Synthesis of Gemcitabine-Threonine Amide Prodrug Effective on Pancreatic Cancer Cells with Improved Pharmacokinetic Properties
Abstract
1. Introduction
2. Results and Discussion
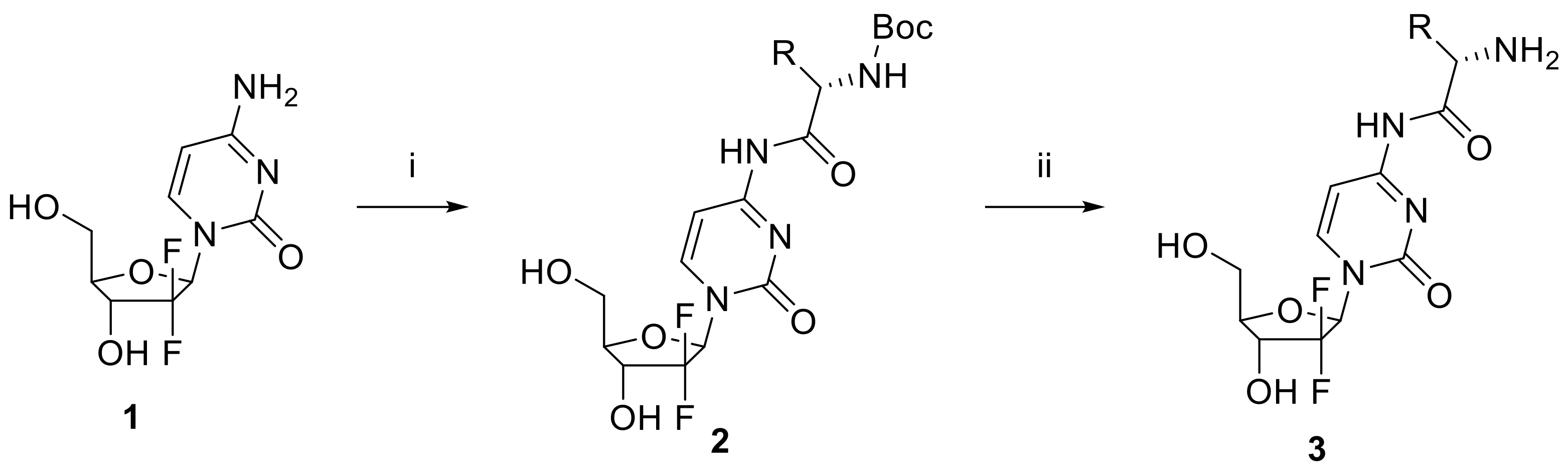
2.1. Synthesis of Gemcitabine Prodrugs with Amino Acids
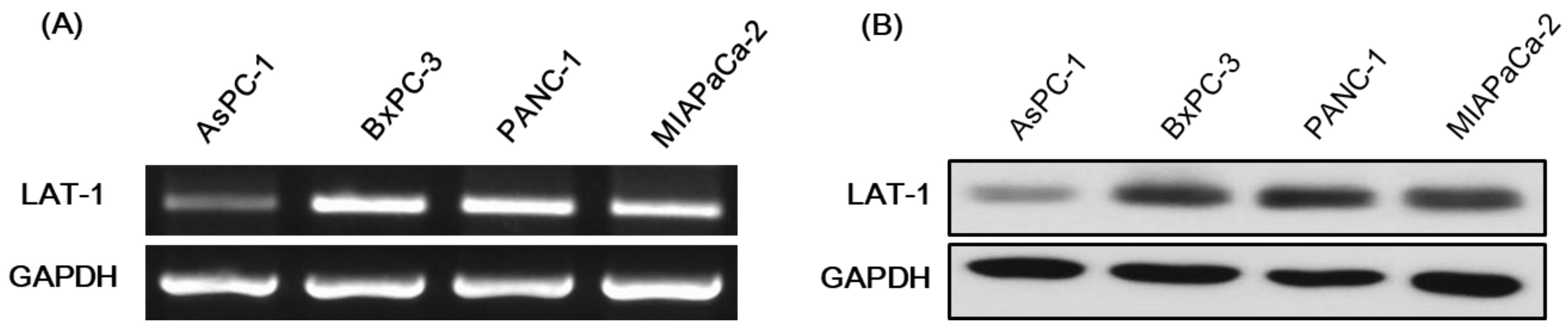
2.2. Expression of LAT-1 in Pancreatic Cancer Cell Lines
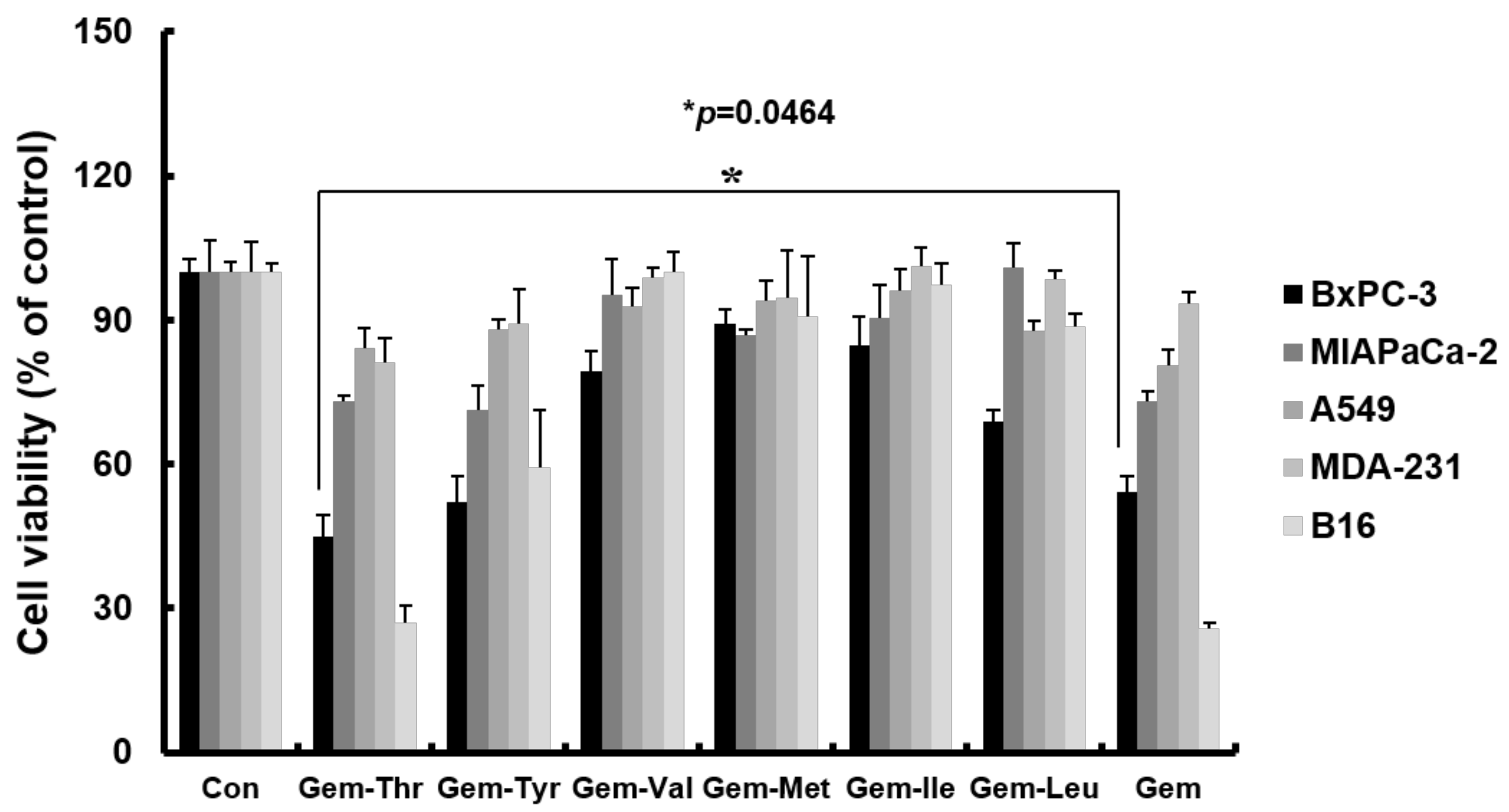
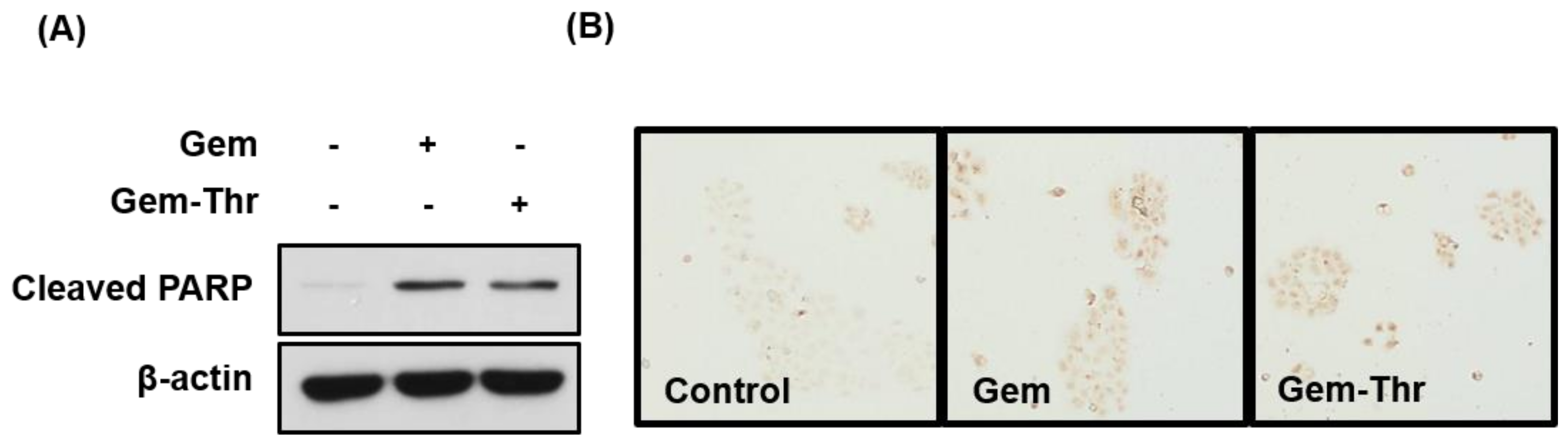
2.3. Anticancer Effects of Prodrugs with Amino Acids in Cancer Cells
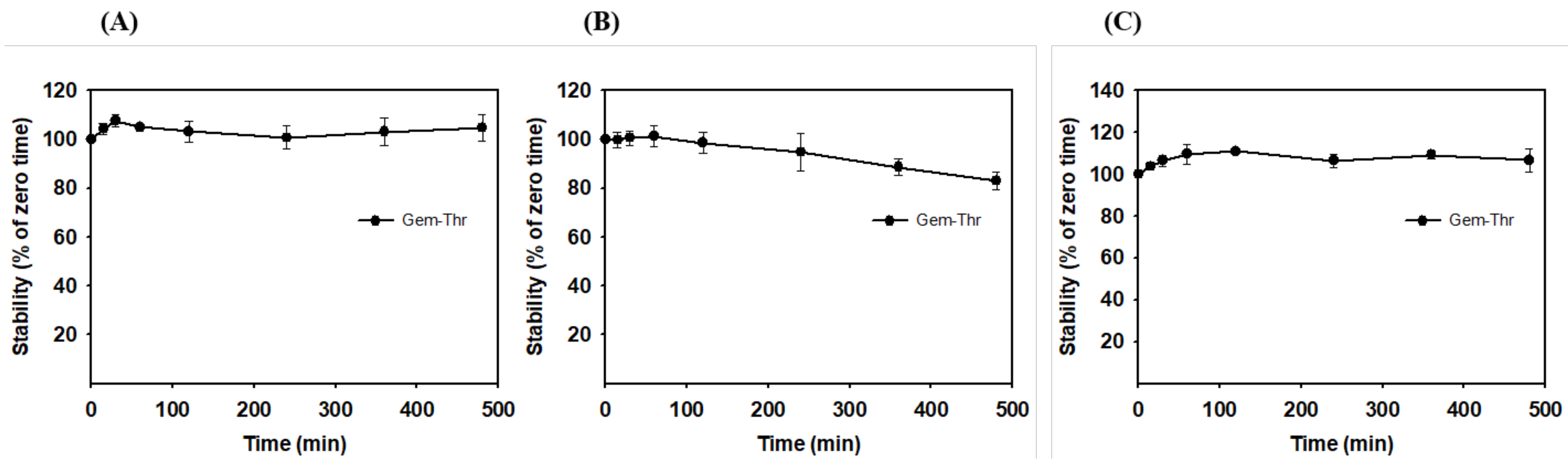
2.4. In Vitro Plasma Stability of Gem-Thr
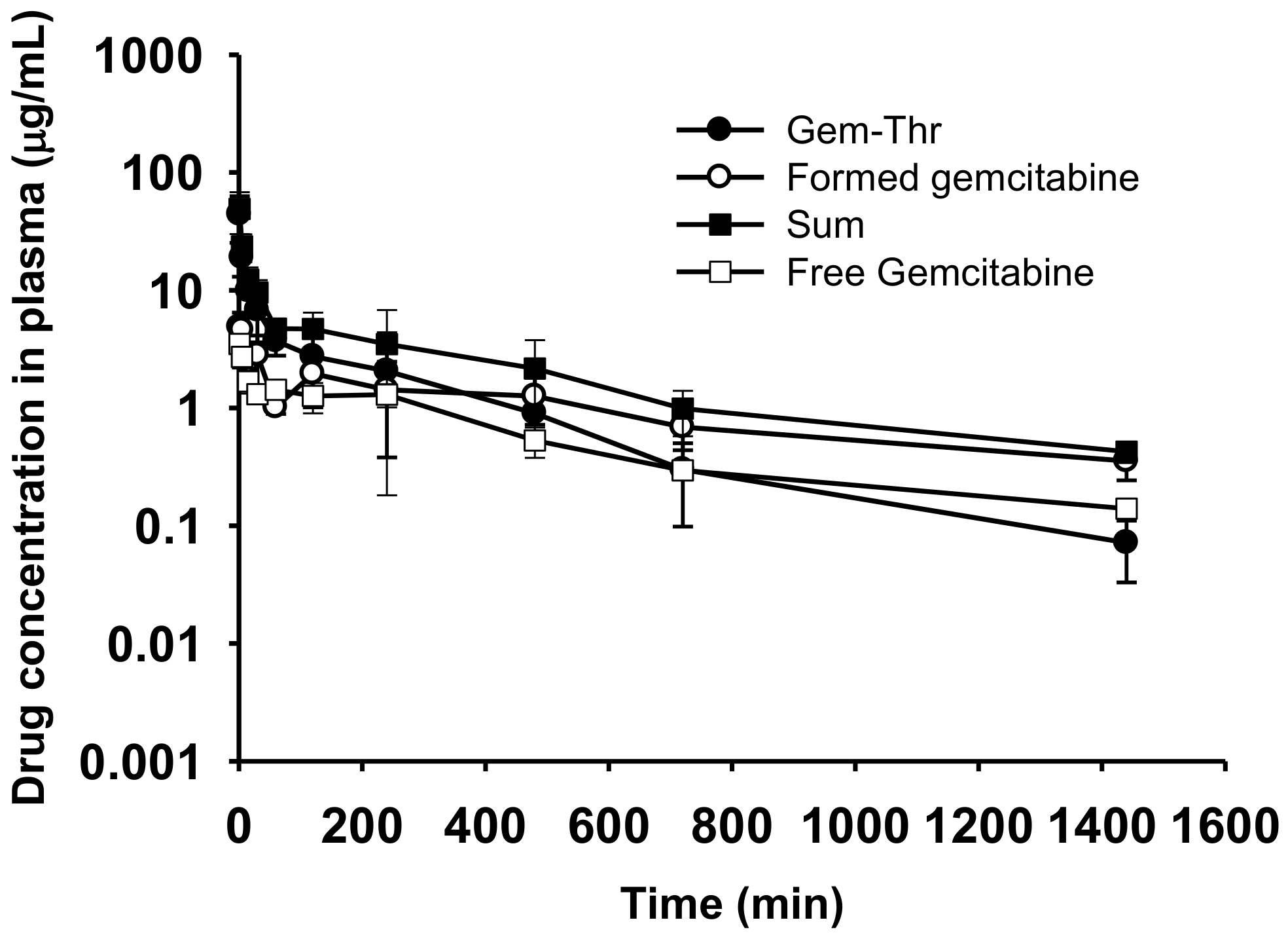
2.5. Comparison of Systemic Pharmacokinetics with Free Gemcitabine
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization of DOX-Val
3.2.1. General Procedure for Preparing Gemcitabine Derivatives
3.2.2. (S)-2-Amino-N-(1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)-3-methylbutanamide (Gem-Val)
3.2.3. (2S,3R)-2-Amino-N-(1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxylmethyl)tetra-hydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)-3-hydroxybutanamide2-(4-((pyridin-3-ylmethyl)amino)quinazolin-2-yl)phenol (Gem-Thr)
3.2.4. (S)-2-Amino-N-(1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)-3-(4-hydroxyphenyl)propanamide (Gem-Tyr)
3.2.5. (S)-2-Amino-N-(1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)-4-(methylthio)butanamide (Gem-Met)
3.2.6. (2S,3S)-2-Amino-N-(1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)-3-methylpentanamide (Gem-Ile)
3.2.7. (S)-2-Amino-N-(1-((2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2-oxo-1,2-dihydropyrimidin-4-yl)-4-methylpentanamide (Gem-Leu)
3.3. Characterization of Gemcitabine Prodrugs with Amino Acid
3.3.1. Cell Culture
3.3.2. Reverse Transcription-PCR
3.3.3. Western Blot Assays
3.3.4. Cytotoxicity Assay in Pancreatic Cells
3.3.5. Terminal Deoxynucleotidyl Transferase–Mediated Nick End Labeling (TUNEL) Assay
3.4. In Vitro Metabolic Stability of Gem-Thr
3.5. Systemic Pharmacokinetics Study of Gem-Thr in Rats
3.6. Analysis of Gem-Thr and Gemcitabine by LC-MS/MS
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vitellius, C.; Fizanne, L.; Menager-Tabourel, E.; Nader, J.; Baize, N.; Laly, M.; Lermite, E.; Bertrais, S.; Caroli-Bosc, F.X. The combination of everolimus and zoledronic acid increase the efficacy of gemcitabine in a mouse model of pancreatic adenocarcinoma. Oncotarget 2018, 9, 28069–28082. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 25, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Suzuki, E.; Mikata, R.; Yasui, S.; Abe, M.; Iino, Y.; Ohyama, H.; Chiba, T.; Tsuyuguchi, T.; Kato, N. Five Cases of Interstitial Pneumonitis Due to Gemcitabine and Nab-Paclitaxel Combination Treatment in Pancreatic Cancer Patients. Pancreas 2018, 47, e42–e43. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Robertson, J.M.; Ye, H.; Margolis, J.; Nadeau, L.; Yan, D. Dose-volume analysis of predictors for gastrointestinal toxicity after concurrent full-dose gemcitabine and radiotherapy for locally advanced pancreatic adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.M.; Bao, J.; Dantzig, A.H.; Diseroad, W.D.; Law, K.L.; Magnus, N.A.; Peterson, J.A.; Perkins, E.J.; Pu, Y.J.; Reutzel-Edens, S.M.; et al. Synthesis, crystallization, and biological evaluation of an orally active prodrug of gemcitabine. J. Med. Chem. 2009, 52, 6958–6961. [Google Scholar] [CrossRef] [PubMed]
- Beumer, J.H.; Eiseman, J.L.; Parise, R.A.; Joseph, E.; Covey, J.M.; Egorin, M.J. Modulation of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) pharmacokinetics, metabolism, and bioavailability in mice by 3,4,5,6-tetrahydrouridine. Clin. Cancer Res. 2008, 14, 3529–3535. [Google Scholar] [CrossRef] [PubMed]
- Wickremsinhe, E.; Bao, J.; Smith, R.; Burton, R.; Dow, S.; Perkins, E. Preclinical absorption, distribution, metabolism, and excretion of an oral amide prodrug of gemcitabine designed to deliver prolonged systemic exposure. Pharmaceutics 2013, 5, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Y.; Wen, X.; Ma, H. Current prodrug strategies, for improving oral absorption of nucleoside analogues. Asian J. Pharm. Sci. 2014, 9, 65–74. [Google Scholar] [CrossRef]
- Tsume, Y.; Incecayir, T.; Song, X.; Hilfinger, J.M.; Amidon, G.L. The development of orally administrable gemcitabine prodrugs with D-enantiomer amino acids: Enhanced membrane permeability and enzymatic stability. Eur. J. Pharm. Biopharm. 2014, 86, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Lorenzi, P.L.; Landowski, C.P.; Vig, B.S.; Hilfinger, J.M.; Amidon, G.L. Amino acid ester prodrugs of the anticancer agent gemcitabine: Synthesis, bioconversion, metabolic bioevasion, and hPEPT1-mediated transport. Mol. Pharm. 2005, 2, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Bender, D.M.; Victor, F.; Peterson, J.A.; Boyer, R.D.; Stephenson, G.A.; Azman, A.; McCarthy, J.R. Facile rearrangement of N4-(α-aminoacyl)cytidines to N-(4-cytidinyl)amino acid amides. Tetrahedron Lett. 2008, 49, 2052–2055. [Google Scholar] [CrossRef]
- Wang, G.; Chen, H.; Zhao, D.; Ding, D.; Sun, M.; Kou, L.; Luo, C.; Zhang, D.; Yi, X.; Dong, J.; et al. Combination of l-carnitine with lipophilic linkage-donating gemcitabine derivatives as intestinal novel organic cation transporter 2-targeting oral prodrugs. J. Med. Chem. 2017, 60, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Jin, H.E.; Hong, S.S. Targeting L-type amino acid transporter 1 for anticancer therapy: Clinical impact from diagnostics to therapeutics. Expert Opin. Ther. Targets 2015, 19, 1319–1337. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Sunose, Y.; Arakawa, K.; Ogawa, T.; Sunaga, N.; Shimizu, K.; Tominaga, H.; Oriuchi, N.; Itoh, H.; Nagamori, S.; et al. Prognostic significance of L-type amino acid transporter 1 expression in surgically resected pancreatic cancer. Br. J. Cancer 2012, 107, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, N.; Ichinoe, M.; Mikami, T.; Nakada, N.; Hana, K.; Koizumi, W.; Endou, H.; Okayasu, I. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J. Clin. Pathol. 2012, 65, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Kwak, E.Y.; Shim, W.S.; Chang, J.E.; Chong, S.; Kim, D.D.; Chung, S.J.; Shim, C.K. Enhanced intracellular accumulation of a non-nucleoside anti-cancer agent via increased uptake of its valine ester prodrug through amino acid transporters. Xenobiotica 2012, 42, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Maeng, H.J.; Kim, E.S.; Chough, C.; Joung, M.; Lim, J.W.; Shim, C.K.; Shim, W.S. Addition of amino acid moieties to lapatinib increases the anticancer effect via amino acid transporters. Biopharm. Drug Dispos. 2014, 35, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Park, J.H.; Park, S.; Lee, S.Y.; Cho, K.H.; Kim, D.D.; Shim, W.S.; Yoon, I.S.; Cho, H.J.; Maeng, H.J. Enhanced Cellular Uptake and Pharmacokinetic Characteristics of Doxorubicin-Valine Amide Prodrug. Molecules 2016, 21, 1272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, Y.; Qin, Y.; Wang, R.; Li, G.; Sun, C.; Qu, X.; Li, W. Pharmacokinetics and metabolism of SL-01, a prodrug of gemcitabine, in rats. Cancer Chemother. Pharmacol. 2013, 71, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Son, M.K.; Jung, K.H.; Lee, H.S.; Lee, H.; Kim, S.J.; Yan, H.H.; Ryu, Y.L.; Hong, S.S. SB365, Pulsatilla saponin D suppresses proliferation and induces apoptosis of pancreatic cancer cells. Oncol. Rep. 2013, 30, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.S.; Roh, S.H.; Kim, D.K.; Lee, J.G.; Lee, Y.Y.; Hong, S.S.; Kwon, S.W.; Park, J.H. Anti-cancer effect of Betulin on a human lung cancer cell line: A pharmacoproteomic approach using 2 D SDS PAGE coupled with nano-HPLC tandem Mass Spectrometry. Planta Med. 2009, 75, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.W.; Lin, J.; Hong, X.Y. Cyclin A2 regulates homologous recombination DNA repair and sensitivity to DNA damaging agents and poly (ADP-ribose) polymerase (PARP) inhibitors in human breast cancer cells. Oncotarget 2017, 24, 90842–90851. [Google Scholar] [CrossRef] [PubMed]
- Hatiboglu, M.A.; Kocyigit, A.; Guler, E.M.; Akdur, K.; Nalli, A.; Karatas, E.; Tuzgen, S. Thymoquinone Induces Apoptosis in B16-F10 Melanoma Cell Through Inhibition of p-STAT3 and Inhibits Tumor Growth in a Murine Intracerebral Melanoma Model. World Neurosurg. 2018, 114, e182–e190. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Jung, K.H.; Lee, H.; Son, M.K.; Seo, J.H.; Yan, H.H.; Park, B.H.; Hong, S.; Hong, S.S. Synergistic anticancer activity of HS-173, a novel PI3K inhibitor in combination with Sorafenib against pancreatic cancer cells. Cancer Lett. 2013, 331, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Kim, I.B.; Noh, C.K.; Quach, H.P.; Yoon, I.S.; Chow, E.C.Y.; Kim, M.; Jin, H.E.; Cho, K.H.; Chung, S.J.; et al. Effects of 1α,25-dihydroxyvitamin D3, the natural vitamin D receptor ligand, on the pharmacokinetics of cefdinir and cefadroxil, organic anion transporter substrates, in rat. J. Pharm. Sci. 2014, 103, 3793–3805. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Pharmacokinetic Parameters | Gem-Thr (4 mg/kg) | Free Gemcitabine (4 mg/kg) | ||
|---|---|---|---|---|
| Gem-Thr | Gemcitabine | Sum | ||
| AUC (μg∙min/mL) | 1713.85 ± 1082.40 | 1739.88 ± 282.00 * | 3437.92 ± 1180.56 | 948.38 ± 52.04 |
| Terminal t1/2 (min) | 236.18 ± 50.94 | 666.83 ± 271.49 | 537.23 ± 227.78 | 532.68 ± 177.90 |
| CL (mL/min/kg) | 2.85 ± 1.33 | 0.60 ± 0.10 * | 1.26 ± 0.39 | 4.23 ± 0.23 |
| Vss (mL/kg) | 662.35 ± 281.40 | 545.57 ± 263.01 * | 770.96 ± 435.31 | 2483.64 ± 867.19 |
| MRT (min) | 237.18 ± 20.87 | 907.18 ± 391.68 * | 577.36 ± 212.90 | 582.06 ± 177.90 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Fang, Z.; Jung, H.-Y.; Yoon, J.-H.; Hong, S.-S.; Maeng, H.-J. Synthesis of Gemcitabine-Threonine Amide Prodrug Effective on Pancreatic Cancer Cells with Improved Pharmacokinetic Properties. Molecules 2018, 23, 2608. https://doi.org/10.3390/molecules23102608
Hong S, Fang Z, Jung H-Y, Yoon J-H, Hong S-S, Maeng H-J. Synthesis of Gemcitabine-Threonine Amide Prodrug Effective on Pancreatic Cancer Cells with Improved Pharmacokinetic Properties. Molecules. 2018; 23(10):2608. https://doi.org/10.3390/molecules23102608
Chicago/Turabian StyleHong, Sungwoo, Zhenghuan Fang, Hoi-Yun Jung, Jin-Ha Yoon, Soon-Sun Hong, and Han-Joo Maeng. 2018. "Synthesis of Gemcitabine-Threonine Amide Prodrug Effective on Pancreatic Cancer Cells with Improved Pharmacokinetic Properties" Molecules 23, no. 10: 2608. https://doi.org/10.3390/molecules23102608
APA StyleHong, S., Fang, Z., Jung, H.-Y., Yoon, J.-H., Hong, S.-S., & Maeng, H.-J. (2018). Synthesis of Gemcitabine-Threonine Amide Prodrug Effective on Pancreatic Cancer Cells with Improved Pharmacokinetic Properties. Molecules, 23(10), 2608. https://doi.org/10.3390/molecules23102608