Molecular Targets Modulated by Fangchinoline in Tumor Cells and Preclinical Models
Abstract
1. Introduction
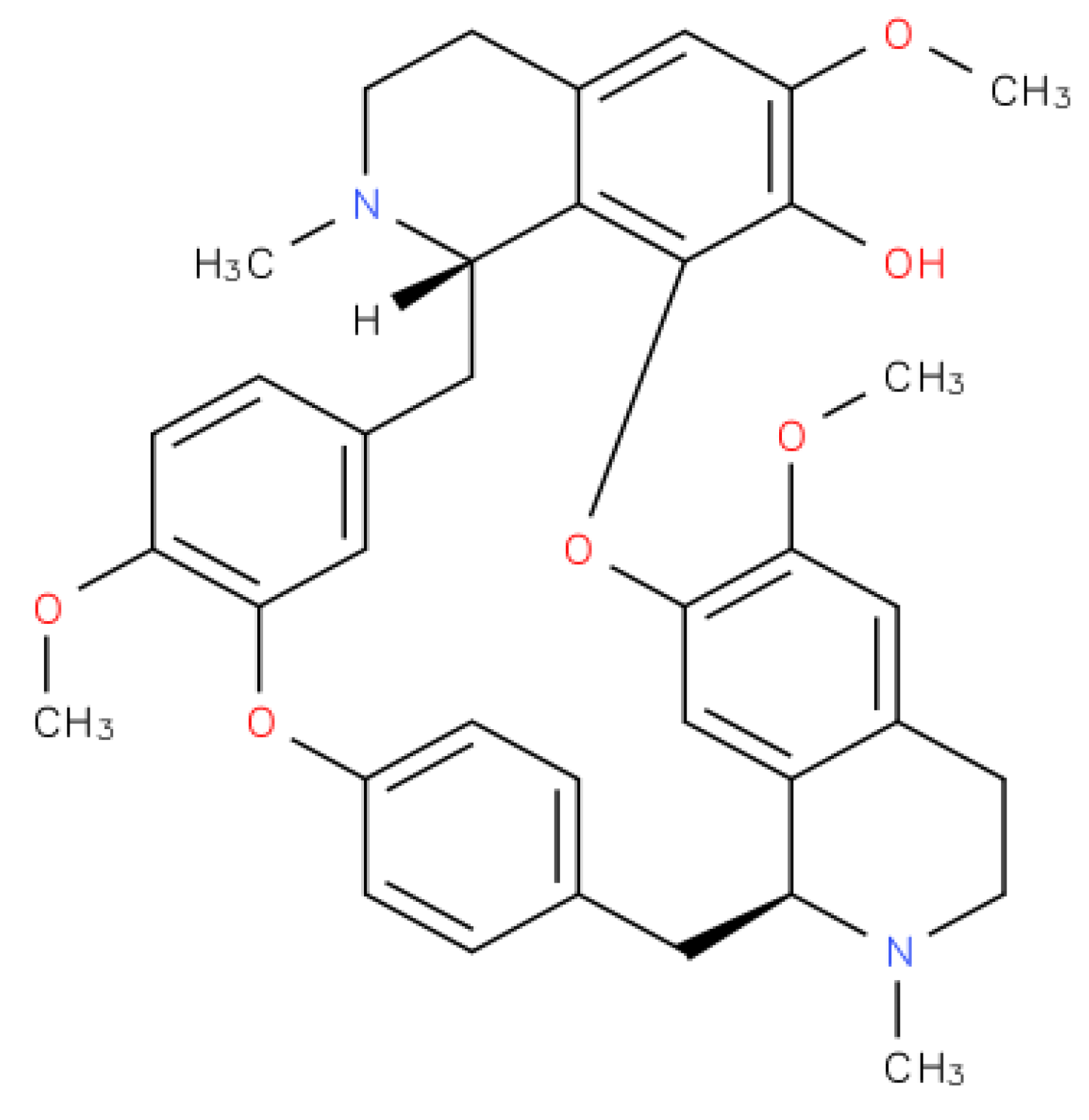
2. Fangchinoline Classification
3. Fangchinoline-Reported Anti-Cancer Effects in Vitro and in Vivo
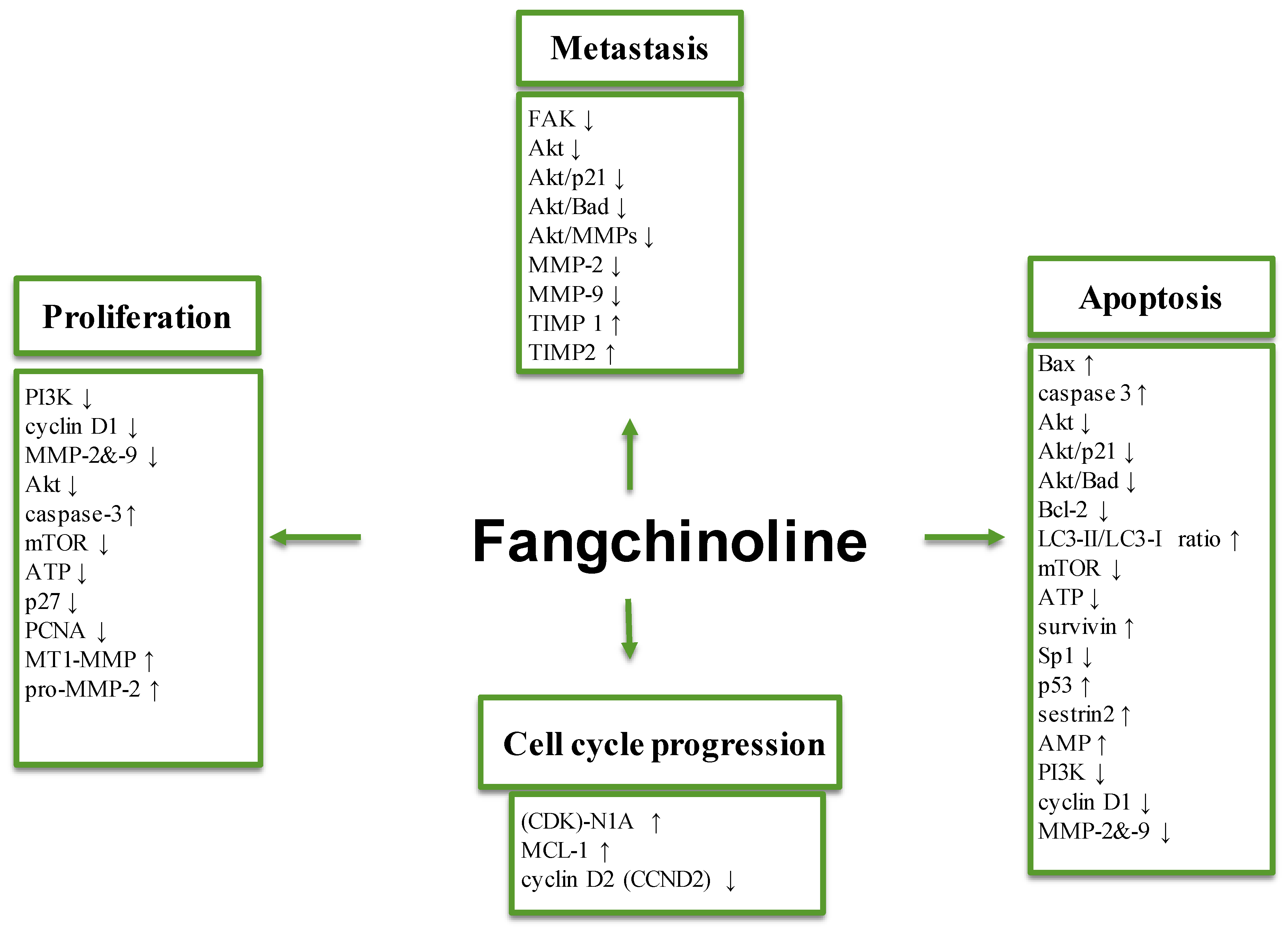
3.1. Effect on Tumor Cell Proliferation
3.2. Anti-Metastatic Effects
3.3. Effects on Apoptosis and Autophagy
3.4. Molecular Targets
3.5. Effects on Multi-Drug Resistance and Other Parameters
3.6. In Vivo Effects
3.7. Effects of the Derivatives of Fangchinoline
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.G.; Chan, T.A. Therapeutic targeting of tumor suppressor genes. Cancer 2015, 121, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Urry, L.A.; Cain, M.L.; Wasserman, S.A.; Minorsky, P.V.; Reece, J.B. Campbell Biology, 10th ed.; Pearson: London, UK, 2013; ISBN-13: 2900321775657. [Google Scholar]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Canc. Res. 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Deorukhkar, A.; Krishnan, S.; Sethi, G.; Aggarwal, B.B. Back to basics: How natural products can provide the basis for new therapeutics. Expert Opin. Inv. Drug 2007, 16, 1753–1773. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Yang, S.F.; Sethi, G.; Hu, D.N. Natural bioactives in cancer treatment and prevention. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Weng, C.J.; Sethi, G.; Hu, D.N. Natural bioactives and phytochemicals serve in cancer treatment and prevention. Evid.-Based Compl. Alt. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Sethi, G.; Kuo, P.L. Novel medicines and strategies in cancer treatment and prevention. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S. Natural Products for Cancer Therapy. Dual Diagn. 2016, 1. [Google Scholar] [CrossRef]
- Baek, S.H.; Lee, J.H.; Kim, C.; Ko, J.H.; Ryu, S.H.; Lee, S.G.; Yang, W.M.; Um, J.Y.; Chinnathambi, A.; Alharbi, S.A.; et al. Ginkgolic Acid C 17:1, Derived from Ginkgo biloba Leaves, Suppresses Constitutive and Inducible STAT3 Activation through Induction of PTEN and SHP-1 Tyrosine Phosphatase. Molecules 2017, 22, E276. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. beta-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, R.; Manu, K.A.; Chen, L.; Li, F.; Rajendran, P.; Subramaniam, A.; Lam, P.; Kumar, A.P.; Sethi, G. Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3 K/Akt signaling pathways. Apoptosis 2011, 16, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, S.M.; Bae, H.; Nam, D.; Lee, J.H.; Lee, S.G.; Shim, B.S.; Kim, S.H.; Ahn, K.S.; Choi, S.H.; et al. Embelin inhibits growth and induces apoptosis through the suppression of Akt/mTOR/S6K1 signaling cascades. Prostate. 2013, 73, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.L.; Osheroff, N. Etoposide, topoisomerase II and cancer. Curr. Med. Chem. Anticancer Agents 2005, 5, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Abernethy, M.L.; Kulp-Shorten, C.; Callen, J.P.; Glazer, S.D.; Huntley, A.; McCray, M.; Monroe, A.B.; Tschen, E.; Wolf, J.E., Jr. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J. Am. Acad. Dermatol. 1991, 24, 738–743. [Google Scholar] [CrossRef]
- Clark, P.I.; Slevin, M.L. The clinical pharmacology of etoposide and teniposide. Clin. Pharmacokinet. 1987, 12, 223–252. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, T.M.; Markman, M. Paclitaxel in cancer therapy. Expert Opin. Pharmacother 2002, 3, 755–766. [Google Scholar] [PubMed]
- Tabaczar, S.; Koceva-Chyla, A.; Matczak, K.; Gwozdzinski, K. Molecular mechanisms of antitumor activity of taxanes. I. Interaction of docetaxel with microtubules. Postep. Hig. Med. Dosw. 2010, 64, 568–581. [Google Scholar]
- Coukell, A.J.; Noble, S.; Faulds, D. Vinorelbine in advanced non-small cell lung cancer. A pharmacoeconomic review. PharmacoEconomics 1999, 15, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Eisenbrand, G. Stephania tetrandra S. Moore. In Chinese Drugs of Plant Origin; Springer: Berlin/Heidelberg, Germany, 1992; pp. 963–978, Print ISBN: 978-3-642-73741-1, online ISBN: 978-3-642-73739-8. [Google Scholar]
- Wang, C.D.; Yuan, C.F.; Bu, Y.Q.; Wu, X.M.; Wan, J.Y.; Zhang, L.; Hu, N.; Liu, X.J.; Zu, Y.; Liu, G.L.; et al. Fangchinoline inhibits cell proliferation via Akt/GSK-3beta/cyclin D1 signaling and induces apoptosis in MDA-MB-231 breast cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kar, S.; Lai, X.; Cai, W.; Arfuso, F.; Sethi, G.; Lobie, P.E.; Goh, B.C.; Lim, L.H.K.; Hartman, M.; et al. Triple negative breast cancer in Asia: An insider’s view. Cancer Treat. Rev. 2018, 62, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Zhang, Y.; Zhang, X.; Yang, Y.; Ma, Y.; Pang, D. Fangchinoline induces G1 arrest in breast cancer cells through cell-cycle regulation. Phytother. Res. 2013, 27, 1790–1794. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Z.; Han, W.; Lu, X.; Jin, S.; Yang, W.; Li, J.; He, W.; Qian, Y. Fangchinoline suppresses the proliferation, invasion and tumorigenesis of human osteosarcoma cells through the inhibition of PI3K and downstream signaling pathways. Int. J. Mol. Med. 2017, 40, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Su, J.; Zhang, T.; Wang, K.; Li, X. Fangchinoline as a kinase inhibitor targets FAK and suppresses FAK-mediated signaling pathway in A549. J. Drug Target 2015, 23, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.D.; Huang, J.G.; Gao, X.; Li, Y.; Zhou, S.Y.; Yan, X.; Zou, A.; Chang, J.L.; Wang, Y.S.; Yang, G.X.; et al. Fangchinoline induced G1/S arrest by modulating expression of p27, PCNA, and cyclin D in human prostate carcinoma cancer PC3 cells and tumor xenograft. Biosci. Biotechnol. Biochem. 2010, 74, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Wang, L.; Huang, Y.; Leng, Y.; Wang, G. Fangchinoline induces G0/G1 arrest by modulating the expression of CDKN1A and CCND2 in K562 human chronic myelogenous leukemia cells. Exp. Ther. Med. 2013, 5, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Zhang, X.; Ma, Y.; Zhang, A. Fangchinoline Induces Apoptosis, Autophagy and Energetic Impairment in Bladder Cancer. Cell. Physiol. Biochem. 2017, 43, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; He, T.; Zhao, K.; Xing, C. Anti-metastatic activity of fangchinoline in human gastric cancer AGS cells. Oncol. Lett. 2017, 13, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Xie, P.; Su, J.; Zhang, T.; Li, X.; Liang, G. Fangchinoline suppresses the growth and invasion of human glioblastoma cells by inhibiting the kinase activity of Akt and Akt-mediated signaling cascades. Tumour Biol. 2016, 37, 2709–2719. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P. What has senescence got to do with cancer? Cancer Cell 2005, 7, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Peng, J.M.; Su, L.D.; Wang, D.Y.; Yu, Y.J. Fangchinoline inhibits the proliferation of SPC-A-1 lung cancer cells by blocking cell cycle progression. Exp. Ther. Med. 2016, 11, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Taran, S.J.; Taran, R.; Malipatil, N.B. Pediatric Osteosarcoma: An Updated Review. Indian J. Med. Paediatr. Oncol. 2017, 38, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Eva, B-C.; Shalonda, W. Melanoma Review: Background and Treatment. Available online: https://www.uspharmacist.com/article/melanoma-review-background-and-treatment (accessed on 5 October 2018).
- Shi, J.; Guo, B.; Hui, Q.; Chang, P.; Tao, K. Fangchinoline suppresses growth and metastasis of melanoma cells by inhibiting the phosphorylation of FAK. Oncol. Rep. 2017, 38, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, A.N.; Jacoby, D.B. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601). Expert Opin. Drug Deliv. 2007, 4, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Brada, M.; van den Bent, M.J.; Tonn, J.C.; Pentheroudakis, G. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, S.; Tobias, D.H. Cancer and its Management, 7th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; ISBN 978-1-118-46873-9. [Google Scholar]
- Butowski, N.A. Epidemiology and diagnosis of brain tumors. Continuum (Minneap. Minn.) 2015, 21, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Drevelegas, A.; Chourmouzi, D.; Papanicolaou, N.; Drevelegas, K. Chapter 38: Malignant Astrocytomas. In Handbook of Neuro-Oncology Neuroimaging, 2nd ed; Newton, H.B., Ed.; Academic Press: SD, USA, 2016; pp. 421–438. ISBN 978-0-12-800945-1. [Google Scholar]
- Holland, E.C. Glioblastoma multiforme: The terminator. Proc. Natl. Acad. Sci. USA 2000, 97, 6242–6244. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [PubMed]
- Nawkar, G.M.; Maibam, P.; Park, J.H.; Sahi, V.P.; Lee, S.Y.; Kang, C.H. UV-Induced cell death in plants. Int. J. Mol. Sci. 2013, 14, 1608–1628. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Gozuacik, D.; Kimchi, A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene 2004, 23, 2891–2906. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Alnemri, E.S.; Altucci, L.; Andrews, D.; Annicchiarico-Petruzzelli, M.; et al. Essential versus accessory aspects of cell death: Recommendations of the NCCD 2015. Cell Death Differ. 2015, 22, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Autophagy in Cancer Therapy. Front. Oncol. 2017, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Vats, S.; Chia, A.Y.; Tan, T.Z.; Deng, S.; Ong, M.S.; Arfuso, F.; Yap, C.T.; Goh, B.C.; Sethi, G.; et al. Dual role of autophagy in hallmarks of cancer. Oncogene 2018, 37, 1142–1158. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Safe, S.; Lee, S.O. Inactivation of the orphan nuclear receptor NR4A1 contributes to apoptosis induction by fangchinoline in pancreatic cancer cells. Toxicol. Appl. Pharm. 2017, 332, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xing, Z.; Wang, F.; Yuan, X.; Zhang, Y. Fangchinoline inhibits migration and causes apoptosis of human breast cancer MDA-MB-231 cells. Oncol. Lett. 2017, 14, 5307–5312. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Pan, W.; Zhu, M.; Zhang, M.; Hao, X.; Liang, G.; Feng, Y. Fangchinoline induces autophagic cell death via p53/sestrin2/AMPK signalling in human hepatocellular carcinoma cells. Br. J. Pharmacol. 2011, 164, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Vogt, P.K.; Hart, J.R.; Gymnopoulos, M.; Jiang, H.; Kang, S.; Bader, A.G.; Zhao, L.; Denley, A. Phosphatidylinositol 3-kinase: The oncoprotein. Curr. Top. Microbiol. 2010, 347, 79–104. [Google Scholar]
- Vincent, E.E.; Elder, D.J.; Thomas, E.C.; Phillips, L.; Morgan, C.; Pawade, J.; Sohail, M.; May, M.T.; Hetzel, M.R.; Tavare, J.M. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br. J. Cancer 2011, 104, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Samuels, Y.; Ericson, K. Oncogenic PI3K and its role in cancer. Curr. Opin. Oncol. 2006, 18, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Peloponese, J.M., Jr.; Jeang, K.T. Role for Akt/protein kinase B and activator protein-1 in cellular proliferation induced by the human T-cell leukemia virus type 1 tax oncoprotein. J. Biol. Chem. 2006, 281, 8927–8938. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Nito, C.; Kamada, H.; Nishi, T.; Chan, P.H. Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J. Cerebr. Blood Flow Met. 2006, 26, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Rossig, L.; Jadidi, A.S.; Urbich, C.; Badorff, C.; Zeiher, A.M.; Dimmeler, S. Akt-dependent phosphorylation of p21(Cip1) regulates PCNA binding and proliferation of endothelial cells. Mol. Cell. Biol. 2001, 21, 5644–5657. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Zhang, D.; Brodt, P. Type 1 insulin-like growth factor regulates MT1-MMP synthesis and tumor invasion via PI 3-kinase/Akt signaling. Oncogene 2003, 22, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sounni, N.E.; Devy, L.; Hajitou, A.; Frankenne, F.; Munaut, C.; Gilles, C.; Deroanne, C.; Thompson, E.W.; Foidart, J.M.; Noel, A. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. FASEB J. 2002, 16, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guan, J.L. Signal transduction by focal adhesion kinase in cancer. Cancer Metast. Rev. 2009, 28, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guan, J.L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Del. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; ISBN-10: 0-8153-3218-1; ISBN-10: 0-8153-4072-9. [Google Scholar]
- Bai, X.; Li, Y.Y.; Zhang, H.Y.; Wang, F.; He, H.L.; Yao, J.C.; Liu, L.; Li, S.S. Role of matrix metalloproteinase-9 in transforming growth factor-beta1-induced epithelial-mesenchymal transition in esophageal squamous cell carcinoma. Onco Targets Ther. 2017, 10, 2837–2847. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Sogo, S.; Hioki, K.; Tokunaga, R.; Taketani, S. Acquisition of cell adhesion and induction of focal adhesion kinase of human colon cancer Colo 201 cells by retinoic acid-induced differentiation. Differentiation 1998, 62, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, E.; Sattler, M.; Ewaniuk, D.S.; Salgia, R. Role of focal adhesion proteins in signal transduction and oncogenesis. Crit. Rev. Oncogenesis 1997, 8, 343–358. [Google Scholar] [CrossRef] [PubMed]
- McCain, J. The MAPK (ERK) Pathway: Investigational Combinations for the Treatment Of BRAF-Mutated Metastatic Melanoma. Pharmacol. Therapeut. 2013, 38, 96–108. [Google Scholar]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharm. 2016, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, M.C.; Crosariol, M.; Loro, E.; Li, Z.; Pestell, R.G. Cyclins and cell cycle control in cancer and disease. Genes Cancer 2012, 3, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, Z.; Nie, Y.; Shi, Y.; Fan, D. Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer Lett. 2014, 347, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Shine, V.J.; Latha, P.G.; Suja, S.N.; Anuja, G.I.; Raj, G.; Rajasekharan, S.N. Ameliorative effect of alkaloid extract of Cyclea peltata (Poir.) Hook. f. & Thoms. roots (ACP) on APAP/CCl4 induced liver toxicity in Wistar rats and in vitro free radical scavenging property. Asian Pac. J. Trop. Biomed. 2014, 4, 143–151. [Google Scholar] [PubMed]
- Wang, F.P.; Wang, L.; Yang, J.S.; Nomura, M.; Miyamoto, K. Reversal of P-glycoprotein-dependent resistance to vinblastine by newly synthesized bisbenzylisoquinoline alkaloids in mouse leukemia P388 cells. Biol. Pharm. Bull. 2005, 28, 1979–1982. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.E. Identification and function of transmembrane glycoproteins--the red cell model. Tissue Cell 1984, 16, 665–676. [Google Scholar] [CrossRef]
- He, L.; Yang, J.; Hu, L. Transmembrane transport activity of paclitaxel regulated by fangchinoline in MDR1-mDCK II cells. Zhongguo Zhong Yao Za Zhi 2010, 35, 1478–1481. [Google Scholar] [PubMed]
- Jang, S.H.; Wientjes, M.G.; Au, J.L. Kinetics of P-glycoprotein-mediated efflux of paclitaxel. J. Pharm. Exp. Ther. 2001, 298, 1236–1242. [Google Scholar]
- Sun, Y.F.; Wink, M. Tetrandrine and fangchinoline, bisbenzylisoquinoline alkaloids from Stephania tetrandra can reverse multidrug resistance by inhibiting P-glycoprotein activity in multidrug resistant human cancer cells. Phytomedicine 2014, 21, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.O.; Seong, Y.H. Protective effect of fangchinoline on cyanide-induced neurotoxicity in cultured rat cerebellar granule cells. Arch. Pharm. Res. 2002, 25, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liu, G.; Hu, N.; Yuan, C.; Liu, Z.; Li, F.; Pan, J.; Wang, C. Fangchinoline Inhibits Breast Tumor Proliferation and Induces Apoptosis in MDA-MB-231 Cell Line in Vivo. J. Cancer Sci. Clin. Oncol. 2015, 2, 201. [Google Scholar]
- Li, D.; Lu, Y.; Sun, P.; Feng, L.X.; Liu, M.; Hu, L.H.; Wu, W.Y.; Jiang, B.H.; Yang, M.; Qu, X.B.; et al. Inhibition on Proteasome beta1 Subunit Might Contribute to the Anti-Cancer Effects of Fangchinoline in Human Prostate Cancer Cells. PLoS ONE 2015, 10, e0141681. [Google Scholar]
- Sheng, Z.F.; Cui, X.Y.; Cui, S.Y.; Yu, B.; Zhang, X.Q.; Li, S.J.; Cao, Q.; Huang, Y.L.; Xu, Y.P.; Song, J.Z.; et al. Involvement of adrenoceptors, dopamine receptors and AMPA receptors in antidepressant-like action of 7-O-ethylfangchinoline in mice. Acta Pharmacol. Sin. 2015, 36, 949–956. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| Cancer Types | Concentration Range Tested | Pathways/Molecules Altered | References |
|---|---|---|---|
| A549 lung adenocarcinoma | 10–40 µM | FAK ↓; FAK-paxillin/MMP-2&-9 ↓; FAK-Akt ↓; FAK-MEK-ERK1&2 ↓ | [29] |
| MG63 and U20S bone cancer | 10–30 µM | PI3K ↓; Cyclin D1 ↓; MMP2&9 ↓; Akt ↓; Caspase3 &8 ↑ | [28] |
| MDA-MB-231 breast cancer | 6.25–100 µM | MMP-2&-9 ↓ Expression of NF-κB ↓ Phosphorylated Akt ↓ IκB protein ↑ | [25,56,87] |
| SPC-A-1 lung adenocarcinoma | 2.5–10 µM | G0/G1 phase Arrest ↓ | [36] |
| T24 and 5637 bladder cancer | 2.5–40 µM | Caspase-3 ↓ LC3-II/LC3-I ratio ↑ p62 ↓ mTOR ↓ Intracellular ATP level ↓ | [32] |
| PC3 prostate cancer | 10–30 µM | G1/S phase ↓ Cyclin-D ↓ PCNA ↓ p27 expression ↑ | [30] |
| U87MG and U118MG GBM | 10–30 µM | Akt/p21 ↓ Akt/BAD ↓ Akt/MMP2&9↓ | [34] |
| HepG2 and PLC/PRF/5 hepatocellular carcinoma | 2–10 μM | p53 ↑ Sestrin2 ↑ AMP ↑ | [57] |
| AGS gastric cancer | 0–60 μM | Akt↓ MMP2&9 ↓ TIMP1&2 ↑ | [33] |
| A375 and A875 melanoma | 10–20 μM | FAK ↓ | [40] |
| MiaPaCa-2 and Panc-1 pancreatic cancer | 0–15 μM | NR4A1 ↓ Survivin ↓ Sp-1 ↓ | [55] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mérarchi, M.; Sethi, G.; Fan, L.; Mishra, S.; Arfuso, F.; Ahn, K.S. Molecular Targets Modulated by Fangchinoline in Tumor Cells and Preclinical Models. Molecules 2018, 23, 2538. https://doi.org/10.3390/molecules23102538
Mérarchi M, Sethi G, Fan L, Mishra S, Arfuso F, Ahn KS. Molecular Targets Modulated by Fangchinoline in Tumor Cells and Preclinical Models. Molecules. 2018; 23(10):2538. https://doi.org/10.3390/molecules23102538
Chicago/Turabian StyleMérarchi, Myriam, Gautam Sethi, Lu Fan, Srishti Mishra, Frank Arfuso, and Kwang Seok Ahn. 2018. "Molecular Targets Modulated by Fangchinoline in Tumor Cells and Preclinical Models" Molecules 23, no. 10: 2538. https://doi.org/10.3390/molecules23102538
APA StyleMérarchi, M., Sethi, G., Fan, L., Mishra, S., Arfuso, F., & Ahn, K. S. (2018). Molecular Targets Modulated by Fangchinoline in Tumor Cells and Preclinical Models. Molecules, 23(10), 2538. https://doi.org/10.3390/molecules23102538






