The Increase of Triterpene Saponin Production Induced by Trans-Anethole in Hairy Root Cultures of Panax quinquefolium
Abstract
1. Introduction
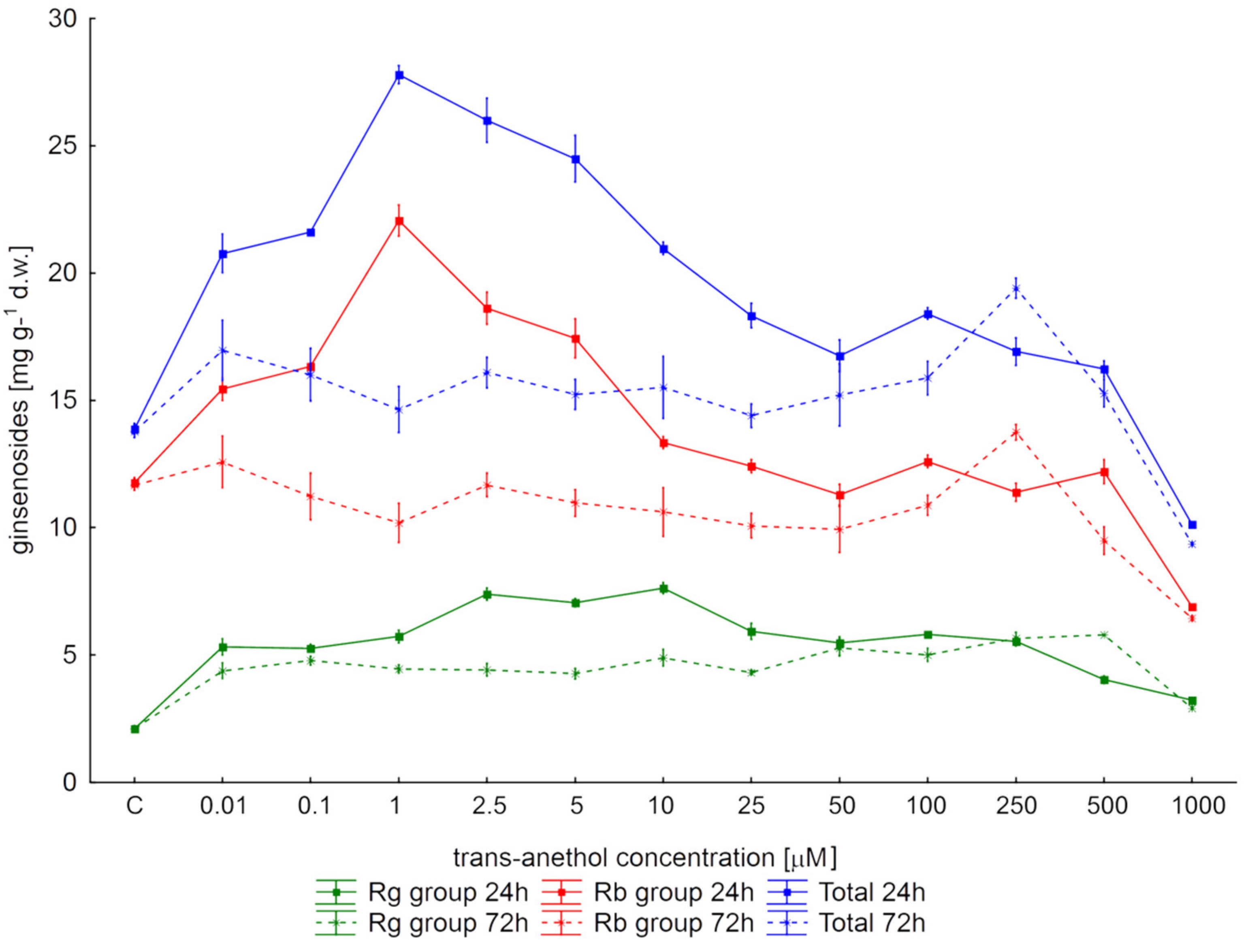
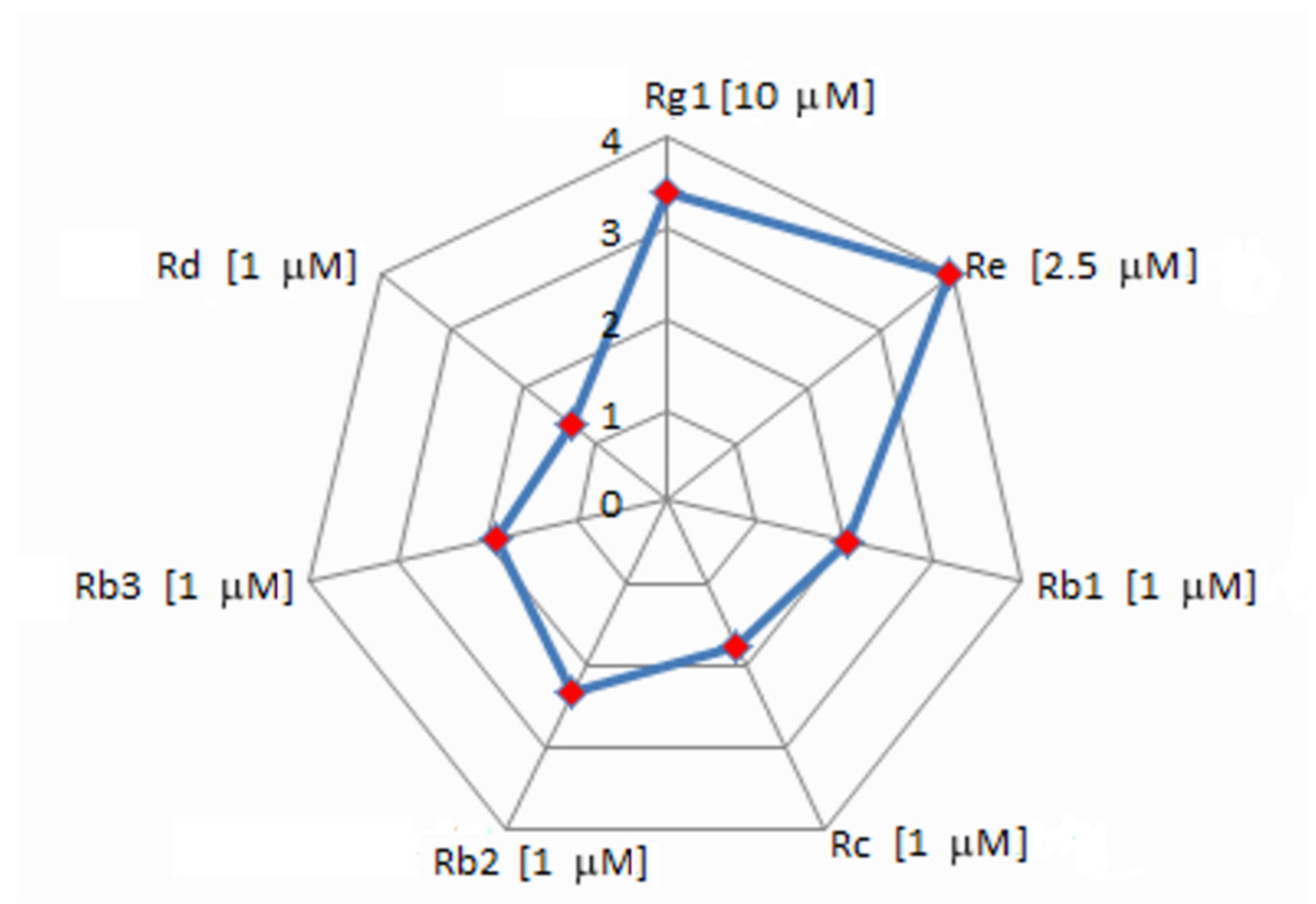
2. Results
3. Discussion
4. Materials and Methods
4.1. Hairy Root Culture
4.2. Elicitation Process
4.3. Ginsenoside Content Determination
4.3.1. Sample Preparation
4.3.2. Standard Solution
4.3.3. HPLC analysis of ginsenosides
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, W.Z.; Hu, Y.; Wu, W.Y.; Ye, M.; Guo, D.A. Saponins in the genus Panax L. (Araliaceae): A systematic review of their chemical diversity. Phytochemistry 2014, 106, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Park, S.Y.; Yin, C.S.; Kim, H.-T.; Kim, Y.M.; Hoo Yi, T.H. Antiaging effects of the mixture of Panax ginseng and Crataegus pinnatifida in human dermal fibroblasts and healthy human skin. J. Ginseng Res. 2017, 41, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Johnke, R.M.; Allison, R.R.; O’Brien, K.F.; Dobbs, L.J., Jr. Radioprotective potential of ginseng. Mutagenesis 2005, 20, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Tansakul, P.; Shibuya, M.; Kushiro, T.; Ebizuka, Y. Dammarenediol-II synthase, the first dedicated enzyme for ginsenoside biosynthesis, in Panax ginseng. FEBS Lett. 2006, 580, 5143–5149. [Google Scholar] [CrossRef] [PubMed]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Lipert, A.; Szymańska, G. Yeast extract stimulates ginsenoside production in hairy root cultures of American ginseng cultivated in shake flasks and nutrient sprinkle bioreactor. Molecules 2017, 22, 880. [Google Scholar] [CrossRef] [PubMed]
- Kochan, E.; Balcerczak, E.; Lipert, A.; Szymańska, G.; Szymczyk, P. Methyl jasmonate as a control factor of the synthase squalene gene promoter and ginsenoside production in American ginseng hairy root cultured in shake flasks and a nutrient sprinkle bioreactor. Ind. Crops Prod. 2018, 115, 182–193. [Google Scholar] [CrossRef]
- Naik, P.M.; Al-Khayri, J.M. Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plants. In Abiotic and Biotic Stress in Plants–Recent Advances and Future Perspectives; Intech: Rijeka, Croatia, 2016; Chapter 10; pp. 247–277. [Google Scholar]
- Ramirez-Estrada, K.; Vidal- Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 3, 182. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Wyslouzil, B.; Weathers, P.J. Secondary metabolism of hairy root cultures in bioreactors. In Vitro Cell. Dev. Biol. Plant 2002, 38, 1–10. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Mnichowska-Polanowska, M.; Pruss, A.; Masiuk, H.; Dzięcioł, M.; Giedrys-Kalemba, S.; Sienkiewicz, M. The effect of fennel essentials oil in combination with antibiotics on Staphylococcus aureus strains isolated from carriers. Burns 2017, 43, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Camurça, V.A.L.F.; Bevilaqua, C.M.L.; Morais, S.M.; Maciel, M.V.; Costa, C.T.C.; Macedo, I.T.F.; Oliveira, L.M.B.; Braga, R.R.; Silva, R.A.; Vieira, L.S. Anthelmintic activity of Croton zehntneri and Lippia sidoides essential oils. Vet. Parasitol. 2007, 148, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Janet, M.; Fatemeh, A.; Jalal, Z. Anethole, a medicinal plant compound, decreases the production of pro-inflammatory TNF-α and IL-1β in a Rat Model of LPS-induced periodontitis. Iran J. Pharm. Res. 2014, 13, 1319–1325. [Google Scholar]
- Massimiliano, T.; Vigilio, B.; Simona, B.; Renato, B.; Mariannina, I.; Elisabetta, B. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol. Res. 2007, 56, 254–260. [Google Scholar]
- Jenner, P.M.; Hagan, E.C.; Taylor, J.M.; Cook, E.L.; Fitzhugh, O.G. Food flavourings and compounds of related structure I. Acute oral toxicity. Food Cosmet. Toxicol. 1964, 2, 27–43. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Boland, R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hahn, E.J.; Murthy, H.N.; Paek, K.Y. Adventitous root growth and ginsenoside accumulation in Panax ginseng cultures as affected by methyl jasmonate. Biotechnol. Lett. 2004, 26, 1789–1792. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wong, H.; Teng, W. Effects of elicitation on the production of saponin in cell culture of Panax ginseng. Plant Cell Rep. 2001, 20, 674–677. [Google Scholar]
- Hu, F.X.; Zhong, J.J. Jasmonic acid mediates gene transcription of ginsenoside biosynthesis in cell cultures of Panax notoginseng treated with chemically synthesized 2-hydroxyethyl jasmonate. Process Biochem. 2008, 43, 113–118. [Google Scholar] [CrossRef]
- Palazón, J.; Cusidó, R.M.; Binfill, M.; Mallol, A.; Moyano, E.; Morales, C.; Pinol, M.T. Elicitation of different Panax ginseng transformed root phenotypes for an improved ginsenoside production. Plant Physiol. Biochem. 2003, 41, 1019–1025. [Google Scholar] [CrossRef]
- Kim, O.T.; Bang, K.H.; Kim, Y.C.; Cha, S.W. Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tissue Organ Cult. 2009, 98, 25–33. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, J.J. Manipulation of ginsenoside heterogeneity in cell cultures of Panax notoginseng by addition of jasmonates. J. Biosci. Bioeng. 2002, 93, 48–53. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.; Li, Y.; Li, J.; Ouyang, Y.; He, Z.; Zhao, S. Enhancement of ginsenoside biosynthesis and secretion by Tween 80 in Panax ginseng hairy roots. Biotechnol. Appl. Biochem. 2015, 62, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Kochan, E.; Królicka, A.; Chmiel, A. Growth and ginsenoside production in Panax quinquefolium hairy roots cultivated in flasks and nutrient sprinkle bioreactor. Acta Physiol. Plant. 2012, 34, 1513–1518. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of sojabean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Szymańska, G. Nitrogen and phosphorus as the factors affecting ginsenoside production in hairy root cultures of Panax quinquefolium cultivated in shake flasks and nutrient sprinkle bioreactor. Acta Physiol. Plant. 2016, 38, 149. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Ginsenosides | Regression Equation | r2 | LOD (µg mL−1) | LOQ (µg mL−1) |
|---|---|---|---|---|
| Rb1 | y = 206.23x − 0.83 | 0.9992 | 2.02 | 6.66 |
| Rb2 | y = 149.39x − 0.19 | 0.9988 | 1.78 | 5.87 |
| Rb3 | y = 699.95x − 0.55 | 0.9988 | 0.26 | 0.86 |
| Rc | y = 142.57x − 0.40 | 0.9981 | 1.35 | 4.46 |
| Rd | y = 166.08x − 0.46 | 0.9993 | 1.80 | 5.94 |
| Rg1 | y = 181.38x + 0.19 | 0.9989 | 0.55 | 1.82 |
| Rg2 | y = 181.18x + 0.32 | 0.9989 | 0.29 | 0.96 |
| Re | y = 155.38x − 0.21 | 0.9989 | 1.44 | 4.75 |
| Rf | y = 202.63x − 0.98 | 0.9998 | 0.18 | 0.59 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Szymańska, G.; Wajs-Bonikowska, A.; Bonikowski, R.; Sienkiewicz, M. The Increase of Triterpene Saponin Production Induced by Trans-Anethole in Hairy Root Cultures of Panax quinquefolium. Molecules 2018, 23, 2674. https://doi.org/10.3390/molecules23102674
Kochan E, Szymczyk P, Kuźma Ł, Szymańska G, Wajs-Bonikowska A, Bonikowski R, Sienkiewicz M. The Increase of Triterpene Saponin Production Induced by Trans-Anethole in Hairy Root Cultures of Panax quinquefolium. Molecules. 2018; 23(10):2674. https://doi.org/10.3390/molecules23102674
Chicago/Turabian StyleKochan, Ewa, Piotr Szymczyk, Łukasz Kuźma, Grażyna Szymańska, Anna Wajs-Bonikowska, Radosław Bonikowski, and Monika Sienkiewicz. 2018. "The Increase of Triterpene Saponin Production Induced by Trans-Anethole in Hairy Root Cultures of Panax quinquefolium" Molecules 23, no. 10: 2674. https://doi.org/10.3390/molecules23102674
APA StyleKochan, E., Szymczyk, P., Kuźma, Ł., Szymańska, G., Wajs-Bonikowska, A., Bonikowski, R., & Sienkiewicz, M. (2018). The Increase of Triterpene Saponin Production Induced by Trans-Anethole in Hairy Root Cultures of Panax quinquefolium. Molecules, 23(10), 2674. https://doi.org/10.3390/molecules23102674







