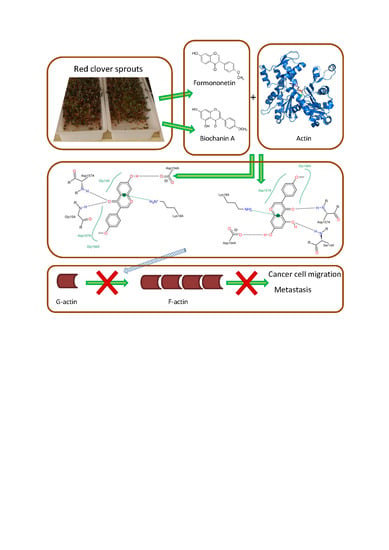
Binding of Red Clover Isoflavones to Actin as A Potential Mechanism of Anti-Metastatic Activity Restricting the Migration of Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Concentration of Isoflavones in Red Clover Sprouts
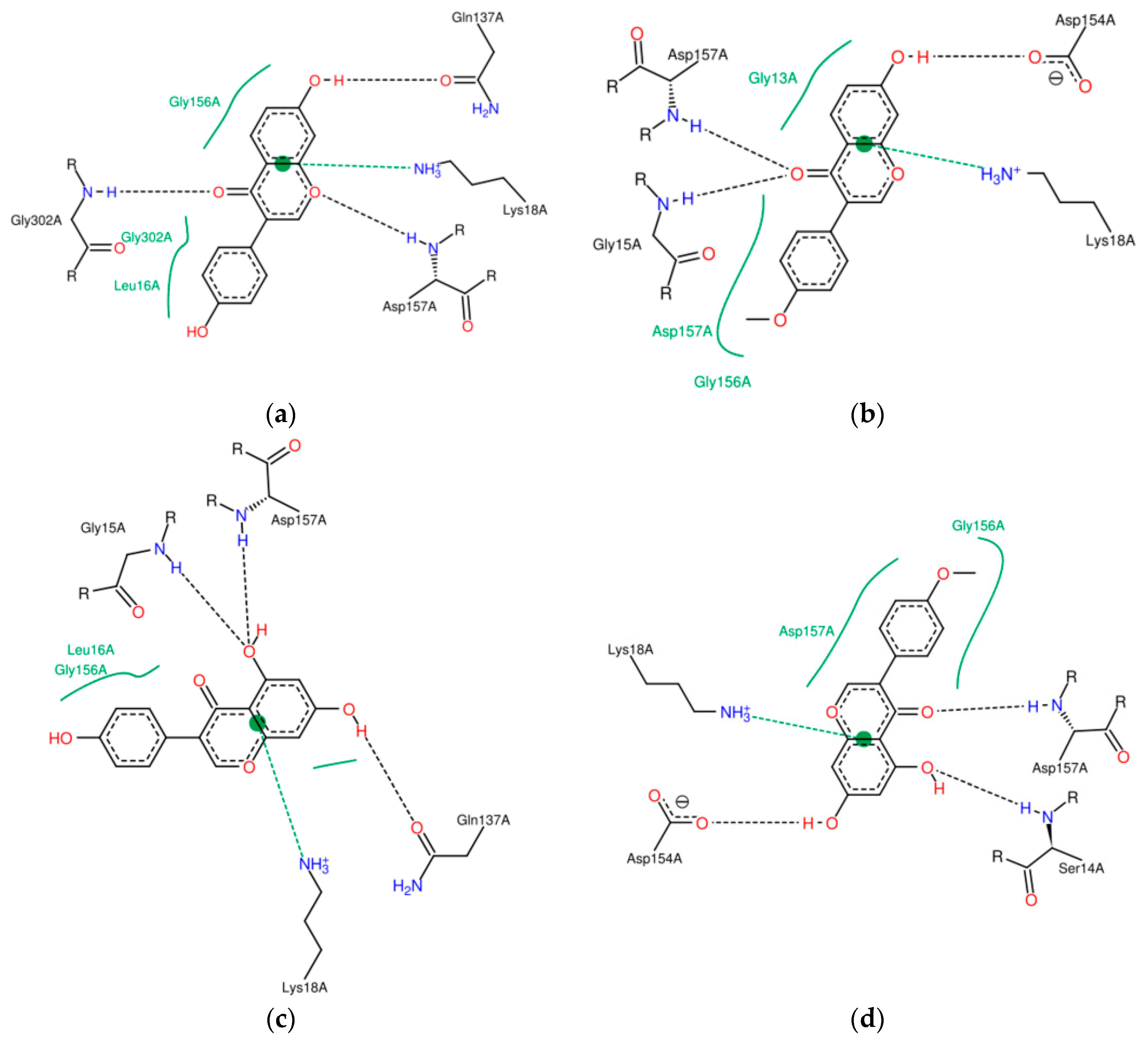
2.2. Characterisation of Complexes of Isoflavones with Actin
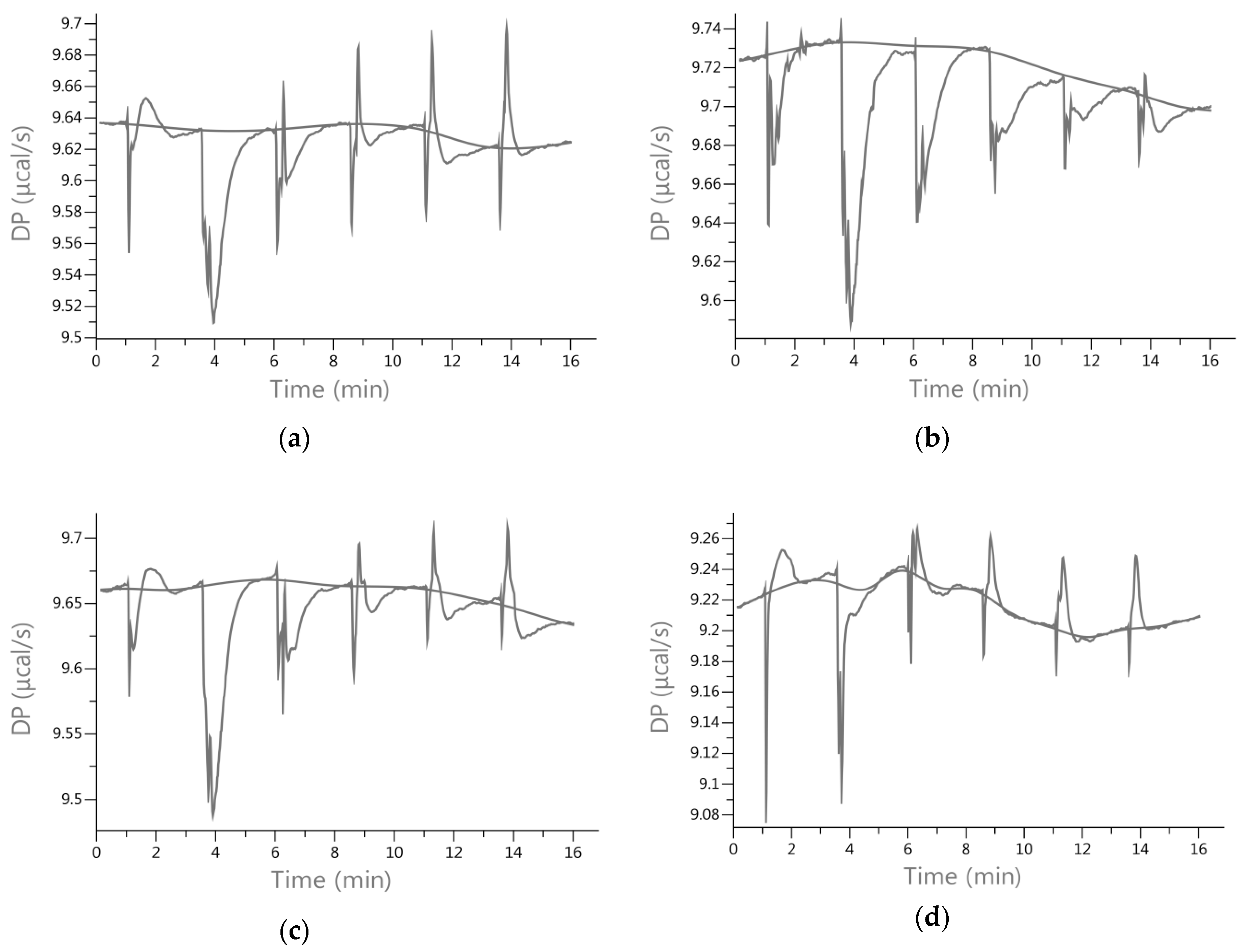
2.3. Energetic Effects of Actin-Isoflavones Interactions
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Isothermal Titration Calorimetry
3.3. Cultivation of Sprouts
3.4. Extraction and LC-ESI-MS Analysis of Isoflavones
3.5. Molecular Modelling
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belmont, L.D.; Orlova, A.; Drubin, D.G.; Egelman, E.H. A change of actin conformation associated with filament instability after Pi release. Proc. Natl. Acad. Sci. USA 1999, 96, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Rouabhia, D.; Park, H.J.; Giasson, L.; Zhang, Z. Effect of soft foods on primary human gingival epithelial cell growth and the wound healing process. Food Res. Int. 2017, 100, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-H.; Ho, C.-T.; Chen, Z.-F.; Chen, L.-C.; Whang-Peng, J.; Lin, T.-N.; Ho, Y.-S. The apple polyphenol phloretin inhibits breast cancer cell migration and proliferation via inhibition of signals by type 2 glucose transporter. J. Food Drug Anal. 2018, 26, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, A.; Jung, W.K.; Jeon, T.J. Effect of fucoidan on cell morphology and migration in osteoblast. Food Sci. Biotechnol. 2015, 24, 699–704. [Google Scholar] [CrossRef]
- Reutzel, R.; Yoshioka, C.; Govindasamy, L.; Yarmola, E.G.; Agbandje-McKenna, M.; Bubb, M.R.; McKenna, R. Actin crystal dynamics: Structural implications for F-actin nucleation, polymerization, and branching mediated by the anti-parallel dimer. J. Struct. Biol. 2004, 146, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Dedova, I.V.; Dedov, V.N.; Nosworthy, N.J.; Hambly, B.D.; Remedios, C.G. Cofilin and Dnase I affect the conformation of the small domain of actin. Biophys. J. 2002, 82, 3134–3143. [Google Scholar] [CrossRef]
- Kienchin, V.A.; King, R.; Tanaka, J.; Marriott, G.; Rayment, I. Structural basis of swinholide A binding to actin. Chem. Biol. 2005, 12, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Brayford, S.; Schevzov, G.; Vos, J.; Gunning, P. The role of actin cytoskeleton in cancer and its potential use as a therapeutic target. In The Cytoskeleton in Health and Disease; Schatten, H., Ed.; Springer Science+Business Media: New York, NY, USA, 2015; pp. 373–391. ISBN 978-1-4939-2903-0. [Google Scholar]
- Pawlik, A.; Szczepański, M.A.; Klimaszewska, A.; Gackowska, L.; Zuryn, A.; Grzanka, A. Phenethyl isothiocyanate-induced cytoskeleton changes and cell death in lung cancer cell. Food Chem. Toxicol. 2012, 50, 3577–3594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.Z.; Cao, F.K.; Du, S.W. The apoptotic pathways in the curcumin analog MHMD-induced lung cancer cell death and the essential role of actin polymerization during apoptosis. Biomed. Pharmacother. 2015, 71, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Böhl, M.; Tietze, S.; Sokoll, A.; Madathil, S.; Pfennig, F.; Apostolakis, J.; Fahmy, K.; Gutzeit, H.O. Flavonoids affect actin functions in cytoplasm and nucleus. Biophys. J. 2007, 93, 2767–2780. [Google Scholar] [CrossRef] [PubMed]
- Miralles, F.; Visa, N. Actin in transcription and transcription regulation. Curr. Opin. Cell Biol. 2006, 18, 261–299. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Z.; Wang, L.; Deng, L.; Sun, J.; Jiang, X.; Zhang, Y.; Tian, L.; Wag, Y.; Bai, W. Scandenolone, a natural isoflavone derivative from Cudrania tricuspidata fruit, targets EGFR to induce apoptosis and block autophagy flux in human melanoma cells. J. Funct. Foods 2017, 37, 229–240. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Wang, D.; Fang, F.; Lai, J.; Wu, T.; Tsao, R. Isoflavone, γ-aminobutyric acid contents and antioxidant activities are significantly increased during germination of three Chinese soybean cultivars. J. Funct. Foods 2015, 14, 596–604. [Google Scholar] [CrossRef]
- Budryn, G.; Gałązka-Czarnecka, I.; Brzozowska, E.; Grzelczyk, J.; Mostowski, R.; Żyżelewicz, Ż.; Cerón-Carrasco, J.P.; Pérez-Sánchez, H. Evaluation of estrogenic activity of red clover (Trifolium pratense L.) sprouts cultivated under different conditions by content of isoflavones, calorimetric study and molecular modeling. Food Chem. 2018, 245, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Pałecz, B.; Rachwal-Rosiak, D.; Oracz, J.; Zaczyńska, D.; Sylwia, B.; Inmaculada, N.-G.; Josefina María, V.M.; Horacio, P.-S. Effect of inclusion of hydroxycinnamic and chlorogenic acids from green coffee bean in beta-cyclodextrin on their interactions with whey, egg white and soy protein isolates. Food Chem. 2015, 168, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.M.; Wähälä, K.; Adlercreutz, H. Identification of urinary metabolites of the red clover isoflavones formononetin and biochanin A in human subjects. J. Agric. Food Chem. 2004, 52, 6802–6809. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Kai, G. A review of dietary polyphenol-plasma protein interactions: Characterization, influence on the bioactivity, and structure-affinity relationship. Crit. Rev. Food Sci. Nutr. 2012, 52, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Böhl, M.; Czupalla, C.; Tokalov, S.V.; Hoflack, B.; Gutzeit, H.O. Identification of actin as quercetin-binding protein: An approach to identify target molecules for specific ligands. Anal. Biochem. 2015, 346, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Moraczewska, J.; Wawro, B.; Sugero, K.; Strzelecka-Gołaszewska, H. Divalent cation-, nucleotide-, and polymerization-dependent changes in the conformation of subdomain 2 of actin. Biophys. J. 1999, 77, 373–385. [Google Scholar] [CrossRef]
- Frazier, R.A.; Papadopoulou, A.; Green, R.J. Isothermal titration calorimetry study of epicatechin binding to serum albumin. J. Pharm. Biochem. Anal. 2006, 28, 1602–1605. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Y.; Xia, Y.L.; Ai, S.M.; Liang, J.; Sang, P.; Ji, X.L.; Liu, S.Q. Insights into protein–ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Zaczyńska, D.; Pałecz, B.; Rachwał-Rosiak, D.; Belica, S.; den-Haan, H.; Pena-García, J.; Perez-Sanchez, H. Interactions of free and encapsulated hydroxycinnamic acids from green coffee with egg ovalbumin, whey and soy protein hydrolysates. LWT—Food Sci. Technol. 2016, 65, 823–831. [Google Scholar] [CrossRef]
- Delgado-Zamarreño, M.M.; Pérez-Martín, L.; Bustamante-Rangel, M.; Carabias-Martínez, R. Pressurized liquid extraction as a sample preparation method for the analysis of isoflavones in pulses. Anal. Bioanal. Chem. 2012, 404, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yao, Y.; Zhu, Y.; Ren, G. Isoflavone content and composition in chickpea (Cicer arietinum L.) sprouts germinated under different conditions. J. Agric. Food Chem. 2015, 63, 2701–2707. [Google Scholar] [CrossRef] [PubMed]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aid. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity—A rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the red clover sprouts are available from the authors. |
| Red Clover Sprouts * | Daidzein | Formononetin | Genistein | Biochanin A | Total |
|---|---|---|---|---|---|
| White light 10 | 40.14 ± 2.38 | 1449.87 ± 50.92 | 118.49 ± 4.88 | 212.08 ± 11.92 | 1820.58 ± 55.92 |
| UVA3 | 3.72 ± 0.17 | 721.37 ± 32.36 | 84.47 ± 6.39 | 192.84 ± 6.18 | 1002.40 ± 38.48 |
| UVA8 | 11.85 ± 0.36 | 794.36 ± 35.59 | 70.81 ± 3.18 | 152.10 ± 5.74 | 1348.32 ± 49.09 |
| UVB11 | 115.35 ± 7.39 | 496.15 ± 19.61 | 188.96 ± 6.96 | 353.15 ± 15.95 | 1153.61 ± 37.71 |
| Isoflavone/Red Clover Sprouts * | KA × 103 (L/mol) | ∆H (kJ/mol) | ∆S (J/mol·K) | ∆G (kJ/mol) | ∆Gpredicted (kJ/mol) |
|---|---|---|---|---|---|
| single isoflavones | |||||
| Daidzein | 1.34 ± 0.09 | −0.04 ± 0.01 | 57.85 ± 3.12 | −17.29 ± 0.71 | −35.98 |
| Formononetin | 2.41 ± 0.11 | 0.11 ± 0.02 | 62.99 ± 4.05 | −18.67 ± 0.90 b | −38.07 |
| Genistein | 0.82 ± 0.06 | 23.03 ± 1.38 a | 131.11 ± 10.92 | −16.08 ± 0.55 | −37.66 |
| Biochanin A | 2.87 ± 0.15 | 23.49 ± 1.08 a | 142.77 ± 9.15 | −19.09 ± 0.87 b | −38.49 |
| pairs of isoflavones | |||||
| Daidzein + Formononetin | - | −8.21 ± 0.60 | 36.03 ± 2.55 | −18.59 ± 0.92 a | - |
| Daidzein + Genistein | - | 0.28 ± 0.04 | 66.50 ± 5.96 | −18.88 ± 0.74 a | - |
| Daidzein + Biochanin A | - | −11.26 ± 0.71 | 15.55 ± 0.83 | −15.74 ± 0.39 | - |
| Formononetin + Genistein | - | 0.85 ± 0.07 | 60.94 ± 5.39 b | −16.71 ± 0.59 | - |
| Formononetin + Biochanin A | - | 5.11 ± 0.41 | 91.10 ± 7.50 | −21.14 ± 0.85 | - |
| Genistein + Biochanin A | - | 0.15 ± 0.01 | 62.98 ± 4.08 b | −18.00 ± 0.81 a | - |
| red clover sprouts extracts | |||||
| White light 10 | - | −22.57 ± 0.92 b | −35.89 ± 1.18 | −12.23 ± 0.43 | - |
| UVA3 | - | −22.19 ± 0.78 b | −9.01 ± 0.38 a | −19.59 ± 0.62 a | - |
| UVA8 | - | −21.19 ± 0.65 c | −5.23 ± 0.31 | −19.68 ± 0.71 a | - |
| UVB11 | - | −21.10 ± 1.05 c | −9.88 ± 0.43 a | −18.88 ± 0.65 a | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budryn, G.; Grzelczyk, J.; Pérez-Sánchez, H. Binding of Red Clover Isoflavones to Actin as A Potential Mechanism of Anti-Metastatic Activity Restricting the Migration of Cancer Cells. Molecules 2018, 23, 2471. https://doi.org/10.3390/molecules23102471
Budryn G, Grzelczyk J, Pérez-Sánchez H. Binding of Red Clover Isoflavones to Actin as A Potential Mechanism of Anti-Metastatic Activity Restricting the Migration of Cancer Cells. Molecules. 2018; 23(10):2471. https://doi.org/10.3390/molecules23102471
Chicago/Turabian StyleBudryn, Grażyna, Joanna Grzelczyk, and Horacio Pérez-Sánchez. 2018. "Binding of Red Clover Isoflavones to Actin as A Potential Mechanism of Anti-Metastatic Activity Restricting the Migration of Cancer Cells" Molecules 23, no. 10: 2471. https://doi.org/10.3390/molecules23102471
APA StyleBudryn, G., Grzelczyk, J., & Pérez-Sánchez, H. (2018). Binding of Red Clover Isoflavones to Actin as A Potential Mechanism of Anti-Metastatic Activity Restricting the Migration of Cancer Cells. Molecules, 23(10), 2471. https://doi.org/10.3390/molecules23102471