High Potency of Melaleuca alternifolia Essential Oil against Multi-Drug Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Results
2.1. TTO Chemical Characterization
2.2. Antimicrobial Susceptibility
2.3. Synergistic Activity
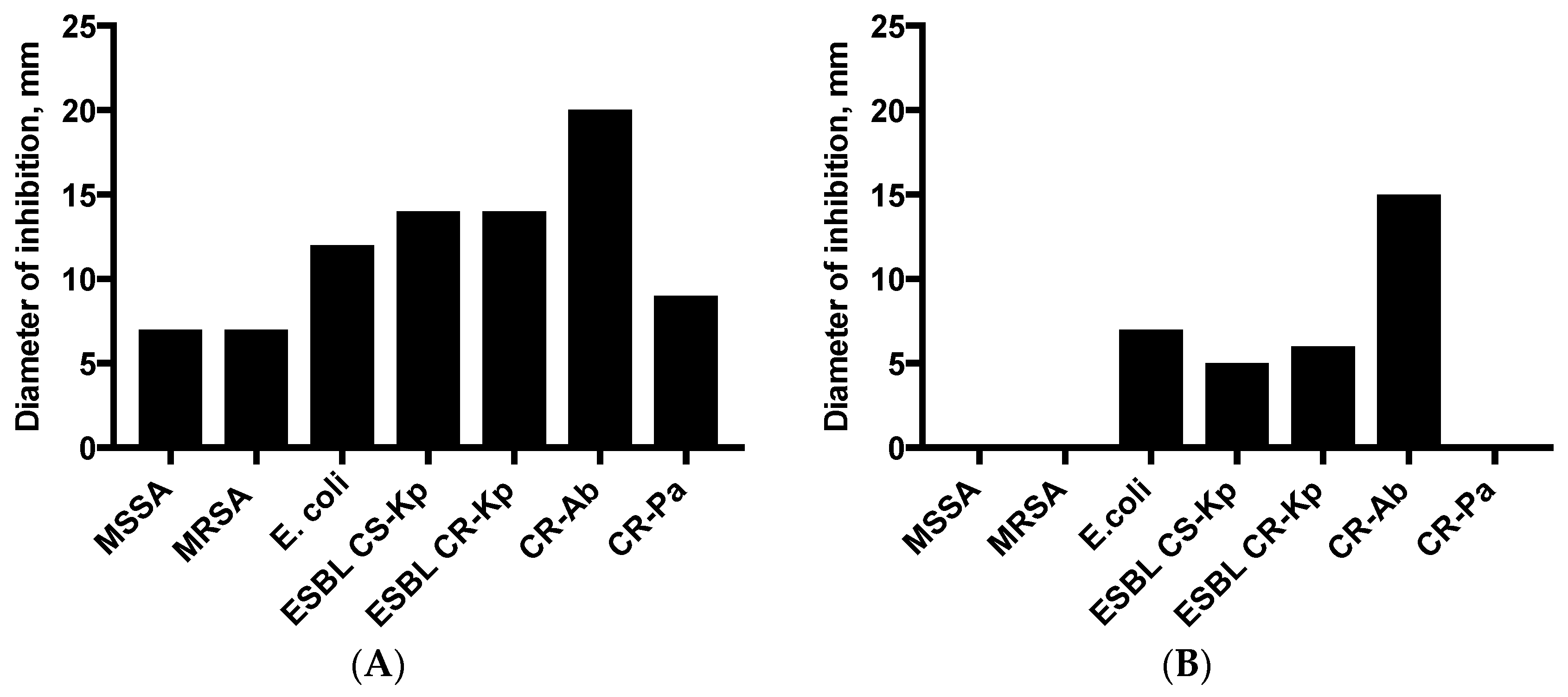
2.4. Disk Diffusion (DD) and VP Assay
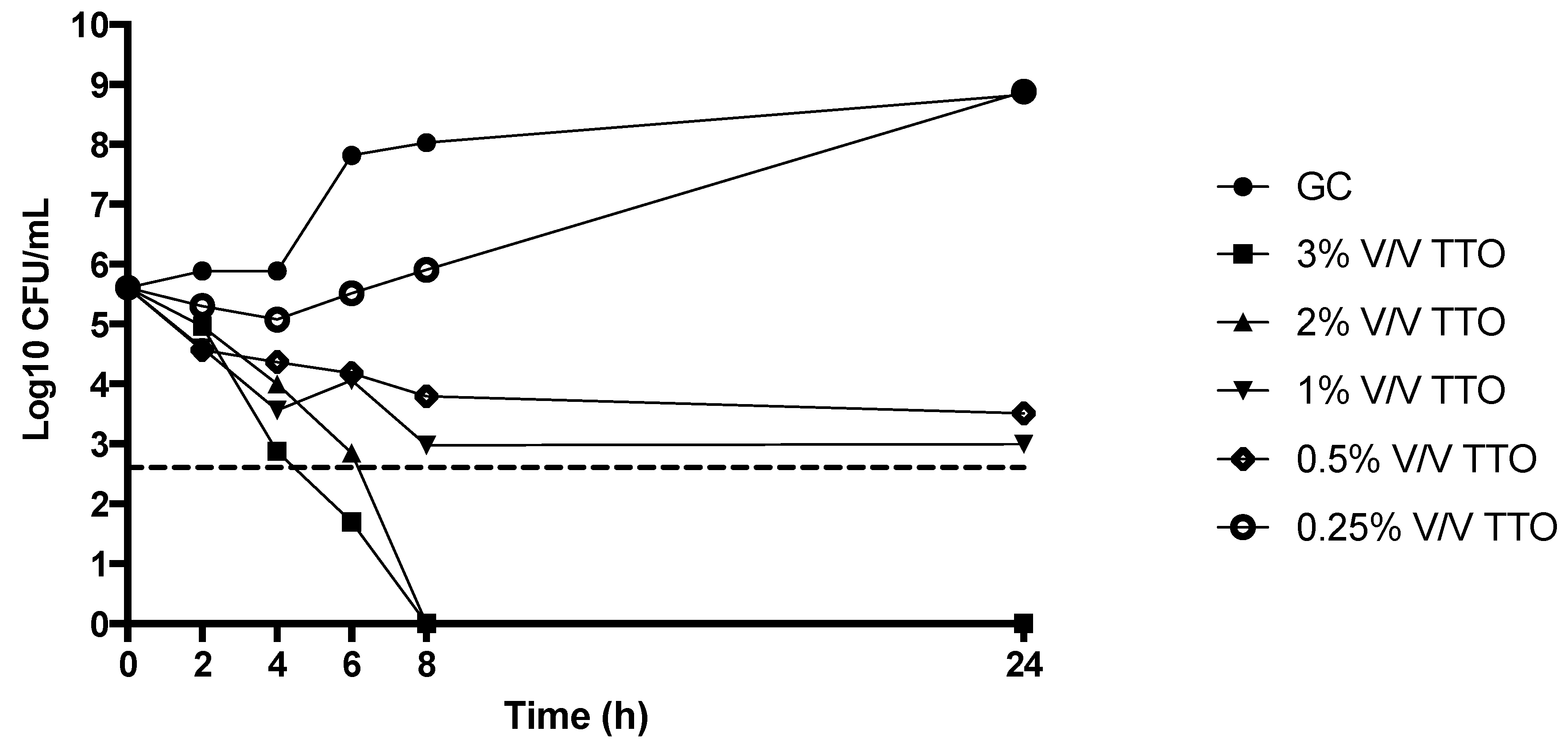
2.5. Time Kill Studies
3. Discussion
4. Materials and Method
4.1. Antimicrobials Agents and TTO
4.2. TTO Chemical Composition Analysis
4.3. Bacterial Strains
4.4. Antimicrobial Activity
4.5. Synergistic Activity of TTO Combined with Antimicrobial Agents
4.6. DD and VP Assay
4.7. Time-Kill Studies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
Abbreviations
| MDR | multidrug-resistant |
| CR | carbapenem-resistant |
| EOs | essential oils |
| TTO | Tea tree oil |
| MSSA | methicillin-susceptible Staphylococus aureus |
| MRSA | methicillin-resistant Staphylococus aureus |
| ESBL | extended-spectrum beta lactamases |
| CS | carbapenem-sensitive |
| Kp | Klebsiella pneumoniae |
| Ab | Acinetobacter baumannii |
| Pa | Pseudomonas aeruginosa |
| HS | Headspace |
| VOCs | volatile organic compounds |
| LRI | Linear retention indices |
| TSB | tryptic soy broth |
| MIC | minimal inhibitory concentration |
| MBC | minimal bactericidal concentration |
| AMK | amikacin |
| CFZ | cefazolin |
| MEM | meropenem |
| OXA | oxacillin |
| COL | colistin |
| RIF | rifampin |
| VAN | vancomicin |
| MHB | Mueller Hinton Broth |
| FICI | fractional inhibitory concentration index |
| DD | disk diffusion |
| VP | vapour phase |
| MHA | Mueller Hinton Agar |
| CFUs | Colony Forming Units |
| PDR | pan-drug resistant |
References
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Falagas, M.E.; Mavroudis, A.D.; Vardakas, K.Z. The antibiotic pipeline for multi-drug resistant gram negative bacteria: What can we expect? Expert Rev. Anti. Infect. Ther. 2016, 14, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Hu, Z.Q.; Hara, Y.; Shimamura, T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Q.; Zhao, W.H.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergy with ampicillin/sulbactam against 28 clinical isolates of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Ozcelik, B.; Kan, Y.; Kartal, M. Inhibitory Effects of Various Essential Oils and Individual Components against Extended-Spectrum Beta-Lactamase (ESBL) Produced by Klebsiella pneumoniae and Their Chemical Compositions. J. Food Sci. 2011, 76, M538–M546. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Azhdarzadeh, F.; Hojjati, M. Chemical Composition and Antimicrobial Activity of Leaf, Ripe and Unripe Peel of Bitter Orange (Citrus aurantium) Essential Oils. Nutr. Food Sci. Res. 2016, 3, 43–50. [Google Scholar] [CrossRef]
- Okoh, O.O.; Sadimenko, A.P.; Afolayan, A.J. Comparative evaluation of the antibacterial activities of the essential oils of Rosmarinus officinalis L. obtained by hydrodistillation and solvent free microwave extraction methods. Food Chem. 2010, 120, 308–312. [Google Scholar] [CrossRef]
- Tadic, V.; Oliva, A.; Bozovic, M.; Cipolla, A.; De Angelis, M.; Vullo, V.; Garzoli, S.; Ragno, R. Chemical and Antimicrobial Analyses of Sideritis romana L. subsp. purpurea (Tal. ex Benth.) Heywood, an Endemic of the Western Balkan. Molecules 2017, 22, 1395. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Sailer, R.; Berger, T.; Reichling, J.; Harkenthal, M. Pharmaceutical and medicinal aspects of Australian tea tree oil. Phytomedicine 1998, 5, 489–495. [Google Scholar] [CrossRef]
- Carson, C.F.; Cookson, B.D.; Farrelly, H.D.; Riley, T.V. Susceptibility of methicillin-resistant Staphylococcus aureus to the essential oil of Melaleuca alternifolia. J. Antimicrob. Chemother. 1995, 35, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- LaPlante, K.L. In vitro activity of lysostaphin, mupirocin, and tea tree oil against clinical methicillin-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2007, 57, 413–418. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, M.; Ginestra, G.; Mandalari, G.; Furneri, P.M.; Bisignano, G. Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea tree) oil against Staphylococcus aureus and Escherichia coli. Phytomedicine 2010, 17, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Artini, M.; Patsilinakos, A.; Papa, R.; Bozovic, M.; Sabatino, M.; Garzoli, S.; Vrenna, G.; Tilotta, M.; Pepi, F.; Ragno, R.; et al. Antimicrobial and Antibiofilm Activity and Machine Learning Classification Analysis of Essential Oils from Different Mediterranean Plants against Pseudomonas aeruginosa. Molecules 2018, 23, 482. [Google Scholar] [CrossRef] [PubMed]
- Bozovic, M.; Garzoli, S.; Sabatino, M.; Pepi, F.; Baldisserotto, A.; Andreotti, E.; Romagnoli, C.; Mai, A.; Manfredini, S.; Ragno, R. Essential Oil Extraction, Chemical Analysis and Anti-Candida Activity of Calamintha nepeta (L.) Savi subsp. glandulosa (Req.) Ball-New Approaches. Molecules 2017, 22, 203. [Google Scholar] [CrossRef]
- Bozovic, M.; Navarra, A.; Garzoli, S.; Pepi, F.; Ragno, R. Esential oils extraction: A 24-hour steam distillation systematic methodology. Nat. Prod. Res. 2017, 31, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, S.; Bozovic, M.; Baldisserotto, A.; Andreotti, E.; Pepi, F.; Tadic, V.; Manfredini, S.; Ragno, R. Sideritis romana L. subsp. purpurea (Tal. ex Benth.) Heywood, a new chemotype from Montenegro. Nat. Prod. Res. 2018, 32, 1056–1061. [Google Scholar] [PubMed]
- Garzoli, S.; Bozovic, M.; Baldisserotto, A.; Sabatino, M.; Cesa, S.; Pepi, F.; Vicentini, C.B.; Manfredini, S.; Ragno, R. Essential oil extraction, chemical analysis and anti-Candida activity of Foeniculum vulgare Miller—New approaches. Nat. Prod. Res. 2018, 32, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Frezza, C.; Salutari, G.; Cecco, M.d.; Ciaschetti, G.; Oliva, A.B.; Angelis, M.D.; Vullo, V.; Sabatino, M.; Garzoli, S.; et al. Composition of the Essential Oil of Coristospermum cuneifolium and Antimicrobial Activity Evaluation. Planta Med. Int. Open 2017, 4, e74–e81. [Google Scholar] [CrossRef]
- Warnke, P.H.; Lott, A.J.; Sherry, E.; Wiltfang, J.; Podschun, R. The ongoing battle against multi-resistant strains: In-vitro inhibition of hospital-acquired MRSA, VRE, Pseudomonas, ESBL E. coli and Klebsiella species in the presence of plant-derived antiseptic oils. J. Craniomaxillofac. Surg. 2013, 41, 321–326. [Google Scholar] [PubMed]
- Oliva, A.; Scorzolini, L.; Cipolla, A.; Mascellino, M.T.; Cancelli, F.; Castaldi, D.; D’Abramo, A.; D’Agostino, C.; Russo, G.; Ciardi, M.R.; et al. In vitro evaluation of different antimicrobial combinations against carbapenemase-producing Klebsiella pneumoniae: The activity of the double-carbapenem regimen is related to meropenem MIC value. J. Antimicrob. Chemother. 2017, 72, 1981–1984. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.; Ferreira, S.; Silva, F.; Domingues, F.C. Synergistic activity of coriander oil and conventional antibiotics against Acinetobacter baumannii. Phytomedicine 2012, 19, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.E.; de Menezes, I.R.; Bezerra Morais Braga, M.F.; Leite, N.F.; Barros, L.M.; Waczuk, E.P.; Pessoa da Silva, M.A.; Boligon, A.; Teixeira Rocha, J.B.; Souza, D.O.; et al. Antimicrobial Activity and Modulatory Effect of Essential Oil from the Leaf of Rhaphiodon echinus (Nees & Mart) Schauer on Some Antimicrobial Drugs. Molecules 2016, 21, 743. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1,8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Theodoridou, I.; Papaemmanouil, V.; Kapsiotis, I.; Panopoulou, M.; Stavropoulou, E.; Bezirtzoglou, E.E.; et al. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health Dis. 2015, 26, 23289. [Google Scholar] [CrossRef] [PubMed]
- Scandorieiro, S.; de Camargo, L.C.; Lancheros, C.A.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.; Duran, N.; Nakazato, G.; Kobayashi, R.K. Synergistic and Additive Effect of Oregano Essential Oil and Biological Silver Nanoparticles against Multidrug-Resistant Bacterial Strains. Front. Microbiol. 2016, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Ekren, P.K.; Ranzani, O.T.; Ceccato, A.; Li Bassi, G.; Munoz Conejero, E.; Ferrer, M.; Niederman, M.S.; Torres, A. Evaluation of the 2016 Infectious Diseases Society of America/American Thoracic Society Guideline Criteria for Risk of Multi-drug Resistant Pathogens in Hospital-acquired and Ventilator-associated Pneumonia Patients in the Intensive Care Unit. Am. J. Respir. Crit. Care Med. 2017, 197, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Doran, A.L.; Morden, W.E.; Dunn, K.; Edwards-Jones, V. Vapour–phase activities of essential oils against antibiotic sensitive and resistant bacteria including MRSA. Lett. Appl. Microbiol. 2009, 48, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, L.; Zhang, T.; Liu, B.; Du, L.; Jin, Y. Pulmonary delivery of tea tree oil-beta-cyclodextrin inclusion complexes for the treatment of fungal and bacterial pneumonia. J. Pharm. Pharmacol. 2017, 69, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V.; Nielsen, J.B. A review of the toxicity of Melaleuca alternifolia (tea tree) oil. Food Chem. Toxicol. 2006, 44, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Halcon, L.; Milkus, K. Staphylococcus aureus and wounds: A review of tea tree oil as a promising antimicrobial. Am. J. Infect. Control. 2004, 32, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Homeyer, D.C.; Sanchez, C.J.; Mende, K.; Beckius, M.L.; Murray, C.K.; Wenke, J.C.; Akers, K.S. In vitro activity of Melaleuca alternifolia (tea tree) oil on filamentous fungi and toxicity to human cells. Med. Mycol. 2015, 53, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, L.; Liu, B.; Du, L.; Jia, X.; Han, L.; Jin, Y. Tea tree oil nanoemulsions for inhalation therapies of bacterial and fungal pneumonia. Colloids Surf. B Biointerfaces 2016, 141, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Calfee, D.P. Trends in Community Versus Health Care-Acquired Methicillin-Resistant Staphylococcus aureus Infections. Curr. Infect. Dis. Rep. 2017, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- David, M.Z.; Daum, R.S. Treatment of Staphylococcus aureus Infections. Curr. Top. Microbiol. Immunol. 2017, 409, 325–383. [Google Scholar] [PubMed]
- Loughlin, R.; Gilmore, B.F.; McCarron, P.A.; Tunney, M.M. Comparison of the cidal activity of tea tree oil and terpinen-4-ol against clinical bacterial skin isolates and human fibroblast cells. Lett. Appl. Microbiol. 2008, 46, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S. Phytochemicals for Bacterial Resistance—Strengths, Weaknesses and Opportunities. Planta Med. 2008, 74, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Lahmar, A.; Bedoui, A.; Mokdad-Bzeouich, I.; Dhaouifi, Z.; Kalboussi, Z.; Cheraif, I.; Ghedira, K.; Chekir-Ghedira, L. Reversal of resistance in bacteria underlies synergistic effect of essential oils with conventional antibiotics. Microb. Pathog. 2017, 106, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Shiota, S.; Mizushima, T.; Ito, H.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Marked potentiation of activity of beta-lactams against methicillin-resistant Staphylococcus aureus by corilagin. Antimicrob. Agents Chemother. 2001, 45, 3198–3201. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, M.; Newall, N.; Carville, K.; Smith, J.; Riley, T.V.; Carson, C.F. Uncontrolled, open-label, pilot study of tea tree (Melaleuca alternifolia) oil solution in the decolonisation of methicillin-resistant Staphylococcus aureus positive wounds and its influence on wound healing. Int. Wound J. 2011, 8, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Septimus, E.J.; Schweizer, M.L. Decolonization in Prevention of Health Care-Associated Infections. Clin. Microbiol. Rev. 2016, 29, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Ferrini, A.M.; Mannoni, V.; Aureli, P.; Salvatore, G.; Piccirillp, E.; Ceddia, T.; Pontieri, E.; Sessa, R.; Oliva, B. Melaleuca Alternifolia Essential Oil Possesses Potent Anti-Staphylococcal Activity Extended to Strains Resistant to Antibiotics. Int. J. Immunopathol. Pharmacol. 2006, 19, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard; Document M7-A7; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Oliva, A.; Gizzi, F.; Mascellino, M.T.; Cipolla, A.; D’Abramo, A.; D’Agostino, C.; Trinchieri, V.; Russo, G.; Tierno, F.; Iannetta, M.; et al. Bactericidal and synergistic activity of double-carbapenem regimen for infections caused by carbapenemase-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2016, 22, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Homer, L.E.; Leach, D.N.; Lea, D.; Slade Lee, L.; Henry, R.J.; Baverstock, P.R. Natural variation in the essential oil content of Melaleuca alternifolia Cheel (Myrtaceae). Biochem. Syst. Ecol. 2000, 28, 367–382. [Google Scholar] [CrossRef]
Sample Availability: Samples of the used TTO are available from the authors. |

| # 1 | Component 2 | LRI 3 | LRIlit 4 | A1% 5 | A2% 6 |
|---|---|---|---|---|---|
| 1 | α-pinene | 1040 | 1039 | 12.4 | 22.5 |
| 2 | β-pinene | 1131 | 1124 | 1.8 | 2.4 |
| 3 | 1,4-cineole | 1192 | 1192 | 0.5 | - |
| 4 | α-terpinene | 1197 | 1195 | 2.8 | 2.9 |
| 5 | d-limonene | 1216 | 1219 | 2.3 | 2.8 |
| 6 | eucalyptol | 1231 | 1230 | 15.2 | 16.5 |
| 7 | γ-terpinene | 1266 | 1265 | 9.8 | 10.7 |
| 8 | o-cymene | 1291 | 1287 | 6.3 | 8.5 |
| 9 | terpinolene | 1306 | 1299 | 1.6 | 1.6 |
| 10 | aromadendrene | 1600 | 1603 | 1.9 | - |
| 11 | terpinen-4-ol | 1631 | 1633 | 35.4 | 28.7 |
| 12 | α-terpineol | 1718 | 1724 | 8.1 | 3.4 |
| 13 | ledene | 1715 | 1707 | 1.1 | - |
| 14 | globulol | 2110 | 2104 | 0.8 | - |
| Total | 100 | 100 |
| Strains | TTO 1 | AMK 2 | OXA 3 | CFZ 4 | VAN 5 | RIF 6 | MEM 7 | COL 8 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC 9 | MBC 10 | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| % v/v | μg/mL | μg/mL | μg/mL | μg/mL | μg/mL | μg/mL | μg/mL | |||||||||
| MSSA 11 | 1 | 2 | 4 | 8 | 0.25 | 0.50 | 0.50 | 0.50 | 0.50 | 1 | 0.007 | 0.007 | NA | NA | ||
| MRSA 12 | 0.50 | 2 | 32 | 32 | 32 | 64 | 64 | 128 | 1 | 1 | 0.007 | 0.007 | NA | NA | ||
| E. coli 13 | 0.25 | 0.25 | 4 | 4 | NA 20 | NA | NA | NA | 0.060 | 0.060 | 0.50 | 0.50 | ||||
| ESBL-CS-Kp 14,15,16 | 0.50 | 0.50 | 0.50 | 0.50 | NA | NA | NA | NA | 0.125 | 0.250 | 256 | 256 | ||||
| ESBL-CR 17 | 0.25 | 0.25 | 64 | 64 | NA | NA | NA | NA | 256 | 512 | 128 | 128 | ||||
| CR-Ab 18 | 0.25 | 0.25 | 8 | 16 | NA | NA | NA | NA | 64 | 128 | 0.25 | 0.25 | ||||
| CR-Pa 19 | 1 | 1 | 8 | 8 | NA | NA | NA | NA | 8 | 16 | 1 | 2 | ||||
| Strains | TTO 1 + AMK 2 | TTO + OXA 3 | TTO + CFZ 4 | TTO + VAN 5 | TTO + RIF 6 | TTO + MEM 7 | TTO + COL 8 |
|---|---|---|---|---|---|---|---|
| MSSA 9 | 0.25 | 0.32 | 0.25 | >0.5 | 0.32 | - | - |
| MRSA 10 | 0.20 | 0.32 | 0.32 | >0.5 | 0.32 | - | - |
| E. coli 11 | 0.25 | NA 18 | NA | NA | NA | >0.50 | 0.13 |
| ESBL-CS-Kp 12,13,14 | >0.50 | NA | NA | NA | NA | 0.50 | 0.32 |
| ESBL-CR-Kp 15 | 0.50 | NA | NA | NA | NA | 0.32 | 0.32 |
| CR-Ab 16 | 0.32 | NA | NA | NA | NA | 0.32 | 0.21 |
| CR-Pa 17 | 0.25 | NA | NA | NA | NA | 0.50 | 0.25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, A.; Costantini, S.; De Angelis, M.; Garzoli, S.; Božović, M.; Mascellino, M.T.; Vullo, V.; Ragno, R. High Potency of Melaleuca alternifolia Essential Oil against Multi-Drug Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 2584. https://doi.org/10.3390/molecules23102584
Oliva A, Costantini S, De Angelis M, Garzoli S, Božović M, Mascellino MT, Vullo V, Ragno R. High Potency of Melaleuca alternifolia Essential Oil against Multi-Drug Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus. Molecules. 2018; 23(10):2584. https://doi.org/10.3390/molecules23102584
Chicago/Turabian StyleOliva, Alessandra, Silvia Costantini, Massimiliano De Angelis, Stefania Garzoli, Mijat Božović, Maria Teresa Mascellino, Vincenzo Vullo, and Rino Ragno. 2018. "High Potency of Melaleuca alternifolia Essential Oil against Multi-Drug Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus" Molecules 23, no. 10: 2584. https://doi.org/10.3390/molecules23102584
APA StyleOliva, A., Costantini, S., De Angelis, M., Garzoli, S., Božović, M., Mascellino, M. T., Vullo, V., & Ragno, R. (2018). High Potency of Melaleuca alternifolia Essential Oil against Multi-Drug Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus. Molecules, 23(10), 2584. https://doi.org/10.3390/molecules23102584