Biochemical Characterization of the Rice Cinnamyl Alcohol Dehydrogenase Gene Family
Abstract
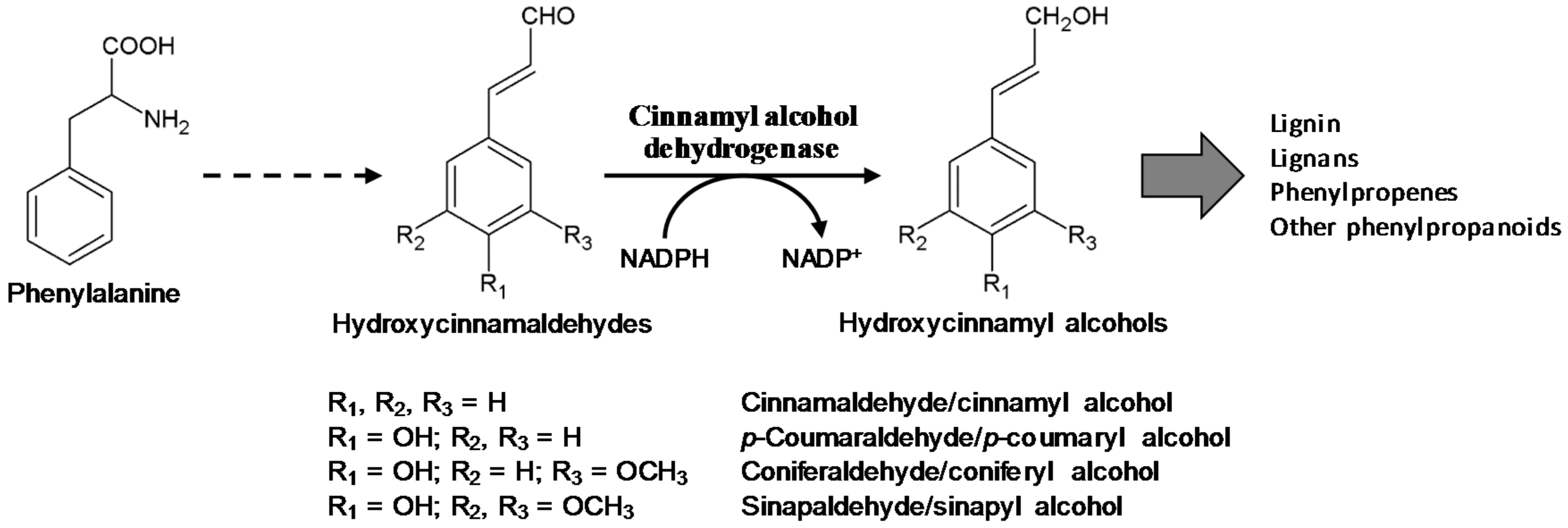
1. Introduction
2. Results
2.1. The CAD Gene Family in Rice
2.2. Sequence Homology and Phylogenetic Analysis of the OsCADs
2.3. Heterologous Expression and Biochemical Characterization of OsCADs
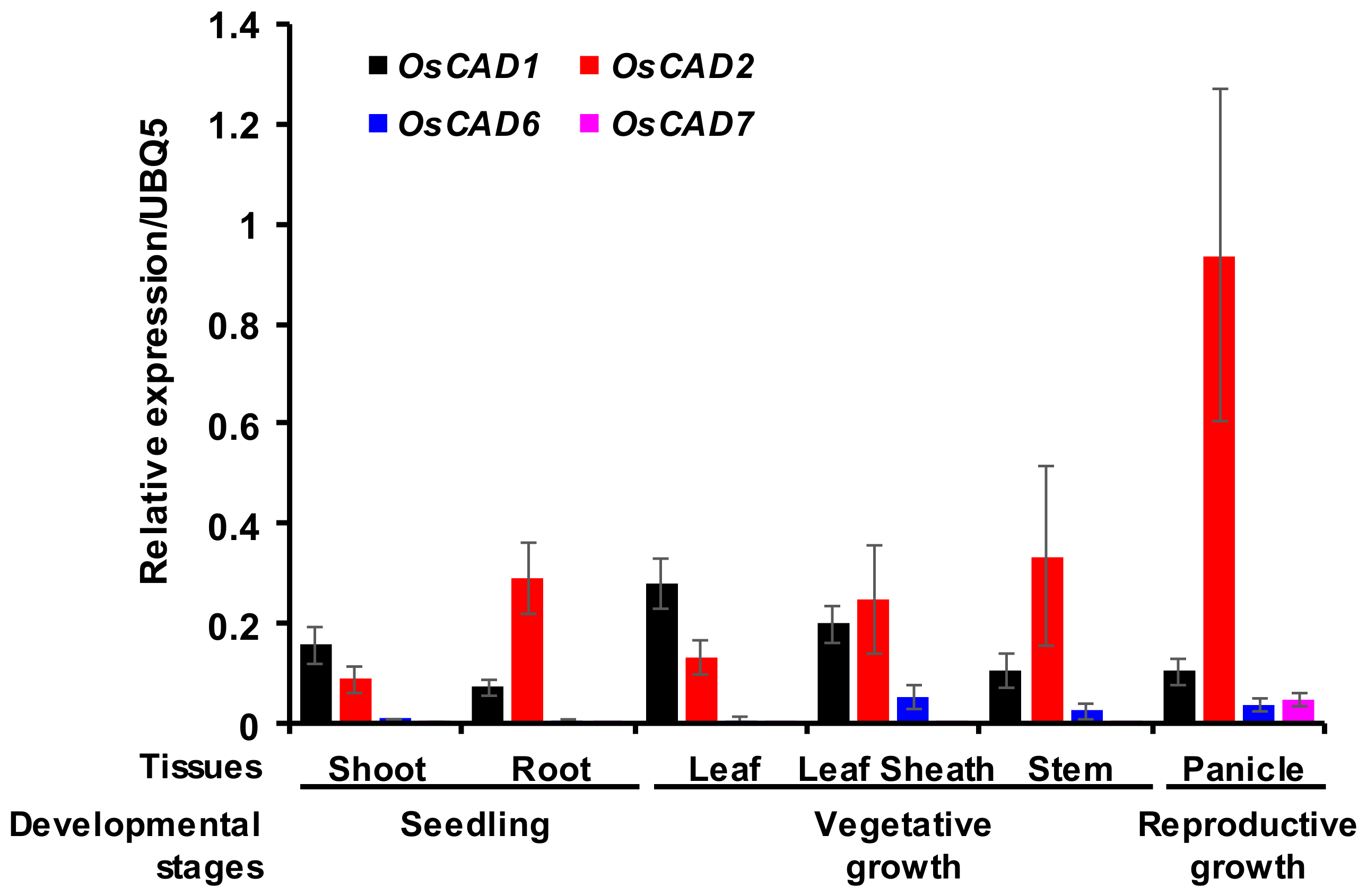
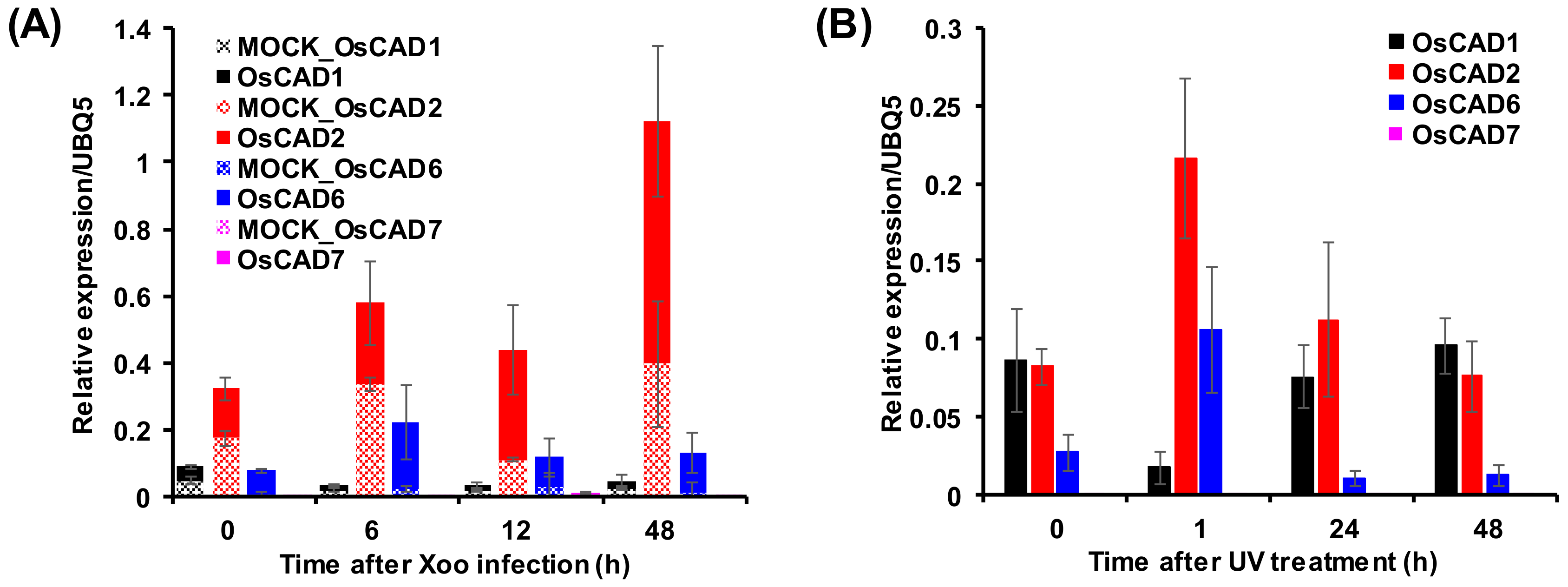
2.4. Expression Analysis of the Biochemically Active OsCADs
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Materials
4.2. Amino Acid Sequence Alignment and Phylogenetic Analysis of OsCADs
4.3. Construction of OsCAD Expression Vectors
4.4. Heterologous Expression and Purification of Recombinant OsCADs
4.5. CAD Activity Assay
4.6. In Silico Transcriptomic and qRT-PCR Analyses of OsCADs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Davin, L.B.; Lewis, N.G. Dirigent proteins and dirigent sites explain the mystery of specificity of radical precursor coupling in lignin and lignin biosynthesis. Plant Physiol. 2000, 123, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.M.; Sederoff, R.R. Variation in Lignin Content and Composition (Mechanisms of Control and Implications for the Genetic Improvement of Plants). Plant Physiol. 1996, 110, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.A. Lignification and lignin topochemistry—An ultrastructural view. Phytochemistry 2001, 57, 859–873. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Chapple, C. The genetics of lignin biosynthesis: Connecting genotype to phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.C.M.S.; Bonine, C.A.V.; Vlana, J.O.F.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in lignin content and composition in plants. J. Int. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Hamann, T. Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front. Plant Sci. 2012, 3, 77. [Google Scholar] [CrossRef] [PubMed]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef] [PubMed]
- König, S.; Feussner, K.; Kaever, A.; Landesfeind, M.; Thurow, C.; Karlovsky, P.; Gatz, C.; Polle, A.; Feussner, I. Soluble phenylpropanoids are involved in the defense response of Arabidopsis against Verticillium longisporum. New Phytol. 2014, 202, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.G.; Davin, L.B. Lignans: Biosynthesis and function. In DHR Barton; Nakanishi, K., Meth-Cohn, O., Eds.; Comprehensive Natural Products Chemistry, Elsevier: London, UK, 1999; Volume 1, pp. 639–712. [Google Scholar]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide analysis of phenylpropanoid defense pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef] [PubMed]
- Begum, A.S.; Kumar, S.S. Advances in the chemistry and pharmacological potential of coumarinolignans. Top. Curr. Chem. 2018, 376, 34. [Google Scholar] [CrossRef] [PubMed]
- Ayres, D.C.; Loike, J.D. Lignans Chemical, Biological and clinical properties. In Chemistry and & Pharmacology of Natural Products; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Zhang, X.; Rakecsh, K.P.; Shantharam, C.S.; Manukumar, H.M.; Asiri, A.M.; Marwani, H.M.; Qin, H.L. Podophyllotoxin derivatives as an excellent anticancer aspirant for future chemotherapy: A key current imminent needs. Bioorg. Med. Chem. 2018, 26, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Gang, D.R.; Wang, J.; Dudareva, N.; Nam, K.H.; Simon, J.E.; Lewinsohn, E.; Pichersky, E. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol. 2001, 125, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Koeduka, T.; Fridman, E.; Gang, D.R.; Vassão, D.G.; Jackson, B.L.; Kish, C.M.; Orlova, I.; Spassova, S.M.; Lewis, N.G.; Noel, J.P.; et al. Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc. Natl. Acad. Sci. USA 2006, 103, 10128–10133. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.G. Phenylpropenes: Occurrence, distribution, and biosynthesis in fruit. J. Agric. Food Chem. 2018, 66, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Koeduka, T. Functional evolution of biosynthetic enzymes that produce plant volatiles. Biosci. Biotechnol. Biochem. 2018, 82, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Sisk, S.B.; Shorey, H.H.; Gerber, R.G.; Gaston, L.K. Semiochemicals that disrupt foraging the argentine ant (Hymenoptera: Formicidae): Laboratory bioassays. J. Chem. Ecol. 2011, 37, 1143–1149. [Google Scholar] [CrossRef]
- Obeng-Ofori, D.; Reichmuth, C.H. Bioactivity of eugenol, a major component of essential oil of Ocimum suave (Wild.) against four species of stored-product Coleoptera. Int. J. Pest Manag. 1997, 43, 89–94. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant. 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.K.; Chapple, C. The origin and evolution of lignin biosynthesis. New Phytol. 2010, 187, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Sibout, R.; Eudes, A.; Pollet, B.; Goujon, T.; Mila, I.; Granier, F.; Séguin, A.; Lapierre, C.; Jouanin, L. Expression pattern of two paralogs encoding cinnamyl alcohol dehydrogenases in Arabidopsis. Isolation and characterization of the corresponding mutants. Plant Physiol. 2003, 132, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, M.R.; Bedgar, D.L.; Moinuddin, S.G.A.; Cardenas, C.L.; Davin, L.B.; Kang, C.H.; Lewis, N.G. Functional reclassification of the putative cinnamyl alcohol dehydrogenase multigene family in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Saballos, A.; Ejeta, G.; Sanchez, E.; Kang, C.H.; Vermerris, W. A genomewide analysis of the cinnamyl alcohol dehydrogenase family in Sorghum [Sorghum bicolar (L) Moench] identifies SbCAD2 as the brown midrib6 gene. Genetics 2009, 181, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Bagniewska-Zadworna, A.; Choi, A.; Plakkat, U.; DiLoreto, D.S.; Yellanki, P.; Carlson, J.E. The cinnamyl alcohol dehydrogenase gene family in Populus: Phylogeny, organization, and expression. BMC Plant Biol. 2009, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Rong, W.; Luo, M.; Shan, T.; Wei, X.; Du, L.; Xu, H.; Zhang, Z. A wheat cinnamyl alcohol dehydrogenase TaCAD12 contributes to host resistance to the sharp eyespot disease. Front. Plant Sci. 2016, 7, 1723. [Google Scholar] [CrossRef] [PubMed]
- Halpin, C.; Holt, K.; Chojecki, J.; Oliver, D.; Chabbert, B.; Monties, B.; Edwards, K.; Barakate, A.; Foxon, G.A. Brown-midrib maize (bm1)—A mutation affecting the cinnamyl alcohol dehydrogenase gene. Plant J. 1998, 14, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.H. Functional analysis of a cinnamyl alcohol dehydrogenase involved in lignin biosynthesis in wheat. J. Exp. Bot. 2010, 61, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
- Chao, N.; Liu, S.X.; Liu, B.M.; Li, N.; Jiang, X.N.; Gai, Y. Molecular cloning and functional analysis of nine cinnamyl alcohol dehydrogenase family members in Populus tomentosa. Planta 2014, 240, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Sibout, R.; Eudes, A.; Mouille, G.; Pollet, B.; Lapierre, C.; Jouanin, L.; Séguin, A. CINNAMYL ALCOHOL DEHYDROGENASE-C and -D are the primary genes involved in lignin biosynthesis in the floral stem of Arabidopsis. Plant Cell 2005, 17, 2059–2076. [Google Scholar] [CrossRef] [PubMed]
- Tobias, C.M.; Chow, E.K. Structure of the cinnamyl-alcohol dehydrogenase gene family in rice and promoter activity of a member associated with lignification. Planta 2005, 220, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Aya, K.; Kondo, M.; Okuno, A.; Morinaka, Y.; Matsuoka, M. OsCAD2is the major CAD gene responsible for monolignol biosynthesis in rice culm. Plant Cell Rep. 2012, 31, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Qian, Q.; Huang, Z.; Wang, Y.; Li, M.; Hong, L.; Zeng, D.; Gu, M.; Chu, C.; Cheng, Z. GOLD HULL AND INTERNODE2 encodes a primarily multifunctional cinnamyl-alcohol dehydrogenase in rice. Plant Physiol. 2006, 140, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Yao, J.; Chen, G.; Li, X.; Zhang, Q.; Wu, C. FLEXIBLE CULM 1 encoding a cinnamyl-alcohol dehydrogenase controls culm mechanical strength in rice. Plant Mol. Biol. 2009, 69, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.; Zhou, S.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Lee, S.S.; Tanaka, T.; Numa, H.; Kim, J.; Kawahara, Y.; Wakimoto, H.; Yang, C.C.; Iwamoto, M.; Abe, T.; et al. Rice Annotation Project Database (RAP-DB): An integrative and interactive database for rice genomics. Plant Cell Physiol. 2013, 54, e6. [Google Scholar] [CrossRef] [PubMed]
- Youn, B.; Camacho, R.; Moinuddin, S.G.A.; Lee, C.; Davin, L.B.; Lewis, N.G.; Kang, C.H. Crystal structures and catalytic mechanism of the Arabidopsis cinnamyl alcohol dehydrogenase AtCAD5 and AtCAD4. Org. Biomol. Chem. 2006, 4, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Porter, S.; Sederoff, R.R. Purification, characterization and cloning of cinnamyl alcohol dehydrogenase in loblolly pine (Pinus taeda L.). Plant Physiol. 1992, 98, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.W.; Boudet, A.M. Purification and characterization of cinnamyl alcohol dehydrogenase isoforms from the periderm of Eucalyptus gunnii hook. Plant Physiol. 1994, 104, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.M.; Ran, J.H.; Wang, X.Q. Evolution of the cinnamyl/sinapyl alcohol dehydrogenase (CAD/SAD) gene family: The emergence of real lignin is associated with the origin of bona fide CAD. J. Mol. Evol. 2010, 71, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Zhang, X.Q.; Li, Y.H.; Xie, X.M. Cloning and in silico analysis of a cinnamyl alcohol dehydrogenase gene in Pennisetum purpureum. J. Genet. 2014, 93, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Guillaumie, S.; Pichon, M.; Martinant, J.P.; Bosio, M.; Goffner, D.; Barrière, Y. Differential expression of phenylpropanoid and related genes in brown-midrib bm1, bm2, bm3, and bm4 young near-isogenic maize plants. Planta 2007, 226, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Saathoff, A.J.; Haas, E.J.; Palmer, N.A.; Funnell-Harris, D.L.; Sarath, G.; Pedersen, J.F. A nonsense mutation in a cinnamyl alcohol dehydrogenase gene is responsible for the sorghum brown midrib6 phentype. Plant Physiol. 2009, 150, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Lee, S.W. Phenolic phytoalexins in rice: Biological functions and biosynthesis. Int. J. Mol. Sci. 2015, 16, 29120–29133. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Bhoo, S.H.; Kwon, M.; Lee, S.W.; Cho, M.H. Biochemical and expression analyses of the rice cinnamoyl-CoA reductase gene family. Front. Plant Sci. 2017, 8, 2099. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Lee, S.W.; Jung, K.H.; Hahn, T.R.; Cho, M.H. Transcriptomic analysis of UV-induced reveals UV-induced phytoalexins biosynthetic pathways and their regulatory networks in rice. Phytochemistry 2013, 96, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Bukh, C.; Nord-Larsen, P.H.; Rasmussen, S.K. Phylogeny and structure of the cinnamyl alcohol dehydrogenase gene family in Brachypodium distachyon. J. Exp. Bot. 2012, 63, 6223–6236. [Google Scholar] [CrossRef] [PubMed]
- Baerson, S.R.; Sánchez-Moreiras, A.; Pedrol-Bonjoch, N.; Schulz, M.; Kagan, I.A.; Agarwal, A.K.; Reigosa, M.J.; Duke, S.O. Detoxification and transctiptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one. J. Biol. Chem. 2005, 280, 21867–21881. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, D.; Chen, J.; Pu, G.; Ji, Y.; Lei, C.; Du, Z.; Liu, B.; Ye, H.; Wang, H. Biochemical characterization and identification of a cinnamyl alcohol dehydrogenase from Artemisia annua. Plant Sci. 2012, 193–194, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Brill, E.M.; Abrahams, S.; Hayes, C.M.; Jenkins, C.L.D.; Watson, J.M. Molecular characterization and expression of a wound-inducible cDNA encoding a novel cinnamyl-alcohol dehydrogenase enzyme in lucerne (Medicago sativa L.). Plant Mol. Biol. 1999, 41, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Mee, B.; Kelleher, D.; Frias, J.; Malone, R.; Tipton, K.F.; Henehan, G.T.M.; Windle, H.J. Characterization of cinnamyl alcohol dehydrogenase of Heloconacter pylori. An aldehyde dismutating enzyme. FEBS J. 2005, 272, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Nimz, H.; Ebel, J.; Grisebach, H. On the structure of lignin from soybean cell suspension cultures. Z. Naturforsch 1975, 30, 442–444. [Google Scholar] [CrossRef]
- Gross, G.G. The biochemistry of lignification. Adv. Bot. Res. 1981, 8, 25–63. [Google Scholar]
- Lüderitz, T.; Grisebach, H. Enzyme synthesis of lignin precursors. Comparison of cinnamoyl-CoA reductase and cinnamyl alcohol:NADP+ dehydrogenase from spruce (Picea abies L.) and soybean (Glycine max L.). Eur. J. Biochem. 1981, 199, 115–124. [Google Scholar] [CrossRef]
- Lacombe, E.; Hawkins, S.; Doorsselaere, J.V.; Piquemal, J.; Goffner, D.; Poeydomenge, O.; Boudet, A.M.; Grima-Pettenati, J. Cinnamoyl-CoA reductase, the first committed enzyme of the linin branch biosynthetic pathway: Cloning, expression and phylogenetic relationships. Plant J. 1997, 11, 426–441. [Google Scholar] [CrossRef]
- Li, L.; Cheng, X.F.; Leshkevich, J.; Umezawa, T.; Harding, S.A.; Chiang, V.L. The last step of syringyl monolignol biosynthesis in angiosperms is regulated by a novel gene encoding sinapyl alcohol dehydrogenase. Plant Cell 2001, 13, 1567–1585. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.H.; Tian, B. Biochemical characterization of a cinnamoyl-CoA reductase from wheat. Biol. Chem. 2005, 386, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.H. Characterization of a cinnamoyl-CoA reductase that is associated with stem development in wheat. J. Exp. Bot. 2007, 58, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Treviño, L.L.; Shen, H.; Uppalapati, S.R.; Ray, T.; Tang, Y.; Hernandez, T.; Yin, Y.; Xu, Y.; Dixon, R.A. Switchgrass (Panicum virgarum) possesses a divergent family of cinnamoyl CoA reductase distinct biochemical properties. New Phytol. 2010, 185, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Eudes, A.; Pollet, B.; Sibout, R.; Do, C.T.; Séguin, A.; Lapierre, C.; Jouanin, L. Evidence for role of AtCAD 1 in lignification of elongating stems of Arabidopsis thaliana. Planta 2006, 225, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Bahabadi, S.E.; Sharifi, M.; Benmanesh, M.; Safaie, N.; Murata, J.; Araki, R.; Yamagaki, T.; Satake, H. Time-course changes in fungal elicitor-induced lignan synthesis and expression of the relevant genes in cell cultures of Linum album. J Plant Physiol. 2012, 169, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Hashimoto, Y.; Tanaka, C.; Dubouzet, J.G.; Nakao, T.; Matsuda, F.; Nishioka, T.; Miyagawa, H.; Wakasa, K. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008, 54, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Yoo, Y.; Hahn, T.R.; Bhoo, S.H.; Lee, S.W.; Cho, M.H. Antimicrobial activity of UV-induced phenylamides from rice leaves. Molecules 2014, 19, 18139–18151. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newton, A.C. Climate change, plant diseases and food security: An overview. Plant Path. 2011, 60, 2–14. [Google Scholar] [CrossRef]
- Kauffman, H.E.; Reddy, A.P.K.; Hsieh, S.P.Y.; Merca, S.D. An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Dis. Rep. 1973, 57, 537–541. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTALW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Wryambik, D.; Grisebach, H. Purification and properties of isoenzymes of cinnamyl-alcohol dehydrogenase from soybean-cell suspension cultures. Eur. J. Biochem. 1975, 59, 9–15. [Google Scholar] [CrossRef]
- Hruz, T.; Laule, O.; Szabo, G.; Wessendorp, F.; Bleuler, S.; Oertle, L.; Widmayer, P.; Gruissem, W.; Zimmermann, P. Genevestigator V3: A reference expression database for the meta-analysis of transcriptomes. Adv. Bioinform. 2008, 2008, 420747. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.C.; Lee, S.; Kim, S.R.; Lee, Y.S.; Liu, C.; Cao, X.; An, G. Trithorax group protein Oryza sativa trithorax1 controls flowering time in rice via interaction with early heading date3. Plant Physiol. 2014, 164, 1326–1337. [Google Scholar] [CrossRef] [PubMed]

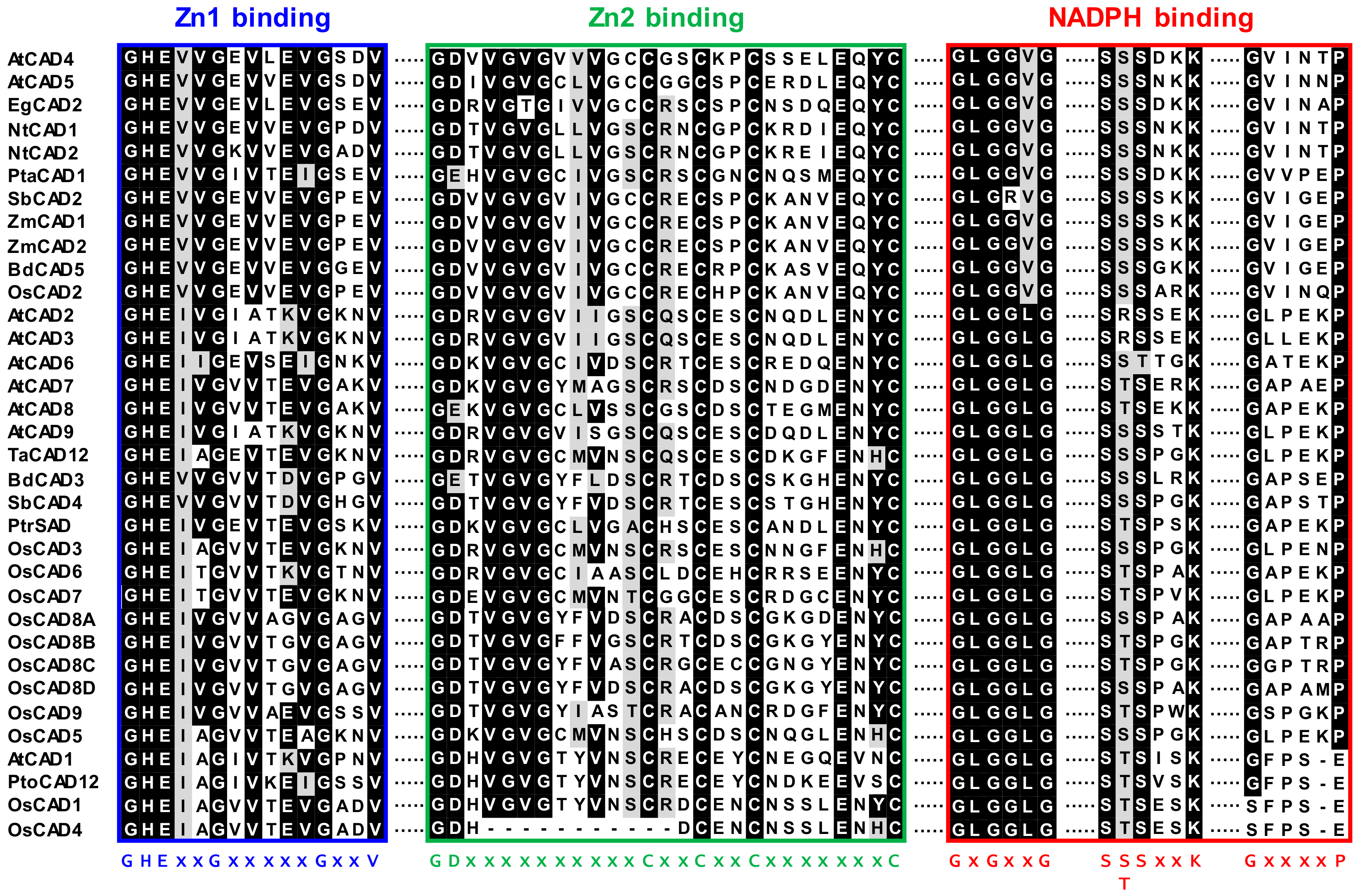

| Locus ID | Name a | Gene Description | ORF b | Protein Size (aa) c | Theoretical MW (kDa) d |
|---|---|---|---|---|---|
| LOC_Os10g11810 | OsCAD1 | dehydrogenase, putative, expressed | 1065 | 354 | 38.5 |
| LOC_Os02g09490 | OsCAD2 | dehydrogenase, putative, expressed | 1092 | 363 | 38.6 |
| LOC_Os10g29470 | OsCAD3 | dehydrogenase, putative, expressed | 1101 | 366 | 38.5 |
| LOC_Os11g40690 | OsCAD4 | dehydrogenase, putative, expressed | 1032 | 343 | 37.5 |
| LOC_Os08g16910 | OsCAD5 | dehydrogenase, putative, expressed | 999 | 332 | 35.0 |
| LOC_Os04g15920 | OsCAD6 | dehydrogenase, putative, expressed | 1083 | 360 | 39.1 |
| LOC_Os04g52280 | OsCAD7 | dehydrogenase, putative, expressed | 1140 | 379 | 39.6 |
| LOC_Os09g23530 | OsCAD8A | dehydrogenase, putative, expressed | 1080 | 359 | 36.8 |
| LOC_Os09g23540 | OsCAD8B | dehydrogenase, putative, expressed | 1311 | 436 | 45.6 |
| LOC_Os09g23550 | OsCAD8C | dehydrogenase, putative, expressed | 1320 | 439 | 45.9 |
| LOC_Os09g23560 | OsCAD8D | dehydrogenase, putative, expressed | 1089 | 362 | 37.2 |
| LOC_Os03g12270 | OsCAD9 | dehydrogenase, putative, expressed | 1089 | 362 | 37.9 |
| Enzyme | Substrate | KM (μM) | Vmax (μmol min−1 mg−1) | kcat (min−1) | kcat/KM (μM−1 min−1) |
|---|---|---|---|---|---|
| OsCAD2 | p-Coumaraldehyde | 9.36 ± 2.50 | 17.79 ± 0.51 | 831.55 | 92.43 |
| Coniferaldehyde | 4.37 ± 0.85 | 38.67 ± 10.08 | 1808.07 | 429.29 | |
| Sinapaldehyde | 8.19 ± 2.62 | 52.86 ± 15.52 | 2471.41 | 321.14 | |
| Cinnamaldehyde | 5.30 ± 3.42 | 11.62 ± 3.85 | 543.15 | 142.14 | |
| OsCAD6 | p-Coumaraldehyde | 10.88 ± 4.29 | 0.11 ±0.01 | 4.54 | 0.45 |
| Coniferaldehyde | 16.18 ± 4.49 | 0.217 ± 0.04 | 9.22 | 0.58 | |
| Sinapaldehyde | 10.72 ± 0.47 | 0.103 ± 0.01 | 4.40 | 0.41 | |
| Cinnamaldehyde | 28.25 ± 11.36 | 0.439 ± 0.13 | 18.66 | 0.69 | |
| OsCAD7 | p-Coumaraldehyde | 2.66 ± 0.49 | 0.09 ± 0.00 | 4.15 | 1.58 |
| Coniferaldehyde | 11.44 ± 2.16 | 0.38 ± 0.04 | 17.09 | 1.52 | |
| Sinapaldehyde | 6.42 ± 1.77 | 0.17 ± 0.04 | 7.65 | 1.27 | |
| Cinnamaldehyde | 14.11 ± 4.00 | 0.25 ± 0.03 | 11.29 | 0.82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.L.; Kim, T.L.; Bhoo, S.H.; Lee, T.H.; Lee, S.-W.; Cho, M.-H. Biochemical Characterization of the Rice Cinnamyl Alcohol Dehydrogenase Gene Family. Molecules 2018, 23, 2659. https://doi.org/10.3390/molecules23102659
Park HL, Kim TL, Bhoo SH, Lee TH, Lee S-W, Cho M-H. Biochemical Characterization of the Rice Cinnamyl Alcohol Dehydrogenase Gene Family. Molecules. 2018; 23(10):2659. https://doi.org/10.3390/molecules23102659
Chicago/Turabian StylePark, Hye Lin, Tae Lim Kim, Seong Hee Bhoo, Tae Hoon Lee, Sang-Won Lee, and Man-Ho Cho. 2018. "Biochemical Characterization of the Rice Cinnamyl Alcohol Dehydrogenase Gene Family" Molecules 23, no. 10: 2659. https://doi.org/10.3390/molecules23102659
APA StylePark, H. L., Kim, T. L., Bhoo, S. H., Lee, T. H., Lee, S.-W., & Cho, M.-H. (2018). Biochemical Characterization of the Rice Cinnamyl Alcohol Dehydrogenase Gene Family. Molecules, 23(10), 2659. https://doi.org/10.3390/molecules23102659





