Time-Dependent Degradation of Polyphenols from Thermally-Processed Berries and Their In Vitro Antiproliferative Effects against Melanoma
Abstract
1. Introduction
2. Results
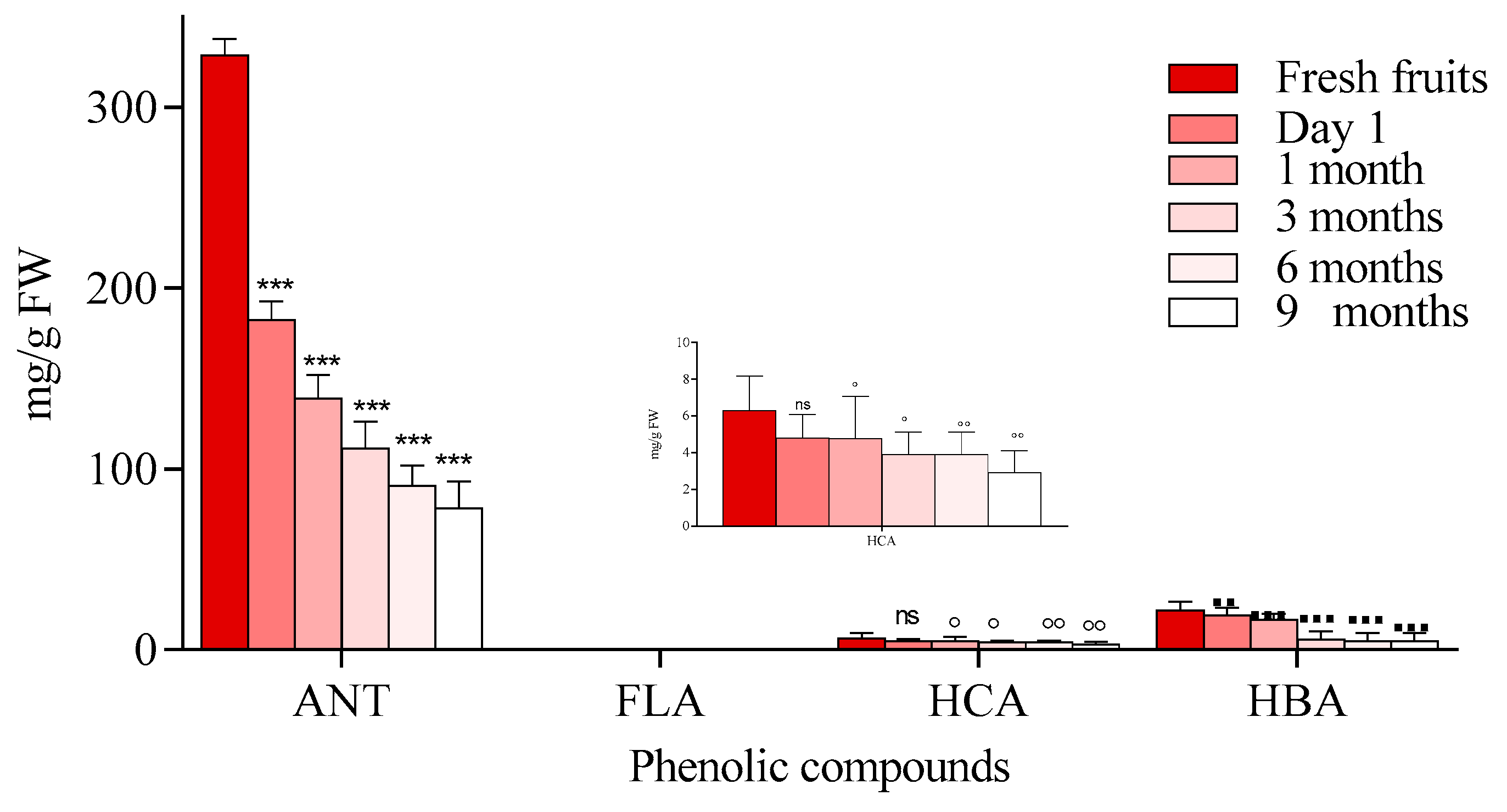
2.1. LC-PDA-ESI/MS Identification and Quantification of Phenolic Compounds
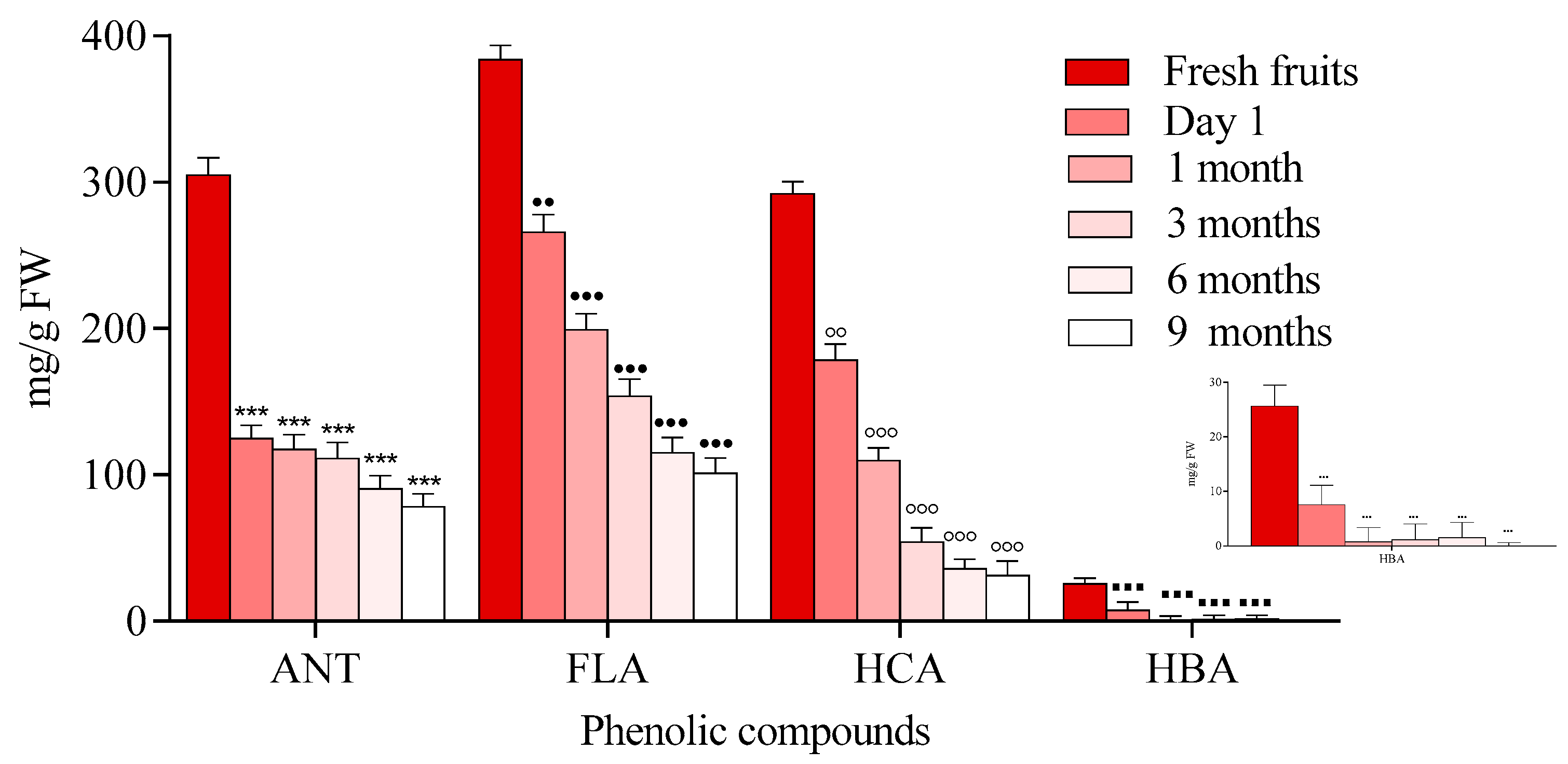
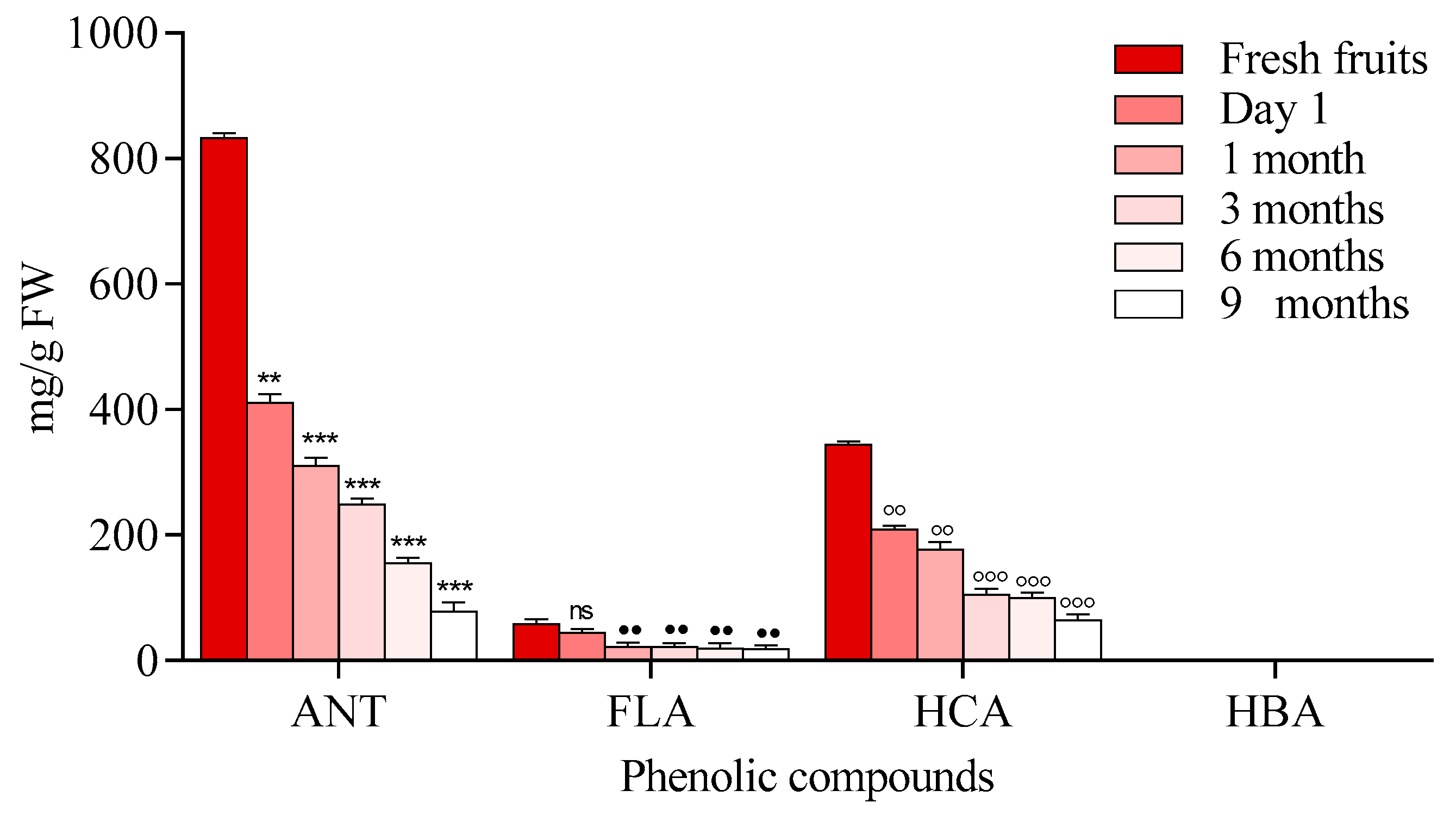
2.1.1. Chokeberry Jam
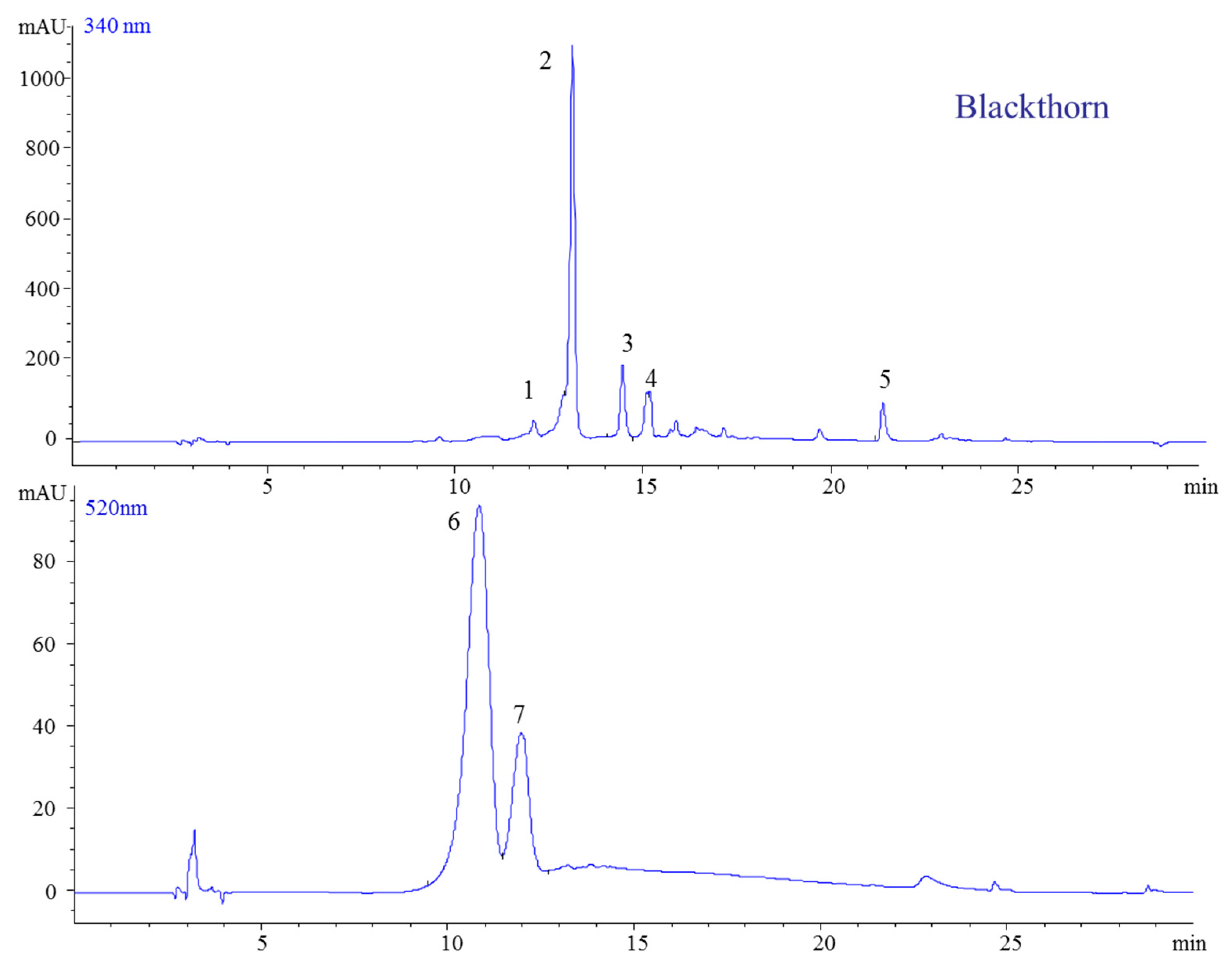
2.1.2. Blackthorn
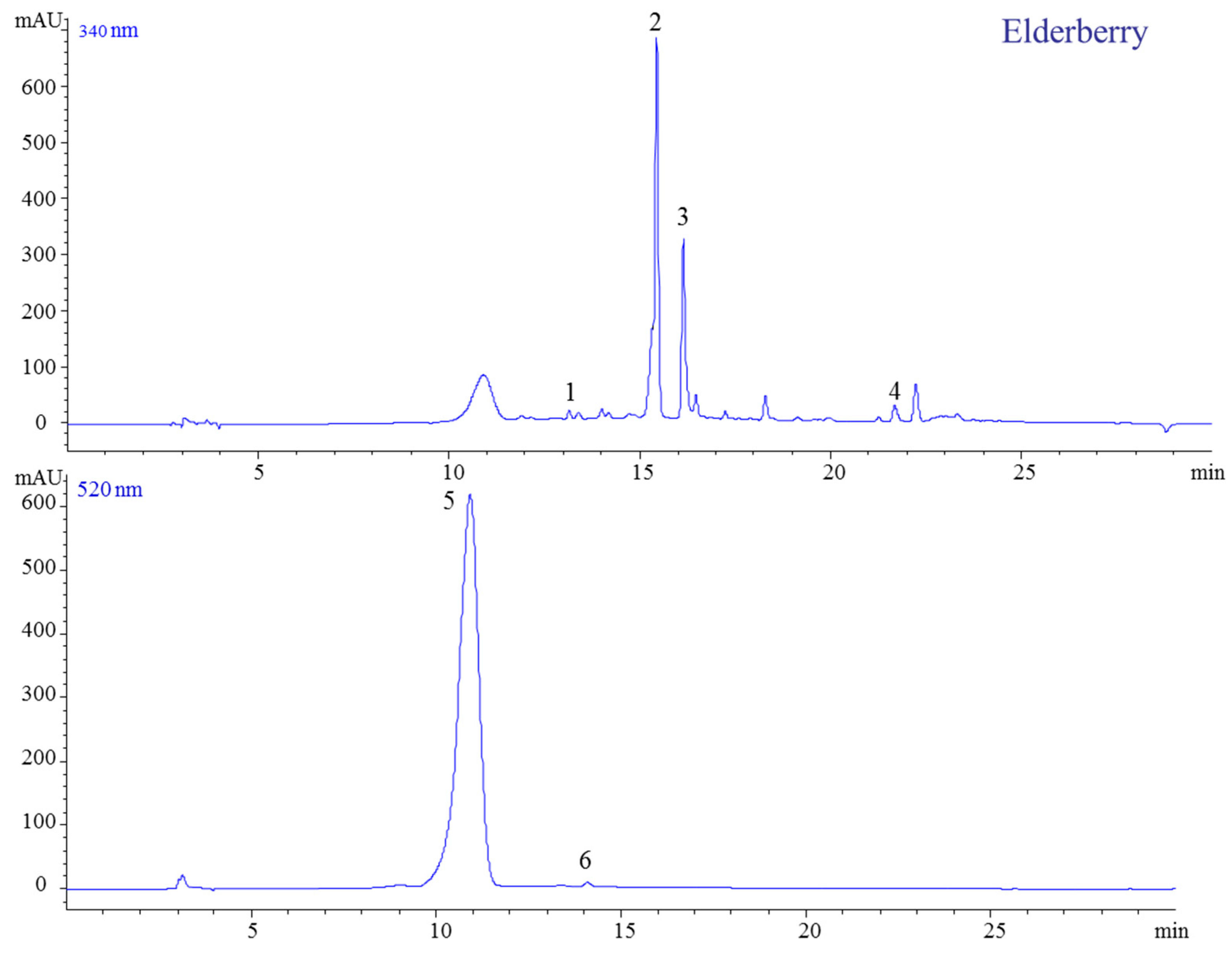
2.1.3. Elderberry
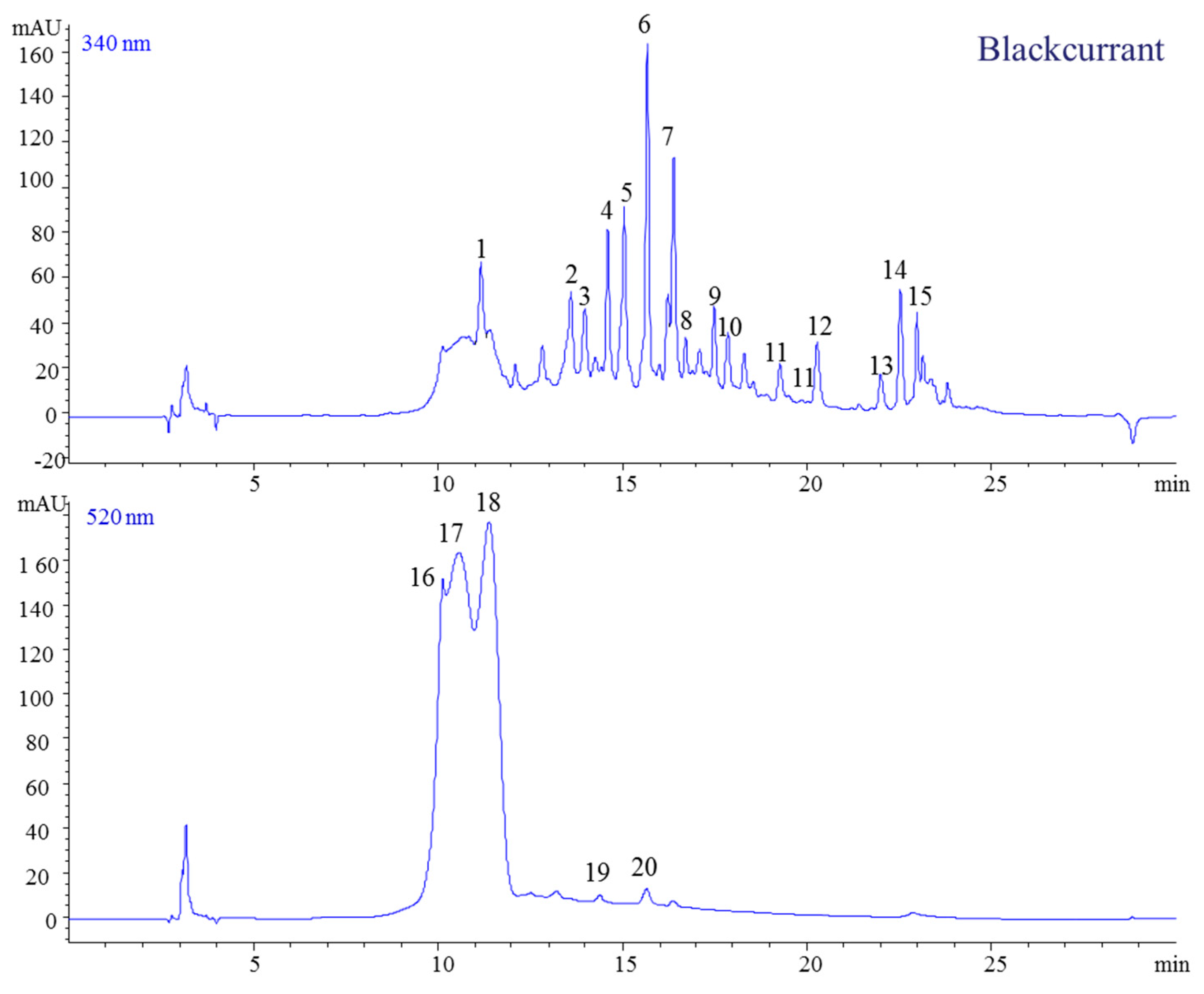
2.1.4. The Blackcurrant
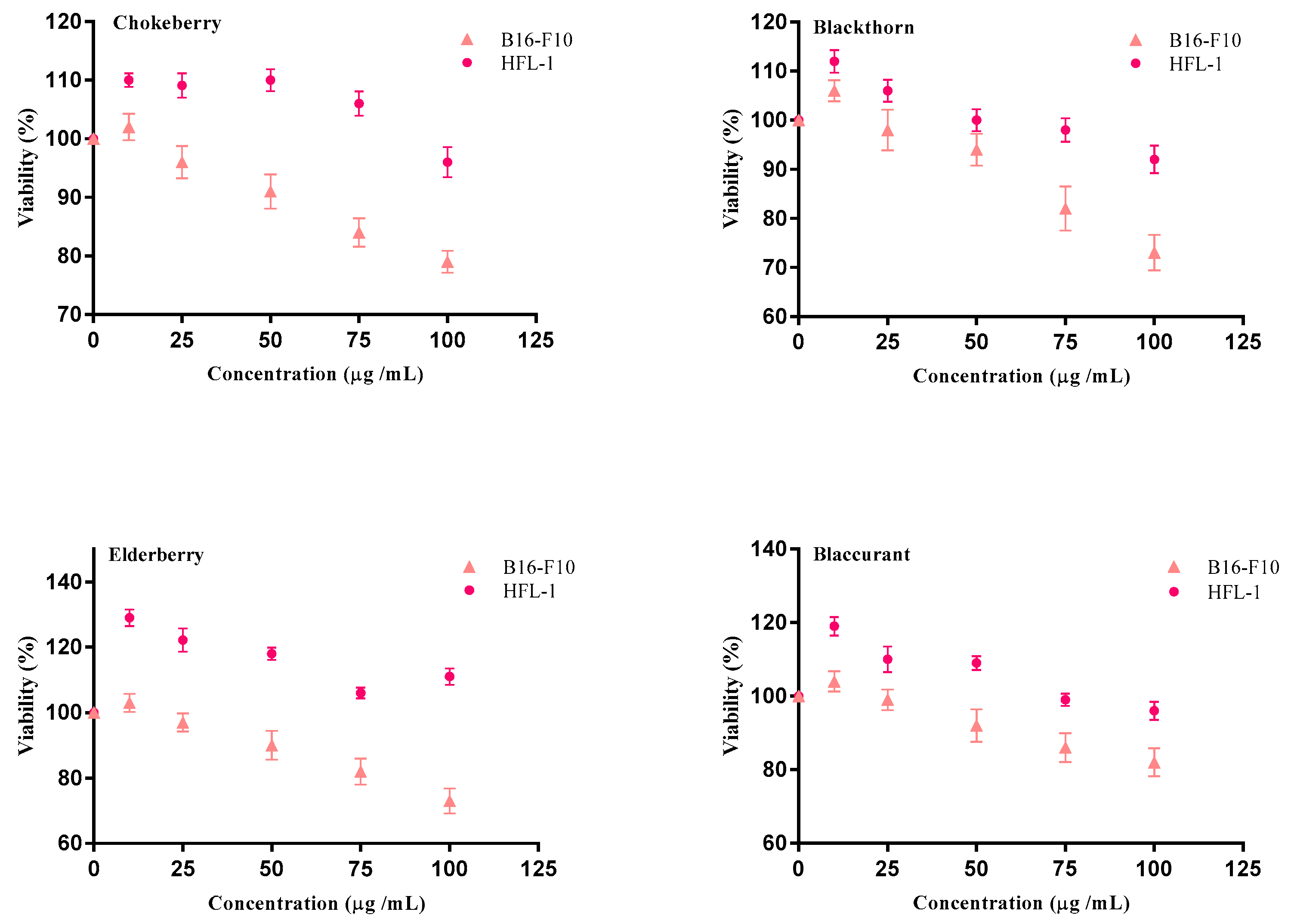
2.2. Cell Proliferation
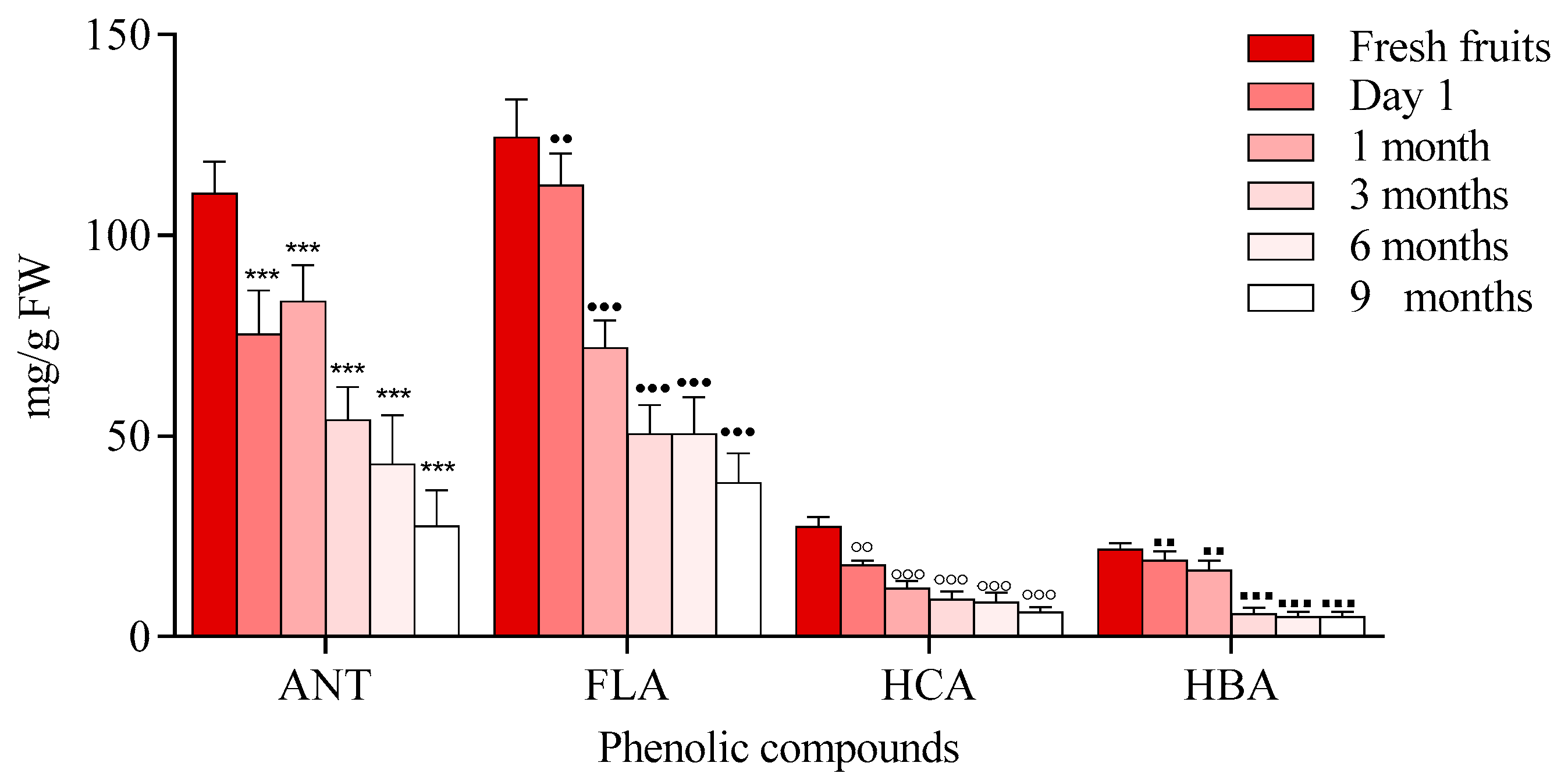
3. Discussion
4. Materials and Methods
4.1. Sampling Procedure
4.2. Extraction of Anthocyanin and Non-Anthocyanin Phenolics from Fresh Berries and Berry Jam
4.3. RP-HPLC-PDA Identification and Quantification of Phenolic Compounds
4.4. HPLC-PDA/-ESI-MS Identification and Quantification of Phenolic Compounds
4.5. Cell Culture
4.6. Analysis of Cell Proliferation
4.7. Statistical Analysis
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide | MTT |
| anthocyanins | ANT |
| Flavonols | FLA |
| Foetal bovin serum | FBS |
| Hydroxybenzoic acids | HBA |
| Hydroxycinnamic acids | HCA |
| Metastatic murine melanoma cell line | B16-F10 |
| Normal fibroblast cell line | HFL-1 |
| Rich polyphenolic extracts | RPE |
References
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.; Andersen, Ø. Radical scavenging and anti-inflammatory activities of representative anthocyanin groupings from pigment-rich fruits and vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of black chokeberry aronia melanocarpa in the prevention of chronic diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Quintin, D.; Garcia-Gomez, P.; Ayuso, M.; Sanmartin, A.M. Active biocompounds to improve food nutritional value. Trends Food Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Berger, R.G. Biotechnology of flavours—The next generation. Biotechnol. Lett. 2009, 31, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2018, 272, 192–200. [Google Scholar] [CrossRef]
- Palmieri, M.G.S.; Cruz, L.T.; Bertges, F.S.; Húngaro, H.M.; Batista, L.R.; da Silva, S.S.; Fonseca, M.J.V.; Rodarte, M.P.; Vilela, F.M.P.; Amaral, M.D.P.H.D. Enhancement of antioxidant properties from green coffee as promising ingredient for food and cosmetic industries. Biocatal. Agric. Biotechnol. 2018, 16, 43–48. [Google Scholar] [CrossRef]
- Nirmala, C.; Bisht, M.S.; Bajwa, H.K.; Santosh, O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018, 77, 91–99. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. As a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Leopold, L.; Rugina, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [PubMed]
- Decareau, R.; Livingston, G.E.; Fellers, C.R. Color changes in strawberry jellies. Food Res. 1956, 10, 125–128. [Google Scholar]
- Markakis, P. (Ed.) Stability of anthocyanins in foods. In Anthocyanins as Food Colors; Academic Press: New York, NY, USA, 1982; pp. 163–181. [Google Scholar]
- Pilando, L.S.; Wrolstad, R.E.; Heatherbell, D.A. Influence of fruit composition, maturity and mold contamination on the color and appearance of strawberry wine. J. Food Sci. 1985, 50, 1121–1125. [Google Scholar] [CrossRef]
- García-Viguera, C.; Zafrilla, P.; Artés, F.; Romero, F.; Abellán, P.; Tomás-Barberán, F.A. Colour and anthocyanin stability of red raspberry jam. J. Sci. Food Agric. 1998, 78, 565–573. [Google Scholar] [CrossRef]
- García-Viguera, C.; Zafrilla, P.; Romero, F.; Abellán, P.; Artés, F.; Tomás-Barberán, F.A. Color stability of strawberry jam as affected by cultivar and storage temperature. J. Food Sci. 1999, 64, 243–247. [Google Scholar] [CrossRef]
- Garzón, G.A.; Wrolstad, R.E. Comparison of the stability of pelargonidin-based anthocyanins in strawberry juice and concentrate. J. Food Sci. 2002, 67, 1288–1299. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Tiwari, B.K.; Butler, F. Stability and degradation kinetics of bioactive compounds and colour in strawberry jam during storage. Food Bioprocess Technol. 2011, 4, 1245–1252. [Google Scholar] [CrossRef]
- Amaro, L.F.; Soares, M.T.; Pinho, C.; Almeida, I.F.; Ferreira, I.M.; Pinho, O. Influence of cultivar and storage conditions in anthocyanin content and radical-scavenging activity of strawberry jams. World Acad. Sci. Eng. Technol. Int. J. Nutr. Food Eng. 2012, 6, 112–116. [Google Scholar]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Bursac Kovacevic, D.; Gajdos Kljusuric, J.; Putnik, P.; Vukusic, T.; Herceg, Z.; Dragovic-Uzelac, V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chem. 2016, 212, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Taheri, R.; Connolly, B.A.; Brand, M.H.; Bolling, B.W. Underutilized chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) accessions are rich sources of anthocyanins, flavonoids, hydroxycinnamic acids, and proanthocyanidins. J. Agric. Food Chem. 2013, 61, 8581–8588. [Google Scholar] [CrossRef] [PubMed]
- Cebulak, T.; Oszmianski, J.; Kapusta, I.; Lachowicz, S. Effect of UV-C radiation, ultra-sonication electromagnetic field and microwaves on changes in polyphenolic compounds in chokeberry (Aronia melanocarpa). Molecules 2017, 22, 1161. [Google Scholar] [CrossRef] [PubMed]
- Ştefănuţ, M.N.; Căta, A.; Pop, R.; Moşoarcă, C.; Zamfir, A.D. Anthocyanins hplc-dad and ms characterization, total phenolics, and antioxidant activity of some berries extracts. Anal. Lett. 2011, 44, 2843–2855. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Rubinskiene, M.; Jasutiene, I.; Venskutonis, P.R.; Viskelis, P. HPLC determination of the composition and stability of blackcurrant anthocyanins. J. Chromatogr. Sci. 2005, 43, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.H.; Hellström, J.; Karhu, S.; Pihlava, J.-M.; Veteläinen, M. High variability in flavonoid contents and composition between different north-european currant (Ribes spp.) varieties. Food Chem. 2016, 204, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Van Camp, J.; Capanoglu, E. Colour retention, anthocyanin stability and antioxidant capacity in black carrot (Daucus carota) jams and marmalades: Effect of processing, storage conditions and in vitro gastrointestinal digestion. J. Funct. Foods 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Howard, L.R.; Castrodale, C.; Brownmiller, C.; Mauromoustakos, A. Jam processing and storage effects on blueberry polyphenolics and antioxidant capacity. J. Agric. Food Chem. 2010, 58, 4022–4029. [Google Scholar] [CrossRef] [PubMed]
- Rababah, T.M.; Al-Mahasneh, M.A.; Kilani, I.; Yang, W.; Alhamad, M.N.; Ereifej, K.; Al-U’datt, M. Effect of jam processing and storage on total phenolics, antioxidant activity, and anthocyanins of different fruits. J. Sci. Food Agric. 2011, 91, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lee, H.; Lee, S.-H.; Lee, C.Y.; Kim, D.-O. Effects of jam processing on anthocyanins and antioxidant capacities of rubus coreanus miquel berry. Food Sci. Biotechnol. 2013, 22, 1607–1612. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bober, I. The effect of addition of chokeberry, flowering quince fruits and rhubarb juice to strawberry jams on their polyphenol content, antioxidant activity and colour. Eur. Food Res. Technol. 2008, 227, 1043–1051. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. RCM 2006, 20, 3229–3235. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.H.; Hellstrom, J.; McDougall, G.; Dobson, G.; Pihlava, J.M.; Tiirikka, T.; Stewart, D.; Karjalainen, R. Polyphenol and vitamin C contents in European commercial blackcurrant juice products. Food Chem. 2011, 127, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Ioku, K.; Aoyama, Y.; Tokuno, A.; Terao, J.; Nakatani, N.; Takei, Y. Various cooking methods and the flavonoid content in onion. J. Nutr. Sci. Vitaminol. 2001, 47, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Liu, Y.; Xu, C.; Liu, J. Antiproliferative and proapoptotic activities of anthocyanin and anthocyanidin extracts from blueberry fruits on B16-F10 melanoma cells. Food Nutr. Res. 2017, 61, 1325308. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Martini, D.; Mena, P.; Tassotti, M.; Calani, L.; Brigati, G.; Brighenti, F.; Holasek, S.; Malliga, D.-E.; Lamprecht, M.; et al. Absorption profile of (poly)phenolic compounds after consumption of three food supplements containing 36 different fruits, vegetables, and berries. Nutrients 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and storage effects on the ellagitannin composition of processed blackberry products. J. Agric. Food Chem. 2010, 58, 11749–11754. [Google Scholar] [CrossRef] [PubMed]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blackberry products. J. Agric. Food Chem. 2008, 56, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Gancel, A.-L.; Feneuil, A.; Acosta, O.; Pérez, A.M.; Vaillant, F. Impact of industrial processing and storage on major polyphenols and the antioxidant capacity of tropical highland blackberry (Rubus adenotrichus). Food Res. Int. 2011, 44, 2243–2251. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Ayvaz, H.; Rugina, D.; Leopold, L.; Stanila, A.; Socaciu, C.; Tabaran, F.; Luput, L.; Mada, D.C.; Pintea, A.; et al. Melanoma inhibition by anthocyanins is associated with the reduction of oxidative stress biomarkers and changes in mitochondrial membrane potential. Plant Foods Hum. Nutr. 2017, 72, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular mechanism and health role of functional ingredients in blueberry for chronic disease in human beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
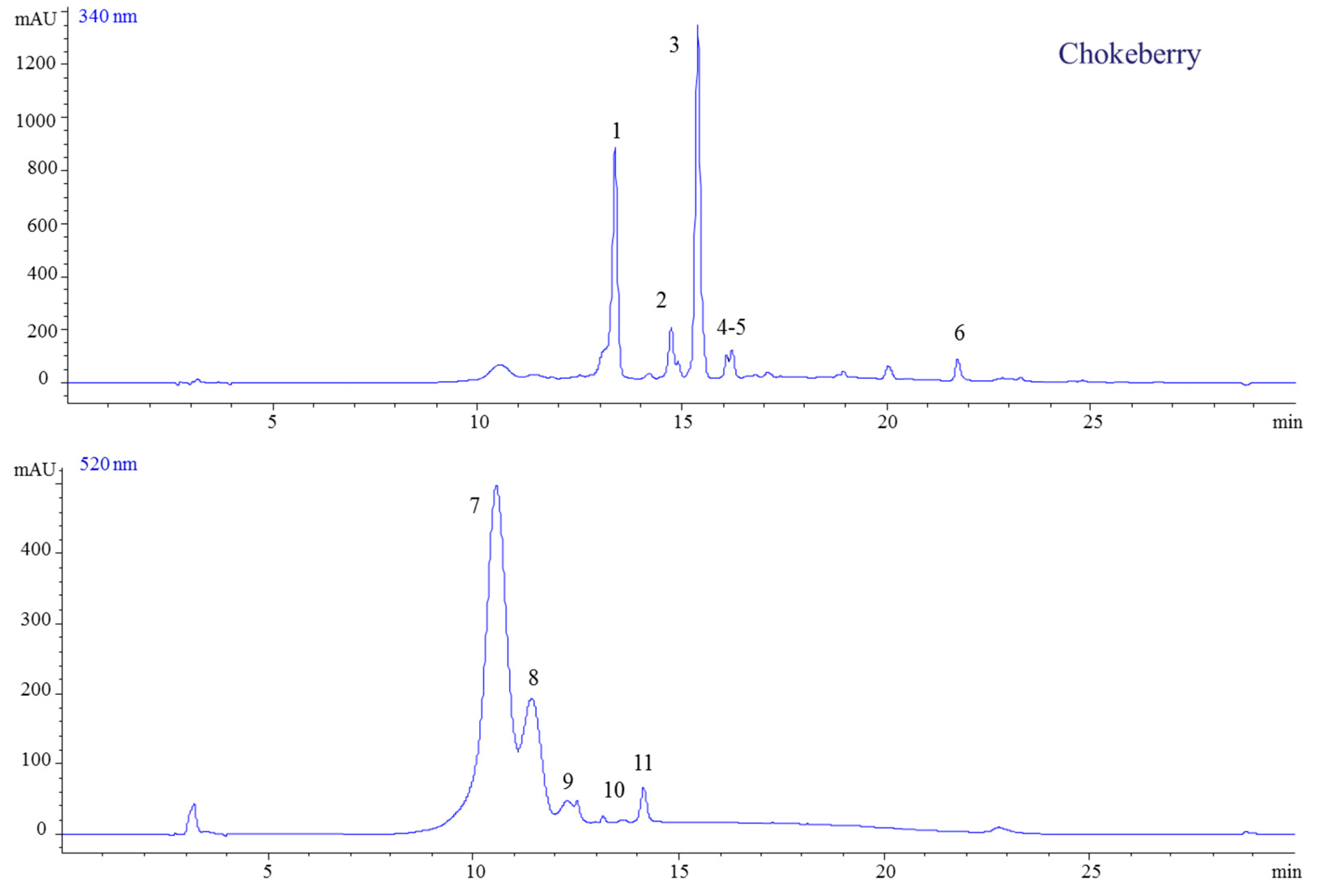
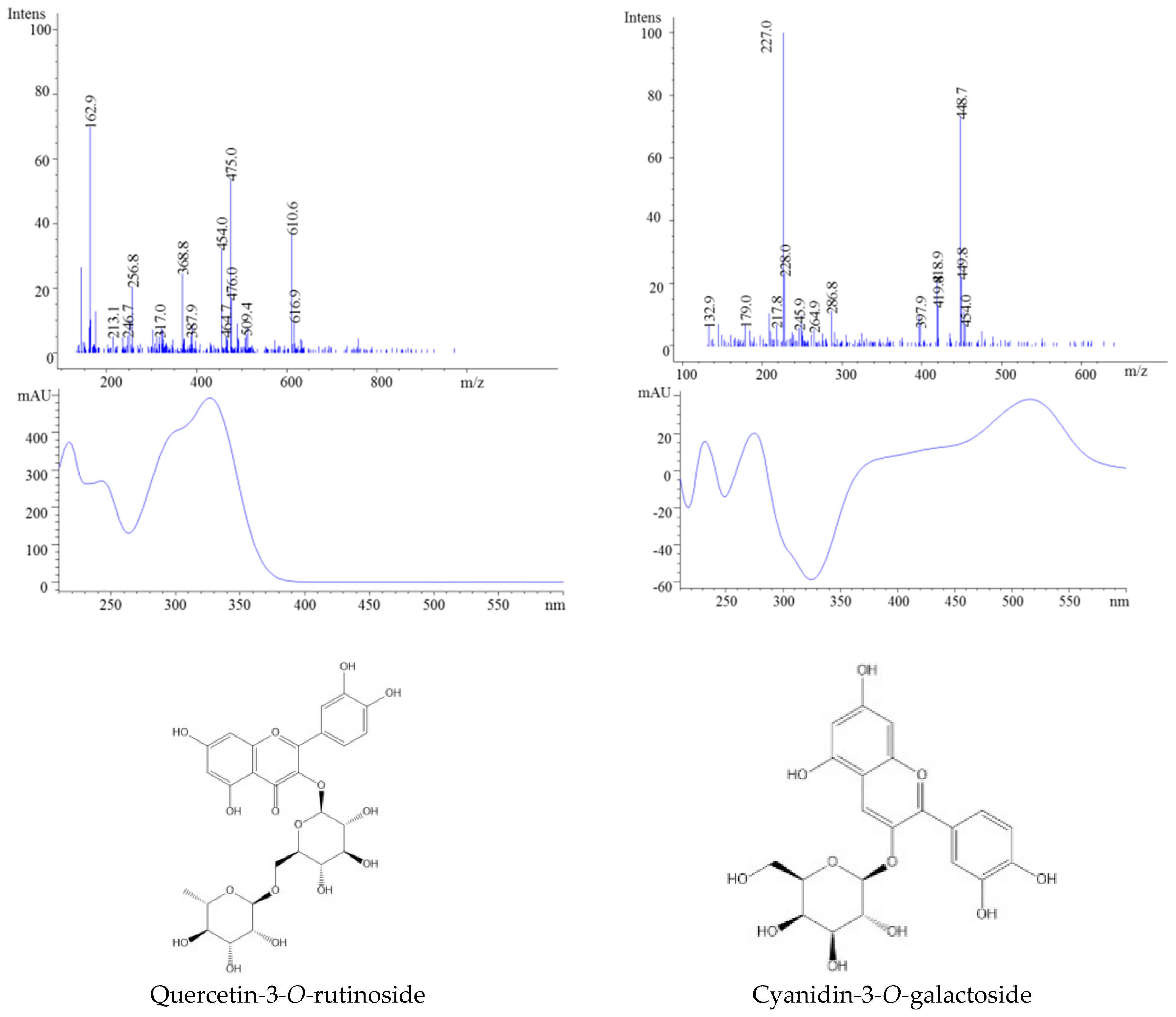
| Peak No. | Rt (min) | Parent Ion | Fragment Ion | MW | UV Spectra | Compound | Ref |
|---|---|---|---|---|---|---|---|
| Chokeberry | |||||||
| 1 | 13.42 | 181 | 163 | 180 | 323 | Caffeic acid | [22,23,25] |
| 2 | 14.79 | 465 | 303 | 464 | 355 | Quercetin-3-O-galactoside | |
| 3 | 15.42 | 611 | 303 | 610 | 354 | Quercetin-3-O-rutinoside (Rutin) | |
| 4 | 16.11 | 465 | 303 | 464 | 355 | Quercetin-3-O-glucoside | |
| 5 | 16.29 | - | 303 | 302.1 | 364 | Ellagic acid | |
| 6 | 21.90 | - | 303 | 302 | 369 | Quercetin | |
| 7 | 10.80 | 449 | 287 | 449 | 528 | Cyanidin-3-O-galactoside | |
| 8 | 11.57 | 419 | 287 | 419 | 517 | Cyanidin-3-O-arabinoside | |
| 9 | 12.58 | 419 | 287 | 454 | 519 | Cyanidin-3-O-xyloside | |
| 10 | 13.20 | 449 | 287 | 449 | 518 | Cyanidin-3-O-glucoside | |
| 11 | 14.31 | - | 287 | 287 | 528 | Cyanidin | |
| Blackthorn | |||||||
| 1 | 13.14 | 181 | 163 | 180 | 319 | Caffeic acid | [26] |
| 2 | 14.47 | 355 | 181, 163 | 354 | 325 | Neochlorogenic acid | |
| 3 | 15.19 | 611 | 303 | 610 | 354 | Quercetin-3-O-rutinoside (rutin) | |
| 4 | 21.38 | 303 | 302 | 369 | Quercetin | ||
| 5 | 449 | 287 | 449 | 528 | Cyanidin-3-O-galactoside | ||
| 6 | 10.84 | 595 | 449, 287 | 595 | 516 | Cyanidin-3-O-rutinoside | |
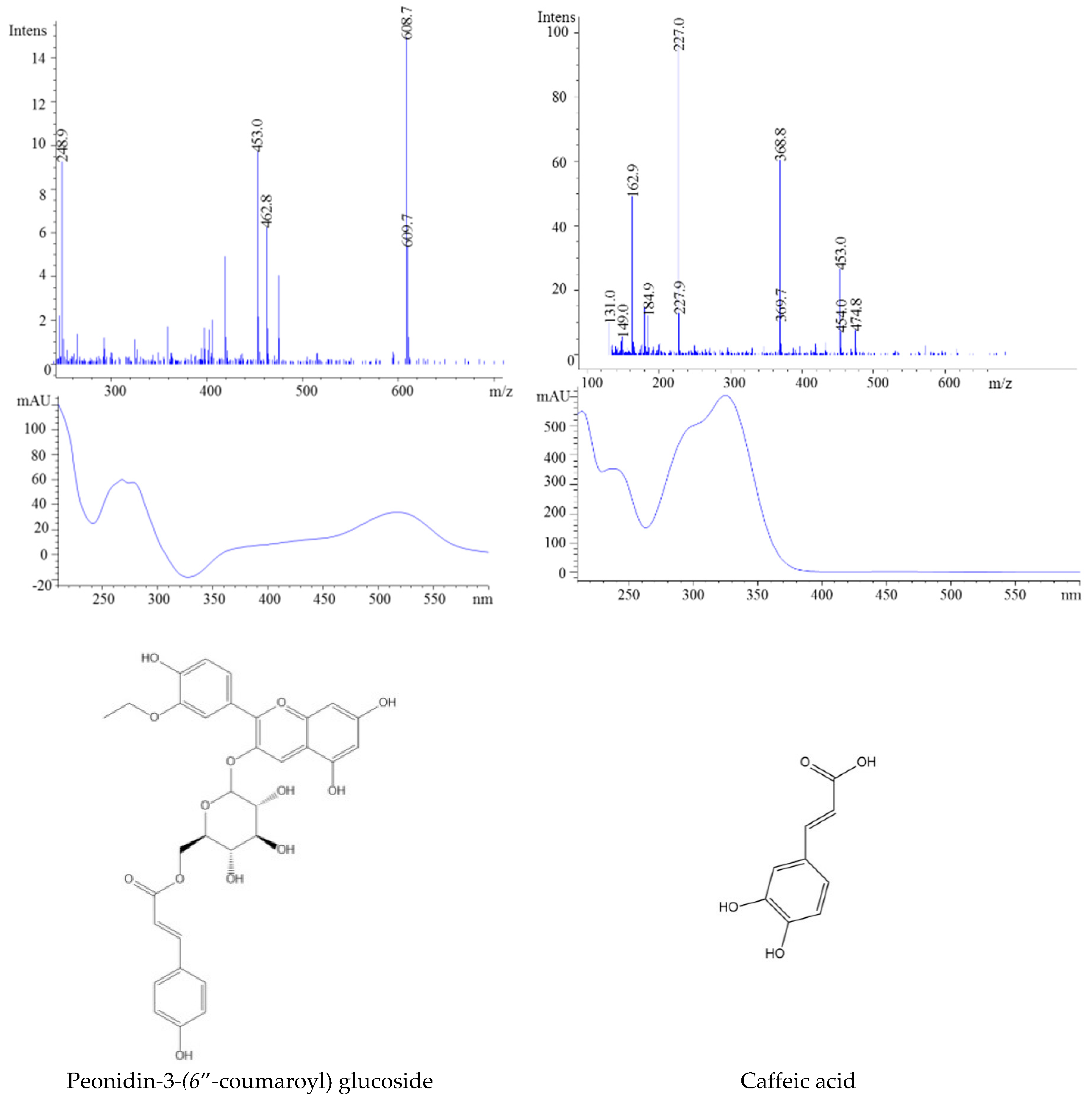
| 7 | 11.96 | 609 | 463, 301 | 611 | 524 | Peonidin-3-(6″-coumaroyl) glucoside | |
| Elderberry | |||||||
| 1 | 13.39 | 355 | 181, 163 | 354 | 352 | Chlorogenic acid | [27] |
| 2 | 15.44 | 611 | 303 | 610 | 354 | Quercetin-3-O-rutinoside | |
| 3 | 16.14 | 465 | 303 | 464 | 355 | Quercetin-3-O-glucoside | |
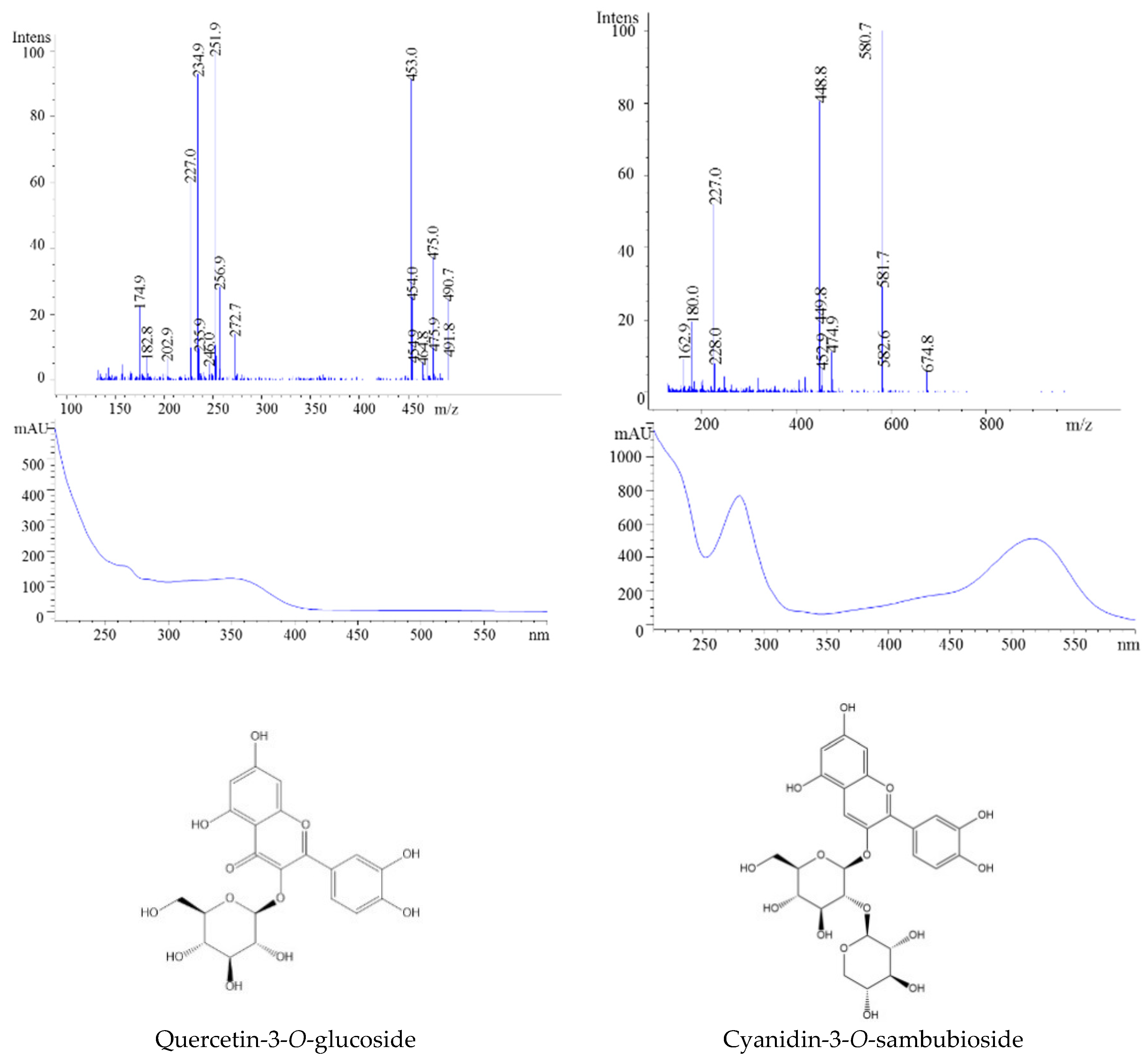
| 4 | 21.38 | - | 303 | 302 | 369 | Quercetin | |
| 5 | 10.94 | 581 | 449, 287 | 616 | 518 | Cyanidin-3-O-sambubioside | |
| 6 | 14.29 | 449, | 287 | 449 | 518 | Cyanidin-3-O-glucoside | |
| Blackcurrant | |||||||
| 1 | 11.16 | 301 | 139 | 300 | 252 | 4-Hydroxybenzoic acid-4-O-glucoside | [12,28,29] |
| 2 | 13.59 | 343 | 181, 163 | 343 | 321 | Caffeic acid-4-O-glucoside | |
| 3 | 13.97 | 595 | 287 | 594 | 346 | Kaempferol-3-O-rutinoside | |
| 4 | 14.59 | 627 | 319 | 626 | 355 | Myricetin-3-O-rutinoside | |
| 5 | 15.04 | 465 | 319 | 646 | 372 | Myricetin-3-O-rhamnoside | |
| 6 | 15.66 | 611 | 303 | 610 | 354 | Quercetin-3-O-rutinoside | |
| 7 | 16.37 | 465 | 303 | 464 | 355 | Quercetin-3-O-glucoside | |
| 8 | 16.70 | 357 | 195 | 194 | 316 | Ferulic acid-4-O-glucoside | |
| 9 | 17.47 | 449 | 287 | 448 | 346 | Kaempferol-3-O-galactoside | |
| 10 | 17.84 | 567 | 319 | 566 | 355 | Myricetin-3-O-(6″-malonyl-glucoside) | |
| 11 | 19.25 | 319 | 300 | 255 | Hydroxybenzoic acid-4 | ||
| 12 | 20.25 | 551 | 303 | 550 | 358 | Quercetin-3-O-(6″-malonyl-glucoside) | |
| 13 | 21.99 | 303 | 302 | 369 | Quercetin | ||
| 14 | 22.53 | 449 | 287 | 448 | 346 | Kaempferol-3-O-glucoside | |
| 15 | 22.98 | 287 | 286 | 365 | Kaempferol | ||
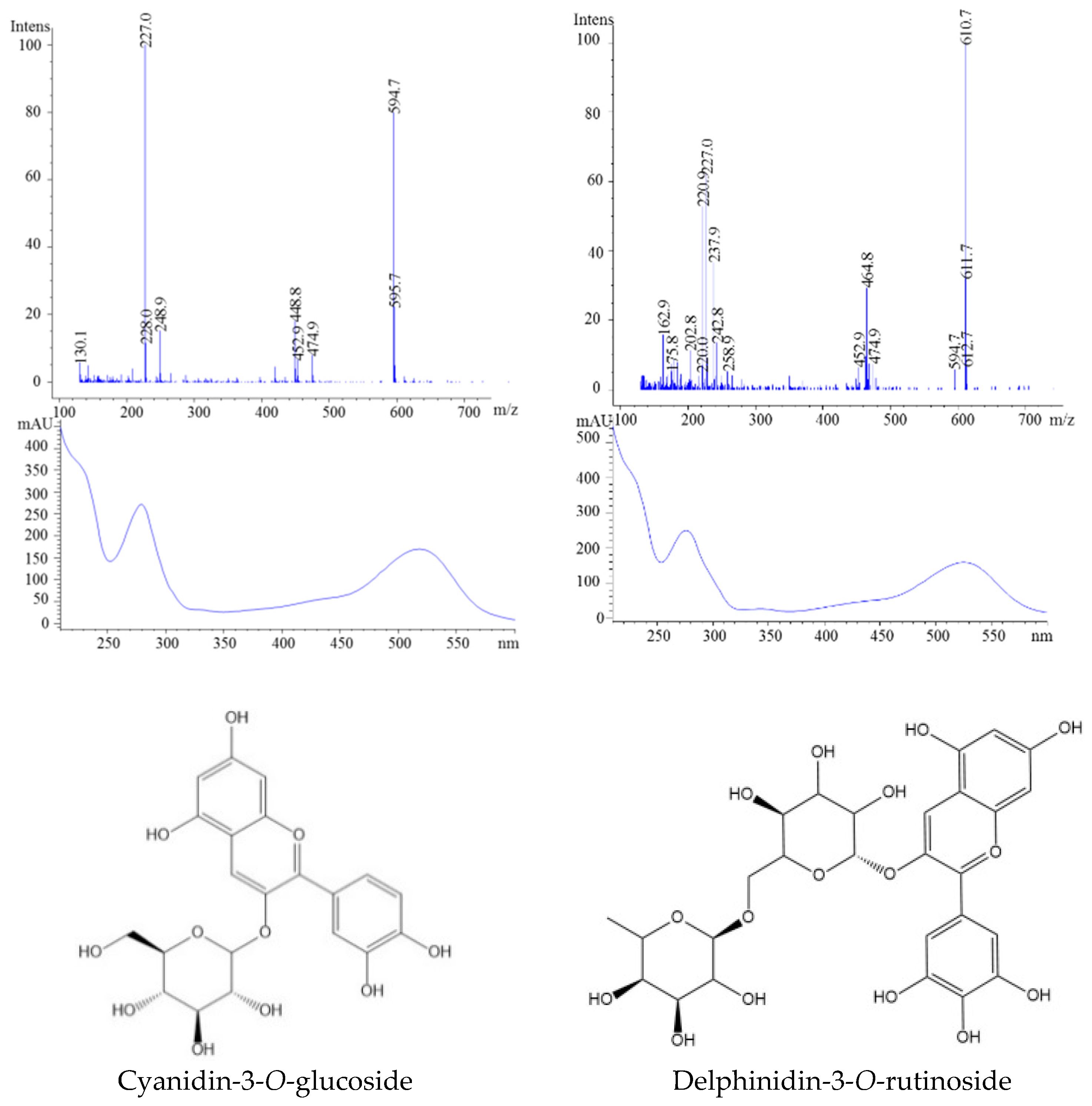
| 16 | 10.12 | 465 | 303 | 465 | 524 | Delphinidin-3-O-glucoside | |
| 17 | 10.57 | 611 | 528 | Delphinidin-3-O-rutinoside | |||
| 18 | 11.38 | 595 | 287 | 449 | 518 | Cyanidin-3-O-glucoside | |
| 19 | 14.38 | 449 | 287 | 287 | 514 | Cyanidin | |
| 20 | 15.64 | 303 | 303 | 520 | Delphinidin | ||
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaconeasa, Z. Time-Dependent Degradation of Polyphenols from Thermally-Processed Berries and Their In Vitro Antiproliferative Effects against Melanoma. Molecules 2018, 23, 2534. https://doi.org/10.3390/molecules23102534
Diaconeasa Z. Time-Dependent Degradation of Polyphenols from Thermally-Processed Berries and Their In Vitro Antiproliferative Effects against Melanoma. Molecules. 2018; 23(10):2534. https://doi.org/10.3390/molecules23102534
Chicago/Turabian StyleDiaconeasa, Zorița. 2018. "Time-Dependent Degradation of Polyphenols from Thermally-Processed Berries and Their In Vitro Antiproliferative Effects against Melanoma" Molecules 23, no. 10: 2534. https://doi.org/10.3390/molecules23102534
APA StyleDiaconeasa, Z. (2018). Time-Dependent Degradation of Polyphenols from Thermally-Processed Berries and Their In Vitro Antiproliferative Effects against Melanoma. Molecules, 23(10), 2534. https://doi.org/10.3390/molecules23102534