | Complex | (X-Si) | (Si-R) | (Si-R’) | (Si-R”) |
|---|---|---|---|---|---|
| 10 | FSiH3···NH3 | 15.7 | 2.4 | 2.4 | 2.4 |
| 15 | SiF2H2···NH3 | 12.7 | 6.2 | 3.1 | 3.1 |
| 16a | SiF3H···NH3 | 16.3 | 11.9 | 11.9 | 7.5 |
| 16b | SiF3H···NH3 | 11.4 | 23.5 | 23.5 | 23.5 |
| 16b | SiHF3···NH3 | 7.9 | 16.8 | 16.8 | 16.8 |
| 17 | SiF4···NH3 | 20.7 | 19.5 | 19.5 | 19.5 |
| Complex | r | CT | n | n | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TA | TA | TA | TA | TA | XT | XT | |||||
| F2···OH2 (Cs) | −1.42 | −1.15 | 2.662 | 0.005 | 0.066 | 0.022 | 0.057 | 0.083 | 4.488 | 0.974 | |
| Cl2···OH2 (Cs) | −2.98 | −2.62 | 2.808 | 0.015 | 0.098 | 0.018 | 0.097 | 0.112 | 2.896 | 0.761 | |
| FCl···OH2 (Cs) | −5.22 | −4.75 | 2.566 | 0.032 | 0.163 | 0.016 | 0.170 | 0.154 | 3.967 | 0.909 | |
| FSH···OH2 (Cs) | −5.69 | −5.15 | 2.659 | 0.028 | 0.138 | 0.010 | 0.152 | 0.144 | 4.011 | 0.914 | |
| FPH2···OH2 (Cs) | −4.63 | −4.02 | 2.780 | 0.021 | 0.107 | 0.006 | 0.118 | 0.125 | 4.198 | 0.938 | |
| F2···NH3 (C3v) | −2.00 | −1.69 | 2.615 | 0.017 | 0.097 | 0.027 | 0.062 | 0.087 | 3.821 | 0.890 | |
| Cl2···NH3 (C3v) | −4.92 | −4.43 | 2.664 | 0.055 | 0.172 | 0.006 | 0.132 | 0.133 | 2.370 | 0.680 | |
| FCl···NH3 (C3v) | −10.13 | −9.39 | 2.320 | 0.145 | 0.358 | −0.058 | 0.311 | 0.216 | 2.687 | 0.729 | |
| FSH···NH3 (Cs) | −8.23 | −7.58 | 2.512 | 0.081 | 0.235 | −0.020 | 0.194 | 0.166 | 3.309 | 0.820 | |
| FPH2···NH3 (Cs) | −6.81 | −6.10 | 2.663 | 0.057 | 0.171 | −0.012 | 0.144 | 0.140 | 3.794 | 0.886 | |
| FCl···Cl− (C∞v) | −30.07 | −28.98 | 2.316 | 0.496 | 0.547 | −0.161 | 0.855 | 0.382 | 1.212 | 0.465 | |
| FSH···Cl− (Cs) | −23.46 | −22.48 | 2.493 | 0.305 | 0.377 | −0.092 | 0.443 | 0.264 | 1.466 | 0.518 | |
| FPH2···Cl− (Cs) | −19.62 | −18.62 | 2.649 | 0.208 | 0.266 | −0.058 | 0.307 | 0.214 | 2.136 | 0.641 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethio, D.; Oliveira, V.; Kraka, E. Quantitative Assessment of Tetrel Bonding Utilizing Vibrational Spectroscopy. Molecules 2018, 23, 2763. https://doi.org/10.3390/molecules23112763
Sethio D, Oliveira V, Kraka E. Quantitative Assessment of Tetrel Bonding Utilizing Vibrational Spectroscopy. Molecules. 2018; 23(11):2763. https://doi.org/10.3390/molecules23112763
Chicago/Turabian StyleSethio, Daniel, Vytor Oliveira, and Elfi Kraka. 2018. "Quantitative Assessment of Tetrel Bonding Utilizing Vibrational Spectroscopy" Molecules 23, no. 11: 2763. https://doi.org/10.3390/molecules23112763
APA StyleSethio, D., Oliveira, V., & Kraka, E. (2018). Quantitative Assessment of Tetrel Bonding Utilizing Vibrational Spectroscopy. Molecules, 23(11), 2763. https://doi.org/10.3390/molecules23112763








