Cytotoxic Effects of Pinnatane A Extracted from Walsura pinnata (Meliaceae) on Human Liver Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Structure of Pinnatane A
2.2. Pinnatane A Induced Cytotoxic Effects in Cancer Cell Lines
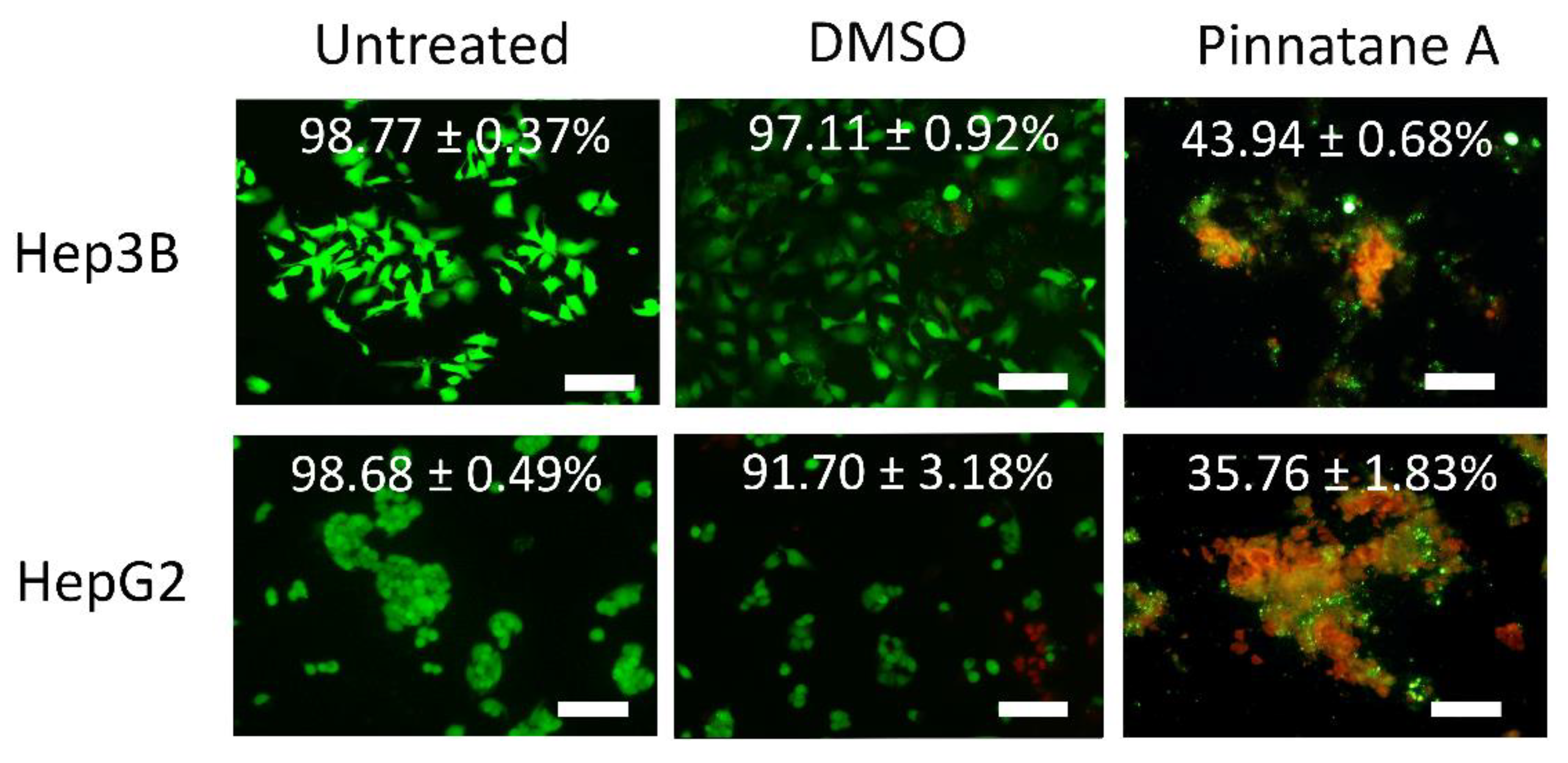
2.3. Pinnatane A Triggered Membrane Integrity Disruption in Liver Cancer Cells
2.4. Pinnatane A Encouraged Cell Cycle Arrest in Liver Cancer Cells
2.5. Pinnatane A Initiated Apoptosis and Necrosis in Liver Cancer Cells
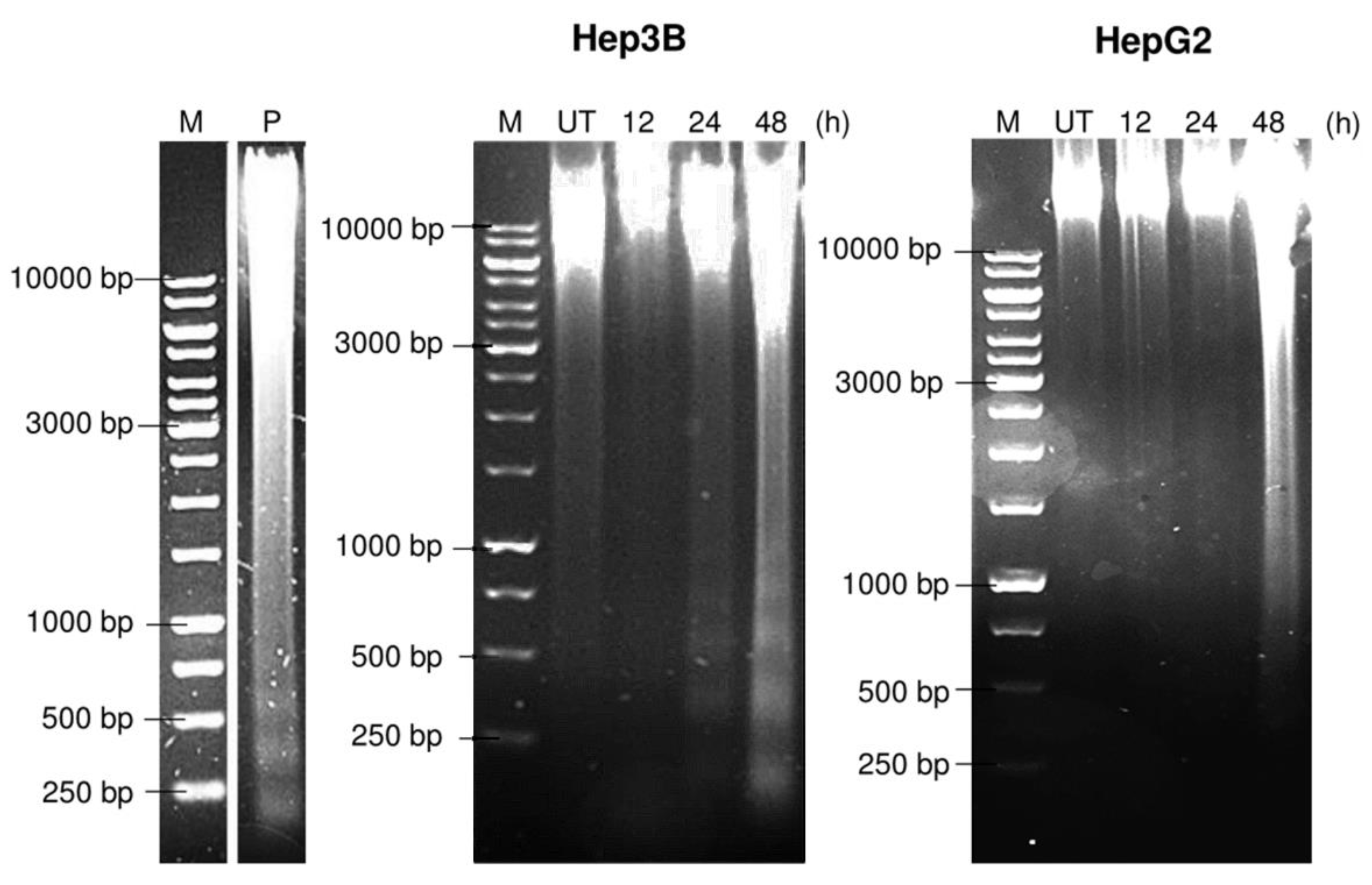
2.6. Pinnatane A Caused Different DNA Degradation Patterns
3. Discussion
4. Materials and Methods
4.1. Reagent and Chemicals
4.2. Plant Materials
4.3. Extraction, Isolation, and Characterization of Pinnatane A
4.4. Cultivation of Cell Lines
4.5. MTT Assay
4.6. Live/Dead Assay
4.7. Cell Cycle Analysis
4.8. Annexin V-FITC/PI Flow Cytometry Assay
4.9. DNA Fragmentation Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeo, W.; Mok, T.S.; Zee, B.; Leung, T.W.; Lai, P.B.; Lau, W.Y.; Koh, J.; Mo, F.K.; Yu, S.C.; Chan, A.T.; et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J. Natl. Cancer Inst. 2005, 97, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Riestra, S.; Rodriguez, M.; Delgado, M.; Suarez, A.; Gonzalez, N.; de la Mata, M.; Diaz, G.; Mino-Fugarolas, G.; Rodrigo, L. Tamoxifen does not improve survival of patients with advanced hepatocellular carcinoma. J. Clin. Gastroenterol. 1998, 26, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Wu, P.C.; Chan, G.C.; Lok, A.S.; Lin, H.J. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer 1988, 62, 479–483. [Google Scholar] [CrossRef]
- Pascual, S.; Herrera, I.; Irurzun., J. New advances in hepatocellular carcinoma. World J. Hepatol. 2016, 8, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M.; Santoro, A. Sorafenib: A review of its use in advanced hepatocellular carcinoma. Drugs 2009, 69, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Raoul, J.-L.; Sherman, M.; Mazzaferro, V.; Bolondi, L.; Craxi, A.; Galle, P.R.; Santoro, A.; Beaugrand, M.; Sangiovanni, A.; et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: Subanalyses of a phase III trial. J. Hepatol. 2012, 57, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Sanoff, H.K.; Chang, Y.; Lund, J.L.; O’Neil, B.H.; Dusetzina, S.B. Sorafenib effectiveness in advanced hepatocellular carcinoma. Oncologist 2016, 21, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.A.; Yu, S.; Mamtani, R.; Mehta, R.; D’Addeo, K.; Wileyto, E.P.; Taddei, T.H.; Kaplan, D.E. Starting dose of sorafenib for the treatment of hepatocellular carcinoma: A retrospective, multi-institutional study. J. Clin. Oncol. 2017, 35, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Zhivotosky, B.; Orrenius, S. Assessment of apoptosis and necrosis by DNA fragmentation and morphological criteria. Curr. Protoc. Cell Biol. 2001, 12, 18.3.1–18.3.23. [Google Scholar] [CrossRef]
- Henriquez, M.; Armisen, R.; Stutzin, A.; Quest, A.F. Cell death by necrosis, a regulated way to go. Curr. Mol. Med. 2008, 8, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Gao, N.; Cheng, S.; Budhraja, A.; Gao, Z.; Chen, J.; Liu, E.H.; Huang, C.; Chen, D.; Yang, Z.; Liu, Q.; et al. Ursolic acid induces apoptosis in human leukaemia cells and exhibits anti-leukaemic activity in nude mice through the PKB pathway. Br. J. Pharmacol. 2012, 165, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N.; Zhang, W.; Wang, Z.; Hai, C. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis 2013, 34, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.P. The species of Walsura and Pseudoclausena genus novum (Meliaceae). Blumea—Biodivers. Evol. Biogeogr. Plants 1994, 38, 247–302. [Google Scholar]
- Soepadmo, E.; Saw, L.G.; Chung, R.C.K. Tree Flora of Sabah and Sarawak; Malaysian Nature Society: Kuala Lumpur, Malaysia, 2002; Volume 4, pp. 212–214. [Google Scholar]
- Mahdzir, M.A.; Shilpi, J.A.; Mahmud, N.; Ramasamy, S.; Awang, K. Chemical constituents from Walsura pinnata (Meliaceae). Nat. Prod. Commun. 2017, 12, 1397–1400. [Google Scholar]
- Leong, K.H.; Mahdzir, M.A.; Din, M.F.; Awang, K.; Tanaka, Y.; Kulkeaw, K.; Ishitani, T.; Sugiyama, D. Induction of intrinsic apoptosis in leukaemia stem cells and in vivo zebrafish model by betulonic acid isolated from Walsura pinnata Hassk (Meliaceae). Phytomedicine 2017, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, M. Chemical Constituents of Walsura pinnata Hassk. Master’s Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2012; pp. 96–99. Available online: http://studentsrepo.um.edu.my/5775/ (accessed on 15 August 2018).
- Mohamad, K.; Yusoff, M.; Awang, K.; Ahmad, K.; Ng, S.W. Pinnatane A from the bark of Walsura pinnata Hassk. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65 Pt 6, o1317. [Google Scholar] [CrossRef]
- Elfita, E.; Muharni, M.; Latief, M.; Darwati, D.; Widiyantoro, A.; Supriyatna, S.; Bahti, H.H.; Dachriyanus, D.; Cos, P.; Maes, L.; et al. Antiplasmodial and other constituents from four Indonesian Garcinia spp. Phytochemistry 2009, 70, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
- Bézivin, C.; Tomasi, S.; Lohézic-Le Dévéhat, F.; Boustie, J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Betulinic acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Žiberna, L.; Šamec, D.; Mocan, A.; Nabavi, S.F.; Bishayee, A.; Farooqi, A.A.; Sureda, A.; Nabavi, S.M. Oleanolic acid alters multiple cell signaling pathways: Implication in cancer prevention and therapy. Int. J. Mol. Sci. 2017, 18, 643. [Google Scholar] [CrossRef] [PubMed]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.; Ayres-Saraiva, D.; Gattass, C.R.; Capella, M.A.M. Oleanolic acid inhibits the activity of the multidrug resistance protein ABCC1 (MRP1) but not of the ABCB1 (P-glycoprotein): Possible use in cancer chemotherapy. Cancer Lett. 2007, 248, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Castilho, R.O.; da Costa, M.R.; Wagner-Souza, K.; Coelho Kaplan, M.A.; Gattass, C.R. Pentacyclic triterpenes from Chrysobalanaceae species: Cytotoxicity on multidrug resistant and sensitive leukemia cell lines. Cancer Lett. 2003, 190, 165–169. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Efferth, T. Cameroonian medicinal plants: Pharmacology and derived natural products. Front. Pharmacol. 2010, 1, 123. [Google Scholar] [CrossRef] [PubMed]
- Boik, J. Natural Compounds in Cancer Therapy; Oregon Medical Press: Princeton, MN, USA, 2001; p. 25. [Google Scholar]
- Kajstura, M.; Halicka, H.D.; Pryjma, J.; Darzynkiewicz, Z. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete “sub-G1” peaks on DNA content histograms. Cytom. A 2007, 71, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Chang, U.M.; Li, C.H.; Lin, L.I.; Huang, C.P.; Kan, L.S.; Lin, S.B. Ganoderiol F, a ganoderma triterpene, induces senescence in hepatoma HepG2 cells. Life Sci. 2006, 79, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.I.; Harper, J.W. Anticancer drug targets: Cell cycle and checkpoint control. J. Clin. Investig. 1999, 104, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Segawa, K.; Nagata, S. An apoptotic ‘eat me’ signal: Phosphatidylserine exposure. Trends Cell Biol. 2015, 25, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Berghe, T.V.; Denecker, G.; Brouckaert, G.; Krysko, D.V.; D’Herde, K.; Vandenabeele, P. More than one way to die. In Tumor Necrosis Factor Methods and Protocols; Humana Press Inc.: Totowa, NJ, USA, 2004; pp. 110–113. [Google Scholar]
- Yan, S.-L.; Huang, C.-Y.; Wu, S.-T.; Yin, M.-C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.W.; Choi, D.S.; Cardone, M.H.; Kim, C.W.; Sinskey, A.J.; Rha, C. Glioblastoma cell death induced by asiatic acid. Cell Biol. Toxicol. 2006, 22, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.-J.; Cheng, J.-S.; Huang, H.-C. Effect of saikosaponin, a triterpene saponin, on apoptosis in lymphocytes: Association with c-myc, p53, and bcl-2 mRNA. Br. J. Pharmacol. 2000, 131, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



| Human Cell Lines | IC50 (µM) | SI a |
|---|---|---|
| Normal lung fibroblast (MRC-5) | 48.8 ± 1.0 | N.A. c |
| Breast adenocarcinoma (MCF-7) | 60.9 ± 2.3 | 0.80 |
| Breast adenocarcinoma (MDA-MB-231) | 92.9 ± 3.1 | 0.53 |
| Bladder carcinoma (EJ-28) | 33.9 ± 3.8 | 1.44 |
| Bladder carcinoma (RT-112) | 48.0 ± 4.6 | 1.02 |
| Cervical adenocarcinoma (HeLa S3) | 59.7 ± 0.9 | 0.82 |
| Cervical carcinoma (SiHa) | >100 b | N.C. d |
| Hepatocellular carcinoma (Hep3B) | 19.0 ± 0.5 | 2.57 |
| Hepatocellular carcinoma (HepG2) | 55.8 ± 2.3 | 0.87 |
| Lung adenocarcinoma (A549) | 50.9 ± 3.1 | 0.96 |
| Lung adenocarcinoma (SK-LU-1) | >100 b | N.C. d |
| Prostate carcinoma (DU 145) | 87.2 ± 2.1 | 0.56 |
| Prostate adenocarcinoma (PC-3) | 55.3 ± 3.9 | 0.88 |
| Time (h) | MRC-5 | Hep3B | HepG2 | ||
|---|---|---|---|---|---|
| IC50 (µM) | IC50 (µM) | SI a | IC50 (µM) | SI a | |
| 12 | 86.0 ± 4.5 | 52.7 ± 5.5 | 1.63 | 70.7 ± 0.5 | 1.22 |
| 24 | 48.6 ± 1.2 | 19.0 ± 0.5 | 2.56 | 55.8 ± 2.3 | 0.87 |
| 48 | 53.3 ± 4.6 | 13.5 ± 1.6 | 3.95 | 17.1 ± 2.1 | 3.12 |
| 72 | 53.1 ± 0.8 | 5.0 ± 0.0 | 10.62 | 8.8 ± 0.6 | 6.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, N.; Mahdzir, M.A.; Yusoff, M.; Mohd Arshad, N.; Awang, K.; Nagoor, N.H. Cytotoxic Effects of Pinnatane A Extracted from Walsura pinnata (Meliaceae) on Human Liver Cancer Cells. Molecules 2018, 23, 2733. https://doi.org/10.3390/molecules23112733
Zakaria N, Mahdzir MA, Yusoff M, Mohd Arshad N, Awang K, Nagoor NH. Cytotoxic Effects of Pinnatane A Extracted from Walsura pinnata (Meliaceae) on Human Liver Cancer Cells. Molecules. 2018; 23(11):2733. https://doi.org/10.3390/molecules23112733
Chicago/Turabian StyleZakaria, Nurhisyam, Mohamad Azrul Mahdzir, Mahfuzah Yusoff, Norhafiza Mohd Arshad, Khalijah Awang, and Noor Hasima Nagoor. 2018. "Cytotoxic Effects of Pinnatane A Extracted from Walsura pinnata (Meliaceae) on Human Liver Cancer Cells" Molecules 23, no. 11: 2733. https://doi.org/10.3390/molecules23112733
APA StyleZakaria, N., Mahdzir, M. A., Yusoff, M., Mohd Arshad, N., Awang, K., & Nagoor, N. H. (2018). Cytotoxic Effects of Pinnatane A Extracted from Walsura pinnata (Meliaceae) on Human Liver Cancer Cells. Molecules, 23(11), 2733. https://doi.org/10.3390/molecules23112733






