Simultaneous Separation and Purification of Five Polymethoxylated Flavones from “Dahongpao” Tangerine (Citrus tangerina Tanaka) Using Macroporous Adsorptive Resins Combined with Prep-HPLC
Abstract
1. Introduction
2. Results and Discussion
2.1. Static Adsorption and Desorption Capacities
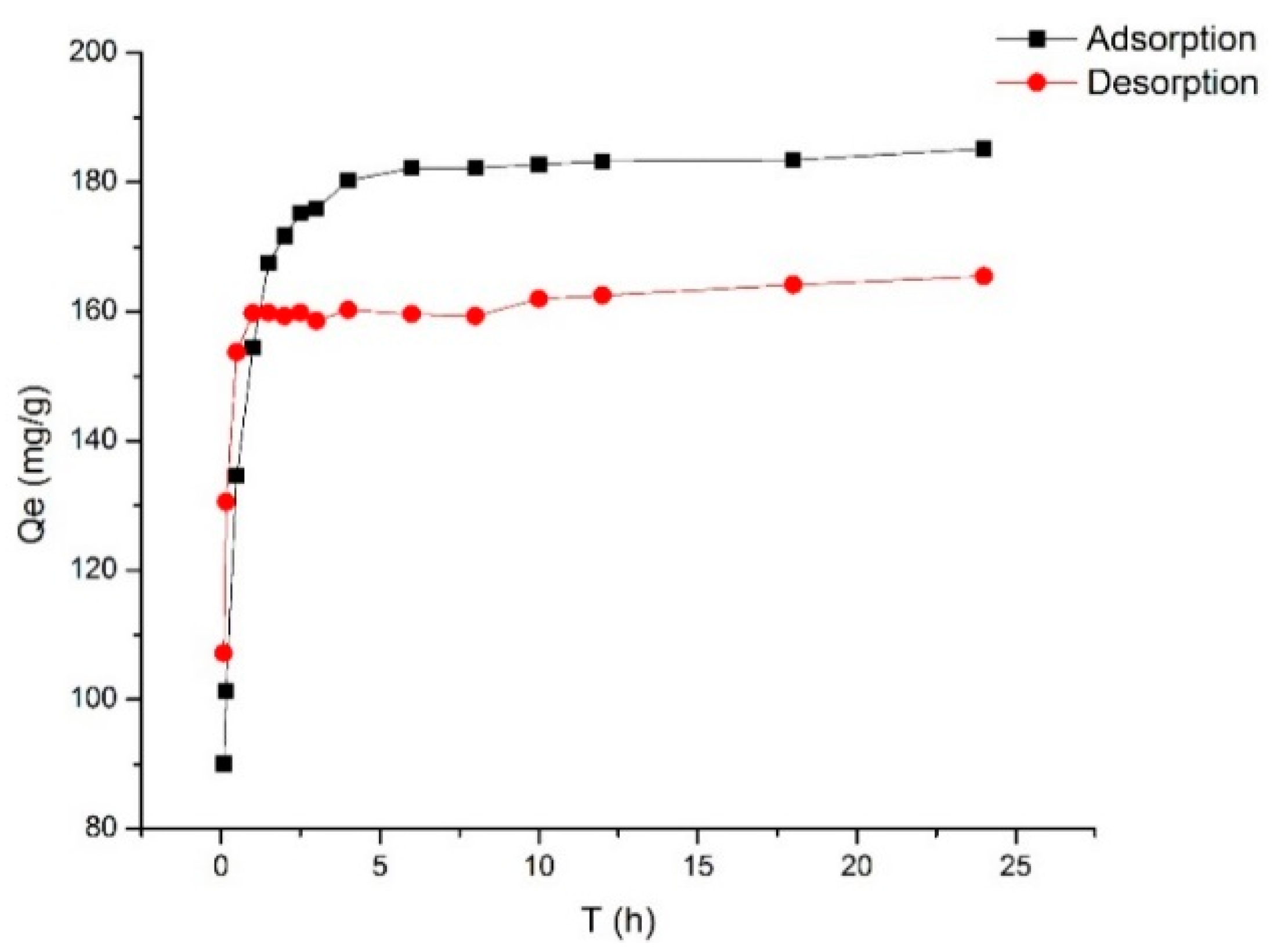
2.2. Adsorption Kinetics
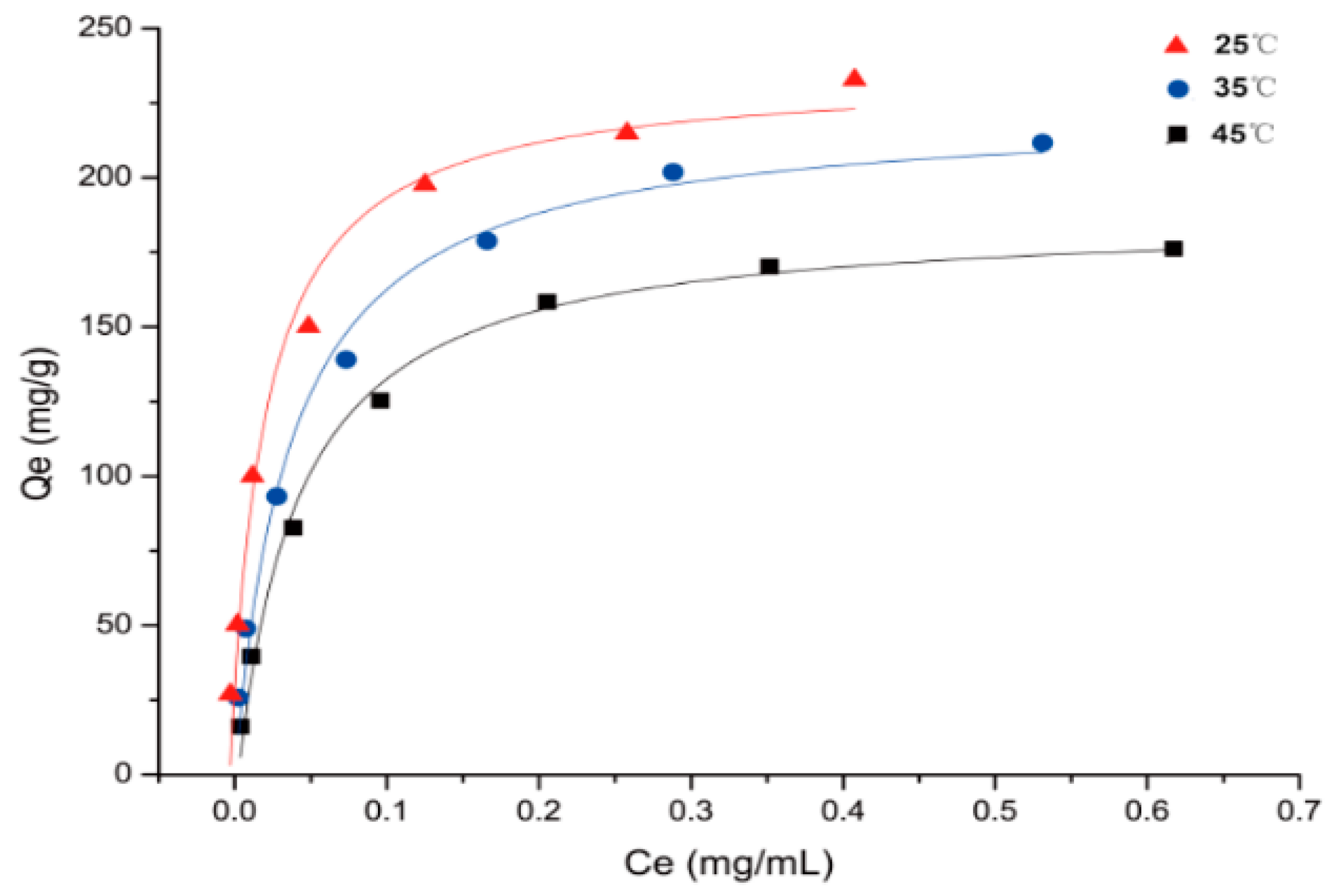
2.3. Adsorption Isotherms
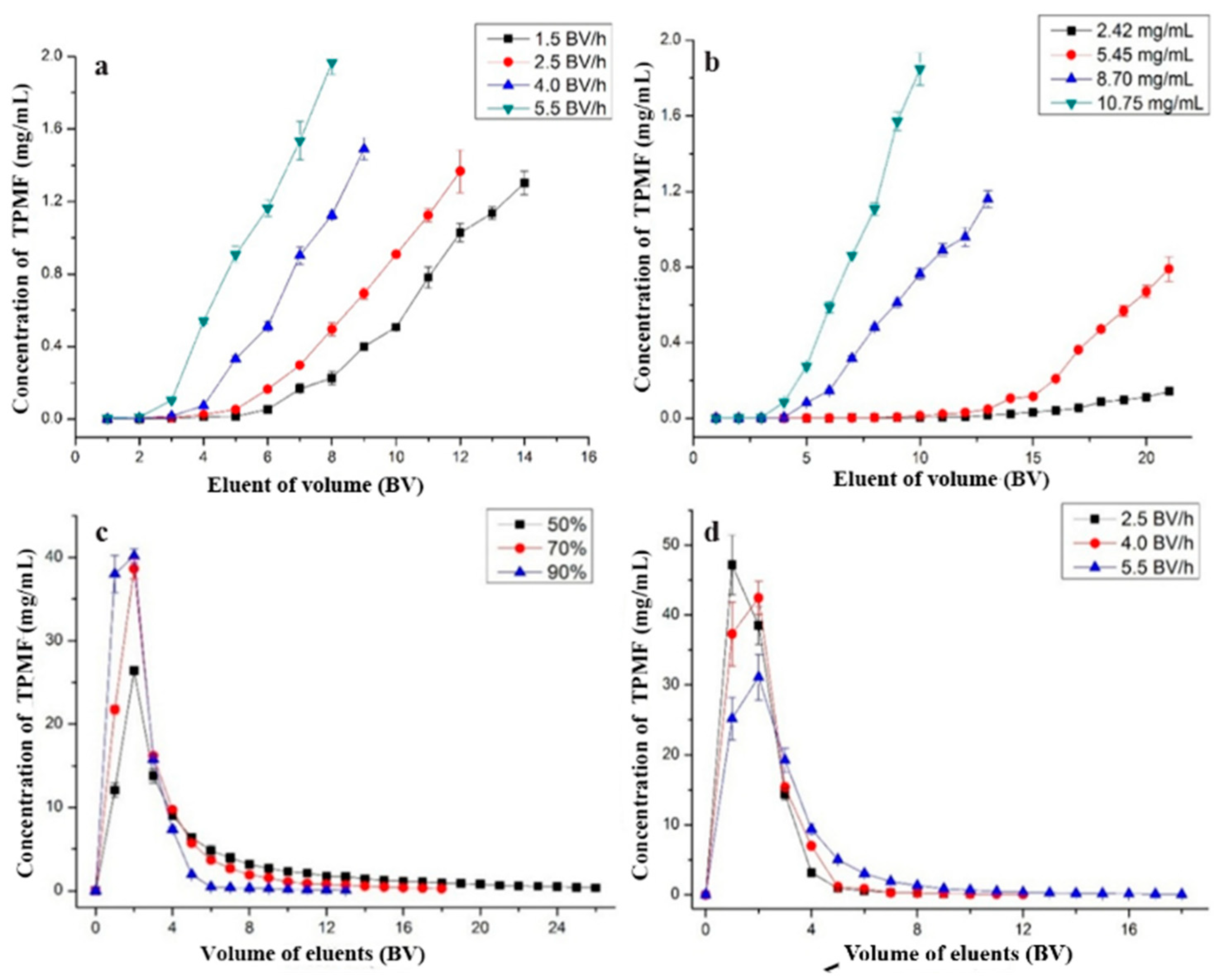
2.4. Dynamic Adsorption and Desorption
2.4.1. Optimal Feeding Speed
2.4.2. Optimal Concentration of Total PMFs
2.4.3. Impurities Removal
2.4.4. Optimal Desorption Ethanol Concentration
2.4.5. Optimal Eluting Speed
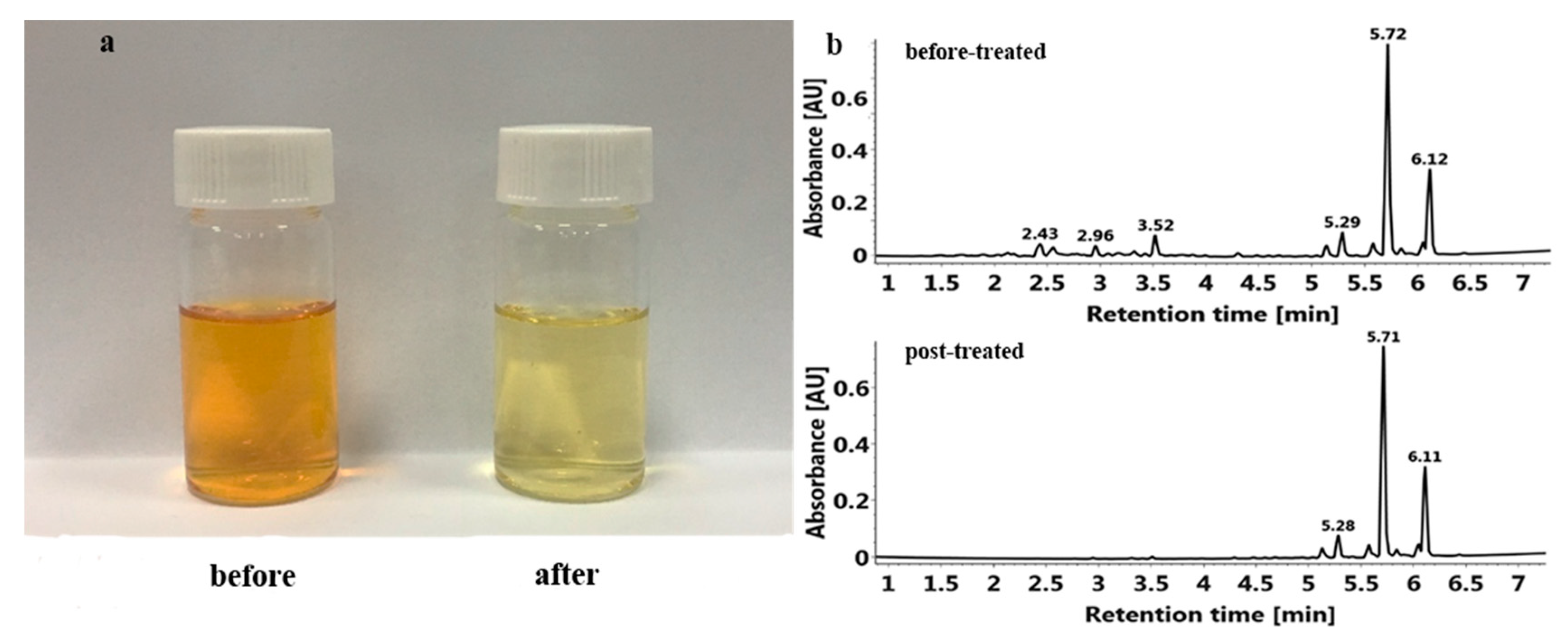
2.5. Effect of Enrichment by HPD 300 Resin on PMFs Extraction
2.6. Separation and Purification of PMFs
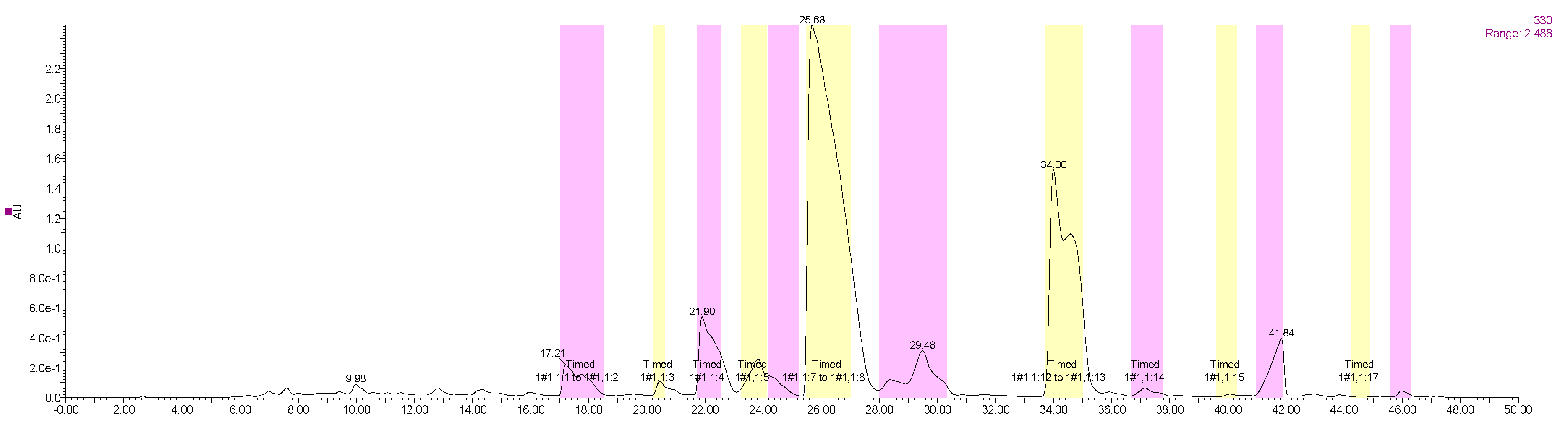
2.6.1. Optimal Conditions of Prep-HPLC
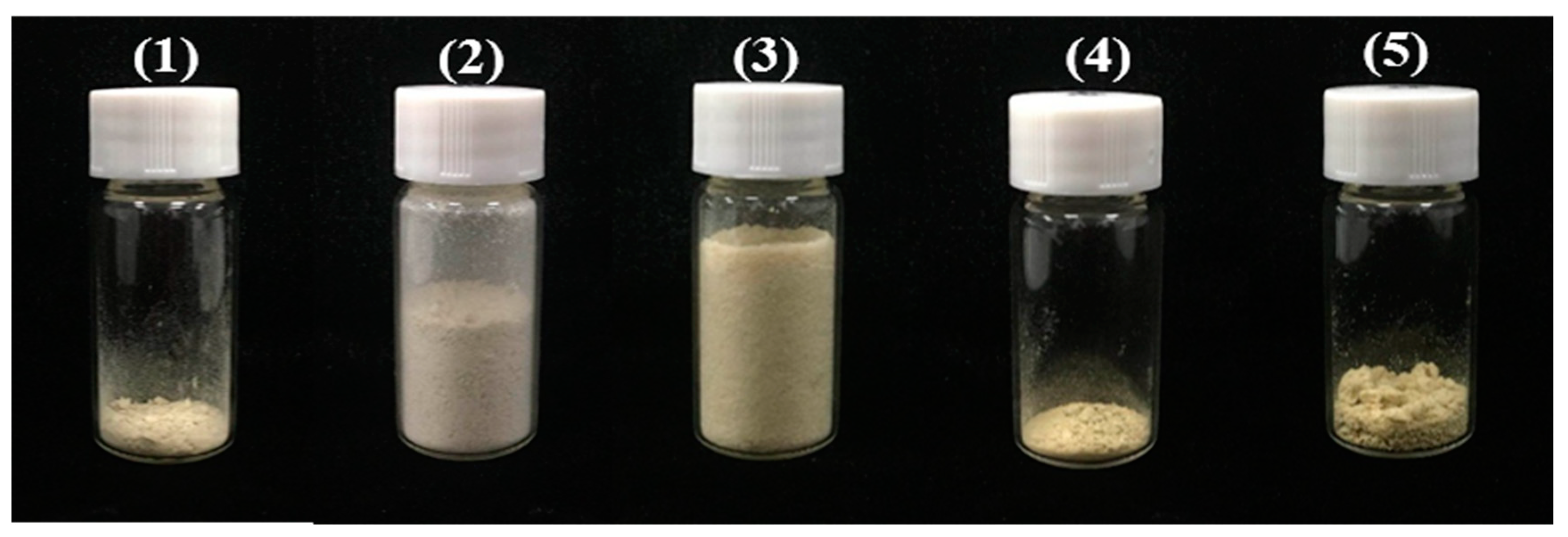
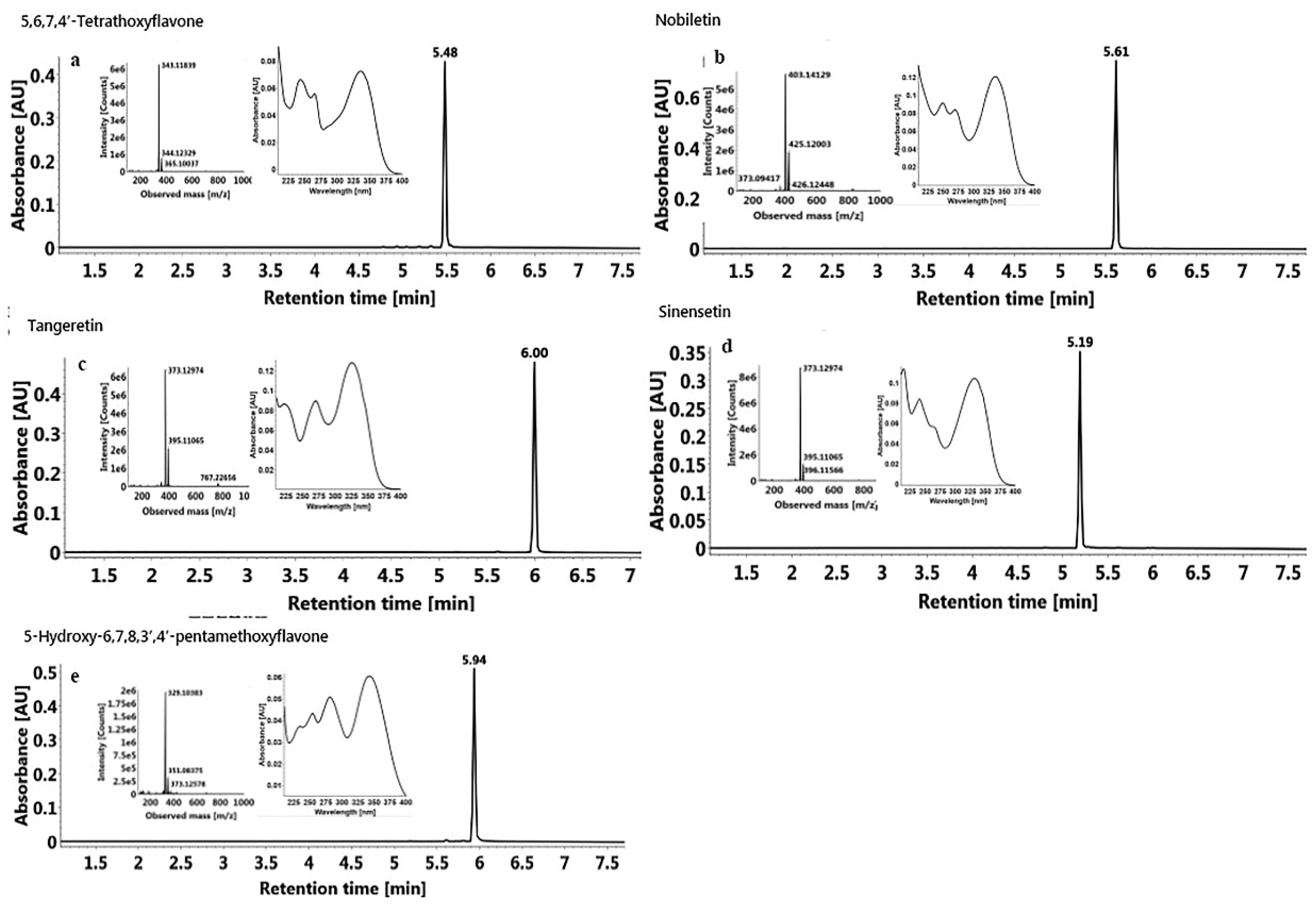
2.6.2. Identification of Isolated PMF Monomers
3. Materials and Methods
3.1. Plant Materials and Reagents
3.2. Preparation of Crude Extracts from “Dahongpao” Tangerine
3.3. Determination of PMF Contents
3.4. Static Adsorption and Desorption Tests
3.4.1. Pretreatment of Macroporous Adsorptive Resins
3.4.2. Static Adsorption and Desorption Properties of the Resins
3.4.3. Adsorption Kinetics of Selected Resin
3.4.4. Adsorption Isotherms on the Selected Resin
3.5. Dynamic Adsorption and Desorption Tests
3.6. Comparison of PMFs before and after Enrichment
3.7. Separation of PMFs by MAR
3.7.1. Preparation of PMF Solutions
3.7.2. Optimization of Experimental Conditions for Prep-HPLC
3.7.3 Identification of PMFs
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Song, M.; Qiu, P.; Li, F.; Wang, M.; Zheng, J.; Wang, Q.; Xu, F.; Xiao, H. A metabolite of nobiletin, 4′-demethylnobiletin and atorvastatin synergistically inhibits human colon cancer cell growth by inducing G0/G1 cell cycle arrest and apoptosis. Food Funct. 2018, 9, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, J.; Liu, A.; Xiao, H.; He, L. Label-free Imaging and Characterization of Cancer Cell Responses to Polymethoxyflavones Using Raman Microscopy. J. Agric. Food Chem. 2016, 64, 9708–9713. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, X.; Cheng, D.; Yao, X.; Pan, S. Preparative separation of polymethoxylated flavones from Ponkan (Citrus reticulata Blanco cv. Ponkan) peel by high-speed countercurrent chromatography and their antifungal activities against Aspergillus niger. Eur. Food Res. Technol. 2012, 235, 631–635. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Gu, S.; Liu, Z.; Zhang, Y.; Zhang, X. Simultaneous Determination of Flavonoids in Different Parts of Citrus reticulata ‘Chachi’ Fruit by High Performance Liquid Chromatography—Photodiode Array Detection. Molecules 2010, 15, 5378–5388. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Guo, L.; Liu, K.; Liu, E.H.; Li, P. Characterization and classification of seven Citrus herbs by liquid chromatography–quadrupole time-of-flight mass spectrometry and genetic algorithm optimized support vector machines. J. Chromatogr. A 2014, 1339, 118–127. [Google Scholar] [CrossRef] [PubMed]
- The State Pharmacopoeia Committee of the People’s Republic of China. Pharmacopoeia of People’s Republic of China; Chemical Industry Press: Beijing, China, 2015; Volume 1, p. 1329. [Google Scholar]
- Choi, M.Y.; Chai, C.; Park, J.H.; Lim, J.; Lee, J.; Kwon, S.W. Effects of storage period and heat treatment on phenolic compound compositionin dried Citrus peels (Chenpi) and discrimination of Chenpi with different storage periods through targeted metabolomic study using HPLC-DAD analysis. J. Pharm. Biomed. Anal. 2011, 54, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Ooghe, W.C.; Ooghe, S.J.; Detavernier, C.M.; Huyghebaert, A. Characterization of Orange Juice (Citrus sinensis) by Flavanone Glycosides. J. Agric. Food Chem. 1994, 42, 2183–2190. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, W.; Zeng, S.; Li, P.; Liu, E. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Chen, X.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Adham, A.N. Comparative Antimicrobrial Activity of Peel and Juice Extract of Citrus Fruits Growing in Kurdistan/Iraq. Am. J. Microbiol. Res. 2015, 5, 155–159. [Google Scholar]
- Ke, Z.; Pan, Y.; Xu, X.; Nie, C.; Zhou, Z. Citrus Flavonoids and Human Cancers. J. Food Nutr. Res. 2015, 3, 341–351. [Google Scholar] [CrossRef]
- Jung, U.J.; Lee, M.; Park, Y.B.; Kang, M.A.; Choi, M. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int. J. Biochem. Cell Biol. 2006, 38, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.J.; Lin, K.C.; Liu, C.P.; Lin, C.Y.; Wu, H.C.; Chou, D.S. Prevention of arterial thrombosis by nobiletin: In vitro and in vivo studies. J. Nutr. Biochem. 2016, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Aoyama, Y.; Shin, E.J.; Nam, Y.; Kim, H.C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Alevels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Tangeretin, a polymethoxylated flavone, modulates lipid homeostasis and decreases oxidative stress by inhibiting NF-κB activation and proinflammatory cytokines in cardiac tissue of streptozotocin-induced diabetic rats. J. Funct. Foods 2015, 16, 315–333. [Google Scholar] [CrossRef]
- Xing, T.T.; Zhao, X.J.; Zhang, Y.D.; Li, Y.F. Fast Separation and Sensitive Quantitation of Polymethoxylated Flavonoids in the Peels of Citrus Using UPLC-Q-TOF-MS. J. Agric. Food Chem. 2017, 65, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, Y.; Chen, H.; Hao, Z.; Wang, J.; Guan, Y.; Zhang, Y.; Feng, W.; Zheng, X. Isolation of two new prenylated flavonoids from Sinopodophyllum emodi fruit by silica gel column and high-speed counter-current chromatography. J. Chromatogr. B 2014, 969, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Wang, Y.; Zhao, X.; Tian, N.; Hu, H.; Li, P. Separation and Identification of Anthocyanin Extracted from Mulberry Fruit and the Pigment Binding Properties toward Human Serum Albumin. J. Agric. Food Chem. 2014, 62, 6813–6819. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Xuan, H.; Sun, A.; Liu, R.; Cui, J. Preparative separation of polyphenols from water-soluble fraction of Chinese propolis using macroporous absorptive resin coupled with preparative high performance liquid chromatography. J. Chromatogr. B 2016, 1012, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zu, Y.; Liu, W.; Hou, C.; Chen, L.; Li, S.; Shi, X.; Tong, M. Preparative separation of vitexin and isovitexin from pigeonpea extracts with macroporous resins. J. Chromatogr. A 2007, 1139, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Chen, X.L.; Qiao, S.Y.; Ma, S.; Peng, L.T. Purification of Red Pigment from Jujube Fruits Peel by Macroporous Resin. Hubei Agric. Sci. 2017, 56, 1924–1927. [Google Scholar]
- Wan, P.; Sheng, Z.; Han, Q.; Zhao, Y.; Cheng, G.; Li, Y. Enrichment and purification of total flavonoids from Flos Populi extracts with macroporous resins and evaluation of antioxidant activities in vitro. J. Chromatogr. B 2014, 945, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, J.; Li, D.; Wang, H. Study on Separation and Purification of Genistein in the Soybean Residue Using Macroporous Resin Adsorption. Ind. Eng. Chem. Res. 2011, 51, 44–49. [Google Scholar] [CrossRef]
- Scordino, M.; Di Mauro, A.; Passerini, A.; Maccarone, E. Adsorption of Flavonoids on Resins: Hesperidin. J. Agric. Food Chem. 2003, 51, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, M.; Sun-Waterhouse, D.; Zhuang, M.; Chen, H.; Feng, M.; Lin, L. Absorption and desorption behaviour of the flavonoids from Glycyrrhiza glabra L. leaf on macroporous adsorption resins. Food Chem. 2015, 168, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, J.; Madhusudhan, M.C.; Raghavarao, K.S.M.S. Extraction of anthocyanins from red cabbage and purification using adsorption. Food Bioprod. Proc. 2012, 90, 615–623. [Google Scholar] [CrossRef]
- Bi, Y.G.; Tan, Y.Q. Study on Macroporous Resin Separation and Purification of Total Flavonoids of Plantago Process. Adv. Mater. Res. 2012, 550, 987–992. [Google Scholar] [CrossRef]
- Jia, G.; Lu, X. Enrichment and purification of madecassoside and asiaticoside from Centella asiatica extracts with macroporous resins. J Chromatogr. A 2008, 1193, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Mu, T.; Sun, H. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Gong, G.; Li, F.; Ren, H.; Liu, Y. Adsorption and desorption properties of macroporous resins for flavonoids from the extract of Chinese wolfberry (Lycium barbarum L.). Food Bioprod. Proc. 2015, 93, 148–155. [Google Scholar] [CrossRef]
- Wang, J.; Lei, H.; Yue, Z.; Gao, R.; Wang, K.; Xu, H. Adsorption characteristics of patulin in jujube juice using macroporous resin. Trans. Chin. Soc. Agric. Eng. 2015, 31, 285–291. [Google Scholar]
- Chao, Y.; Zhu, W.; Yan, B.; Lin, Y.; Xun, S.; Ji, H.; Wu, X.; Li, H.; Han, C. Macroporous polystyrene resins as adsorbents for the removal of tetracycline antibiotics from an aquatic environment. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Mao, J.; Liu, S.; Jun, H.; You, Y.; Mei, L. Kinetic and thermodynamic studies of sulforaphane adsorption on macroporous resin. J. Chromatogr. B 2016, 1028, 231–236. [Google Scholar]
- Zhang, L.; Xu, H.; Liu, Z. Adsorption Characteristics of Lycopene on Macroporous Resin HZ816. Mod. Food Sci. Technol. 2009, 25, 232–236. [Google Scholar]
- Marwani, H.M.; Albishri, H.M.; Jalal, T.A.; Soliman, E.M. Study of isotherm and kinetic models of lanthanum adsorption on activated carbon loaded with recently synthesized Schiff’s base. Arabian J. Chem. 2017, 10, S1032–S1040. [Google Scholar] [CrossRef]
- Raza, M.H.; Sadiq, A.; Farooq, U.; Athar, M.; Hussain, T.; Mujahid, A.; Salman, M. Phragmites karka as a Biosorbent for the Removal of Mercury Metal Ions from Aqueous Solution: Effect of Modification. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Wang, X. Isolation and Purifcation of Anthocyanins from Black Bean Wastewater Using Macroporous Resins. Master’s Thesis, Utah State University, Logan, UT, USA, May 2012. [Google Scholar]
- Li, X.; Fu, Y.; Luo, M.; Wang, W.; Zhang, L.; Zhao, C.; Zu, Y. Preparative separation of dryofragin and aspidin BB from Dryopteris fragrans extracts by macroporous resin column chromatography. J. Pharm. Biomed. Anal. 2012, 61, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, J.; Huang, X.; Tu, Y.; Ni, K. Identification of polymethoxylated flavones from green tangerine peel (Pericarpium Citri Reticulatae Viride) by chromatographic and spectroscopic techniques. J. Pharm. Biomed. Anal. 2007, 44, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.Q.; Xiong, Y.; Zhou, B.; Deng, M.Z.; Deng, K.Z. Isolation and structural identification of flavonoids from Aurantii Fructus. China J. Chin. Mater. Med. 2015, 40, 2352–2356. [Google Scholar]
- Duan, L.; Dou, L.; Yu, K.; Guo, L.; Bai-Zhong, C.; Li, P.; Liu, E. Polymethoxyflavones in peel of Citrus reticulata ‘Chachi’ and their biological activities. Food Chem. 2017, 234, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, X.J.; Pan, Y.; Zhou, Z. Identification of the chemical compositions of Ponkan peel by ultra performance liquid chromatography coupled with quadrupole time of-flight mass spectrometry. Anal. Methods 2016, 8, 893–903. [Google Scholar] [CrossRef]
- Zhao, Z.; He, S.; Hu, Y.; Yang, Y.; Jiao, B.; Fang, Q.; Zhou, Z. Fruit flavonoid variation between and within four cultivated Citrus species evaluated by UPLC-PDA system. Sci. Hortic. 2017, 224, 93–101. [Google Scholar] [CrossRef]
- Toor, M.; Jin, B. Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing diazo dye. Chem. Eng. J. 2012, 187, 79–88. [Google Scholar] [CrossRef]
Sample Availability: 5,6,7,4’- tetramethoxyflavone, nobiletin, tangeretin, sinensetin, and 5-hydroxy -6,7,8,3’,4’-pentamethoxyflavone are available from the authors. |
| MAR Type | Polarity | Particle Diameter (mm) | Surface Area (m2/g) | Average Pore Dimeter (Å) |
|---|---|---|---|---|
| D 101 | Non-polar | 0.315–1.25 | 650–700 | 100–110 |
| HPD 100 | Non-polar | 0.30–1.25 | 650–700 | 85–90 |
| AB-8 | Weak polar | 0.30–1.25 | 480–520 | 130–140 |
| HPD 300 | Weak polar | 0.30–1.25 | 800–870 | 50–55 |
| HPD 400 | Middle polar | 0.30–1.25 | 500–550 | 75–80 |
| DM 130 | Middle polar | 0.30–1.25 | 500–550 | 90–100 |
| NKA-9 | Strong polar | 0.30–1.25 | 250–290 | 155–165 |
| HPD 600 | Strong polar | 0.30–1.20 | 550–600 | 80 |
| MAR Type | Adsorption Capacity (mg/g) | Desorption Capacity (mg/g) | Desorption Rate (%) |
|---|---|---|---|
| D 101 | 227.51 ± 5.87 c | 194.59 ± 19.49 c | 85.45 ± 6.36% ab |
| HPD 100 | 271.52 ± 9.60 b | 248.17 ± 4.01 b | 91.43 ± 1.75% a |
| AB-8 | 275.13 ± 3.67 b | 241.59 ± 1.51 b | 87.82 ± 1.73% ab |
| HPD 300 | 301.75 ± 3.32 a | 274.01 ± 0.40 a | 90.81 ± 0.83% a |
| HPD 400 | 265.57 ± 2.71 b | 239.17 ± 2.51 b | 90.06 ± 0.03% a |
| DM 130 | 236.29 ± 1.82 c | 200.88 ± 2.66 c | 85.02 ± 1.78% ab |
| NKA-9 | 209.03 ± 2.04 d | 173.06 ± 0.65 d | 82.80 ± 1.12% b |
| HPD 600 | 238.03 ± 5.77 c | 206.98 ± 2.72 c | 87.00 ± 3.25% ab |
| Models | Equations | Parameters | Values |
|---|---|---|---|
| Pseudo-first order model | ln(175.2235-Qt) = 5.1661 − 5.5651t | R2 | 0.7920 |
| Qe (mg/g) | 175.2235 | ||
| k1 (L/min) | 5.5651 | ||
| Pseudo-second order model | t/Qt = 0.0054t + 0.0007 | R2 | 0.9999 |
| Qe (mg/g) | 185.1852 | ||
| k2 (g/(mg min)) | 0.0417 | ||
| Intra-particle diffusion model | (first stage) | R2 | 0.9660 |
| k3 (mg/(min1/2·g)) | 75.147 | ||
| C(mg/g) | 73.414 | ||
(second stage) | R2 | 0.6008 | |
| k3(mg/(min1/2·g)) | 2.2816 | ||
| C(mg/g) | 174.45 |
| Models | Equations | T (°C) | Parameters | ||||
|---|---|---|---|---|---|---|---|
| R2 | KL | Qm | KF | 1/n | |||
| Langmuir | Ce/Qe = 0.00010Ce + 0.00425 | 25 | 0.9710 | 44.1981 | 235.1302 | - | - |
| Ce/Qe = 0.00017Ce + 0.00448 | 35 | 0.9845 | 26.6570 | 223.2240 | - | - | |
| Ce/Qe = 0.00021Ce + 0.00537 | 45 | 0.9889 | 26.1808 | 186.2802 | - | - | |
| Freundlich | Qe = 314.5Ce0.2790 | 25 | 0.9637 | - | - | 314.5000 | 0.2790 |
| Qe = 281.7663Ce0.3091 | 35 | 0.9473 | - | - | 281.7663 | 0.3091 | |
| Qe = 224.44Ce0.2927 | 45 | 0.9251 | - | - | 224.4400 | 0.2927 | |
| No. | Contents | Before Enrichment (mg/g) | After Enrichment (mg/g) | Increased Times |
|---|---|---|---|---|
| 1 | Isosinensetin | 1.239 ± 0.046 | 20.59 ± 1.65 | 16.62 |
| 2 | Sinensetin | 1.913 ± 0.066 | 37.56 ± 3.04 | 19.63 |
| 3 | 5,7,3′,4′-Tetrathoxyflavone | 0.056 ± 0.001 | 1.92 ± 0.60 | 34.29 |
| 4 | 5,6,7,4′-Tetrathoxyflavone | 1.207 ± 0.113 | 24.06 ± 3.79 | 19.93 |
| 5 | Nobiletin | 19.27 ± 0.743 | 355.09 ± 18.45 | 18.43 |
| 6 | 3,5,6,7,8,3′,4′-Hetamethoxyflavone | 0.554 ± 0.021 | 12.14 ± 1.56 | 21.91 |
| 7 | 5,7,4′-Trimethoxyflavone | 0.019 ± 0.005 | 1.67 ± 0.16 | 87.89 |
| 8 | 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone | 1.555 ± 0.063 | 30.27 ± 2.57 | 19.47 |
| 9 | Tangeretin | 5.908 ± 0.229 | 111.27 ± 7.10 | 18.83 |
| 10 | Total PMFs | 31.720 ± 1.255 | 594.57 ± 28.05 | 18.74 |
| Contents | CAS No. | Molecular Formula | Monoisotopic Mass (g/mol) | Purity |
|---|---|---|---|---|
| Isosinensetin | 17290-70-9 | C20H20O7 | 372.1209 | >98.0% |
| Sinensetin | 2306-27-6 | C20H20O7 | 372.1209 | ≥98.0% |
| 5,7,3’,4’-Tetrathoxyflavone | 855-97-0 | C19H18O6 | 342.1103 | ≥97.0% |
| 5,6,7,4’-Tetrathoxyflavone | 1168-42-9 | C19H18O6 | 342.1103 | ≥98.0% |
| Nobiletin | 478-01-3 | C21H22O8 | 402.1315 | ≥95.0% |
| 3,5,6,7,8,3’,4’-Hetamethoxyflavone | 1178-24-1 | C22H24O9 | 432.1420 | >98.0% |
| 5,7,4’-Trimethoxyflavone | 5631-70-9 | C18H16O5 | 312.0998 | ≥98.7% |
| 5-Hydroxy-6,7,8,3’,4’-pentamethoxyflavone | 2174-59-6 | C20H20O8 | 388.1158 | ≥98.0% |
| Tangeretin | 481-53-8 | C20H20O7 | 372.1209 | ≥95.0% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhao, Z.; Zhou, Z. Simultaneous Separation and Purification of Five Polymethoxylated Flavones from “Dahongpao” Tangerine (Citrus tangerina Tanaka) Using Macroporous Adsorptive Resins Combined with Prep-HPLC. Molecules 2018, 23, 2660. https://doi.org/10.3390/molecules23102660
Li Z, Zhao Z, Zhou Z. Simultaneous Separation and Purification of Five Polymethoxylated Flavones from “Dahongpao” Tangerine (Citrus tangerina Tanaka) Using Macroporous Adsorptive Resins Combined with Prep-HPLC. Molecules. 2018; 23(10):2660. https://doi.org/10.3390/molecules23102660
Chicago/Turabian StyleLi, Zhenqing, Ziyan Zhao, and Zhiqin Zhou. 2018. "Simultaneous Separation and Purification of Five Polymethoxylated Flavones from “Dahongpao” Tangerine (Citrus tangerina Tanaka) Using Macroporous Adsorptive Resins Combined with Prep-HPLC" Molecules 23, no. 10: 2660. https://doi.org/10.3390/molecules23102660
APA StyleLi, Z., Zhao, Z., & Zhou, Z. (2018). Simultaneous Separation and Purification of Five Polymethoxylated Flavones from “Dahongpao” Tangerine (Citrus tangerina Tanaka) Using Macroporous Adsorptive Resins Combined with Prep-HPLC. Molecules, 23(10), 2660. https://doi.org/10.3390/molecules23102660





