Antrodia cinnamomea Oligosaccharides Suppress Lipopolysaccharide-Induced Inflammation through Promoting O-GlcNAcylation and Repressing p38/Akt Phosphorylation
Abstract
1. Introduction
2. Results
2.1. Composition Analysis of ACCO and ACHO
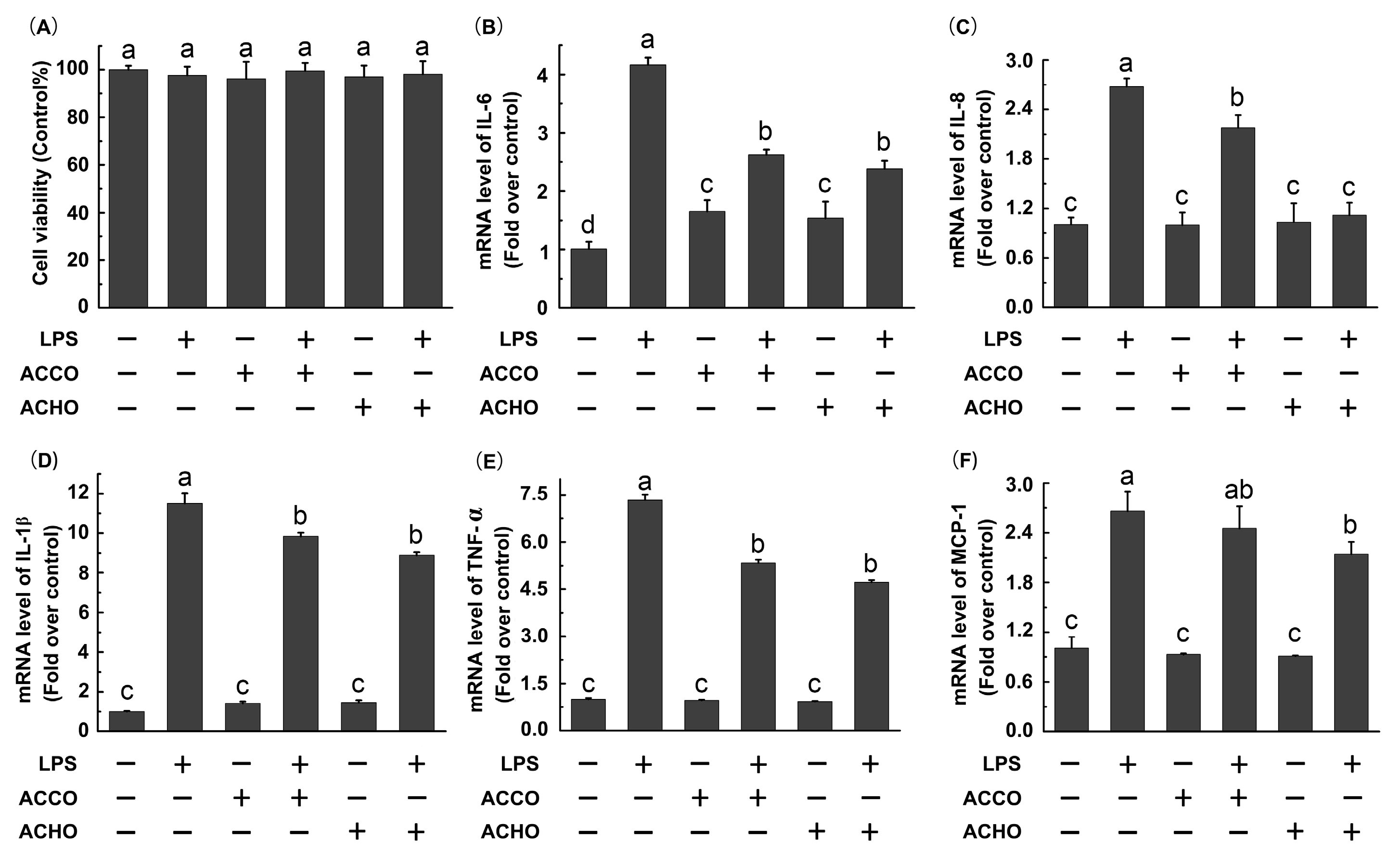
2.2. ACCO and ACHO Suppressed LPS-Induced Inflammation in Macrophage Cells
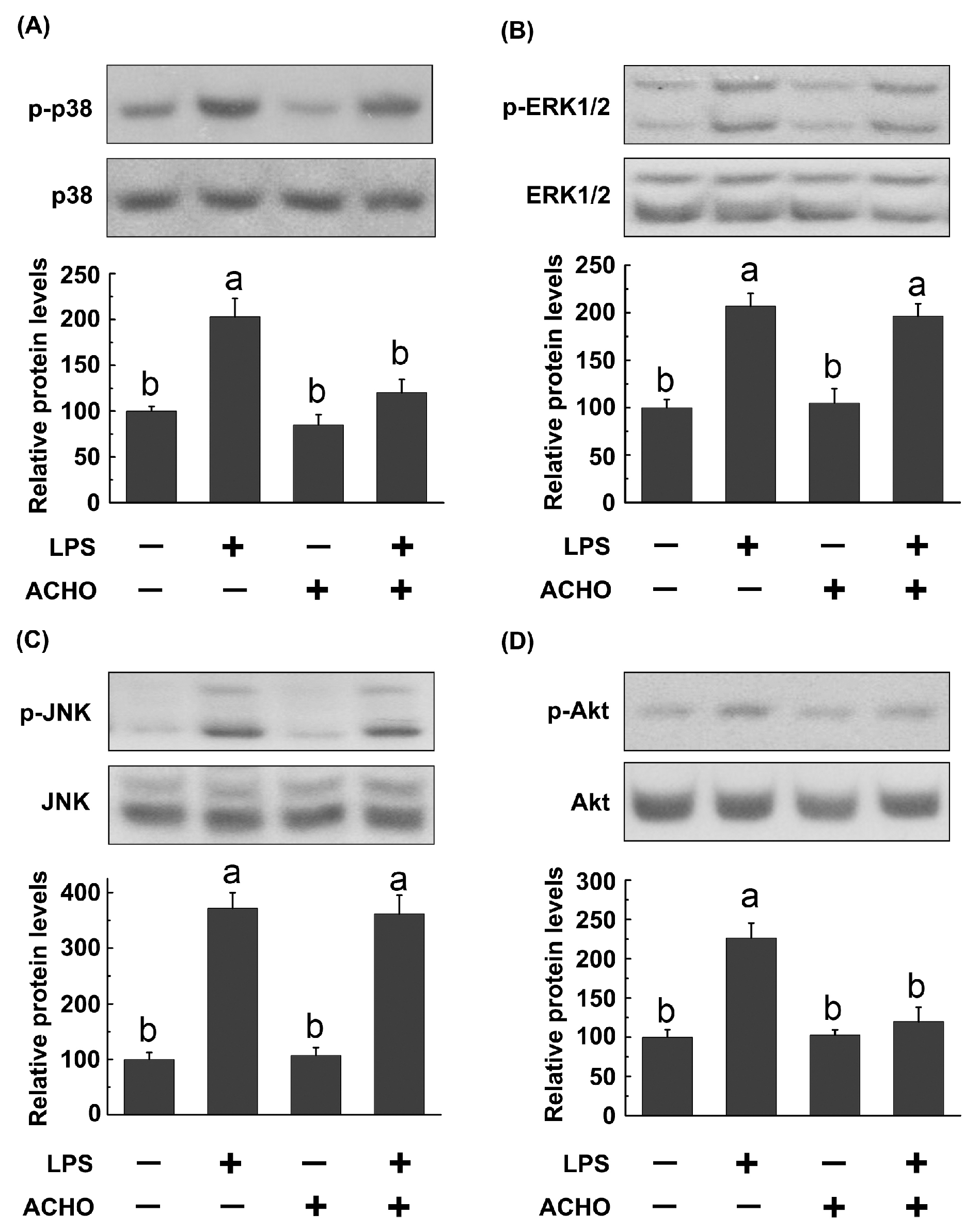
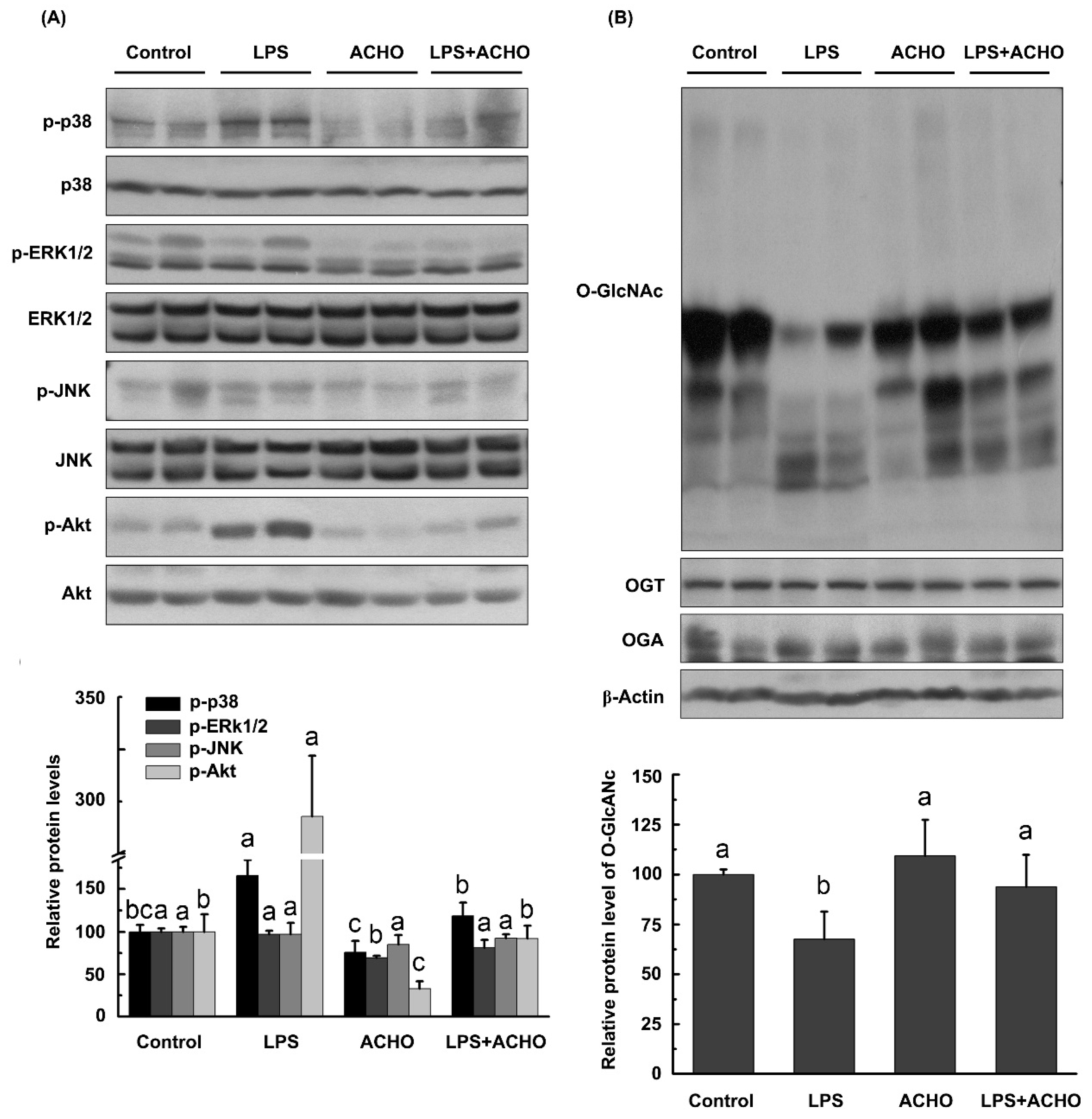
2.3. ACHO Inhibited the Phosphorylation of p38 and Akt in LPS-Induced Macrophage Cells
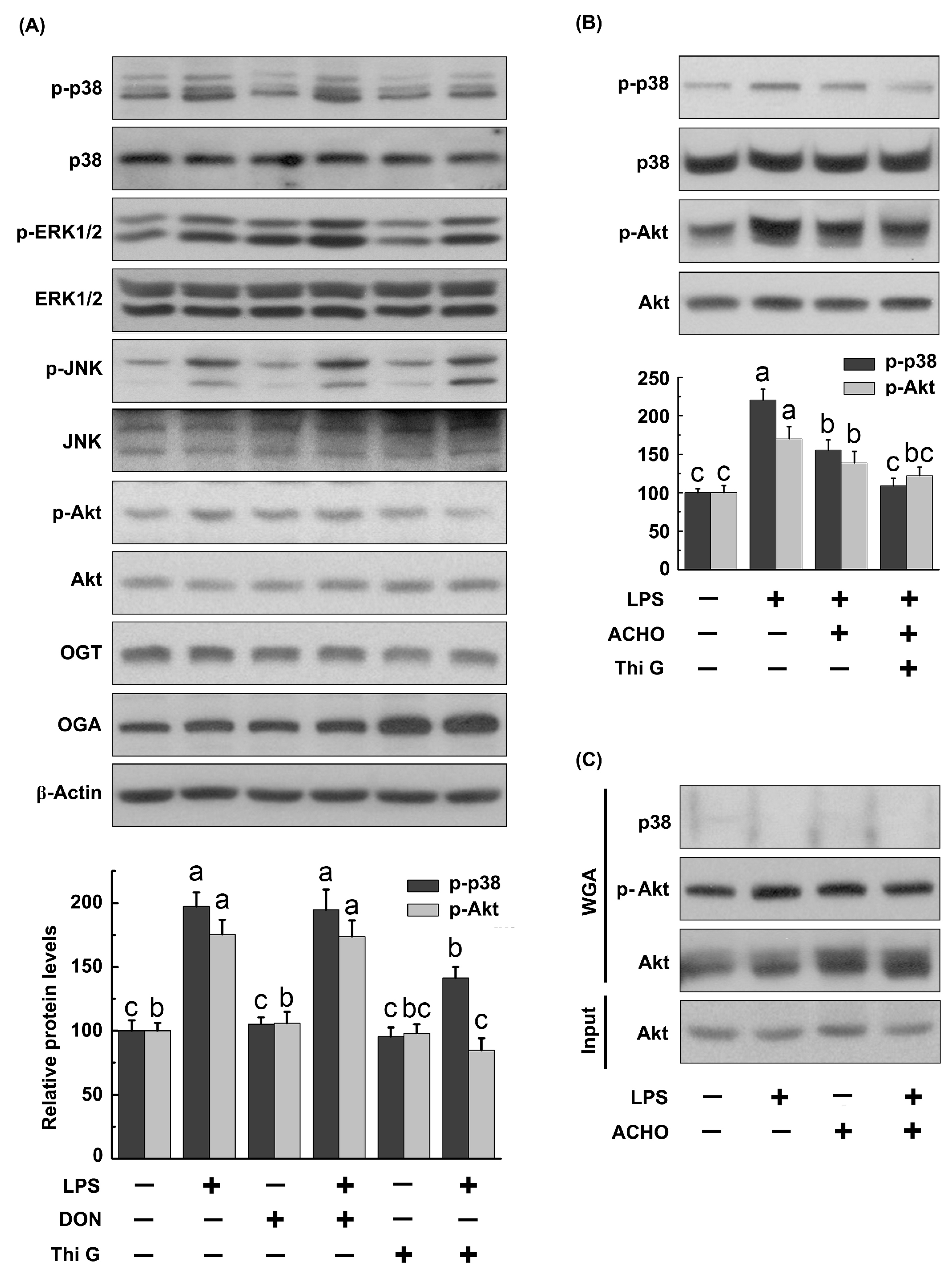
2.4. ACHO Reversed Down-Regulation of O-GlcNAcylation in LPS-Induced Macrophage Cells
2.5. ACHO Inhibited p38 and Akt Phosphorylation by Promoting Protein O-GlcNAcylation in LPS-Induced Macrophage Cells
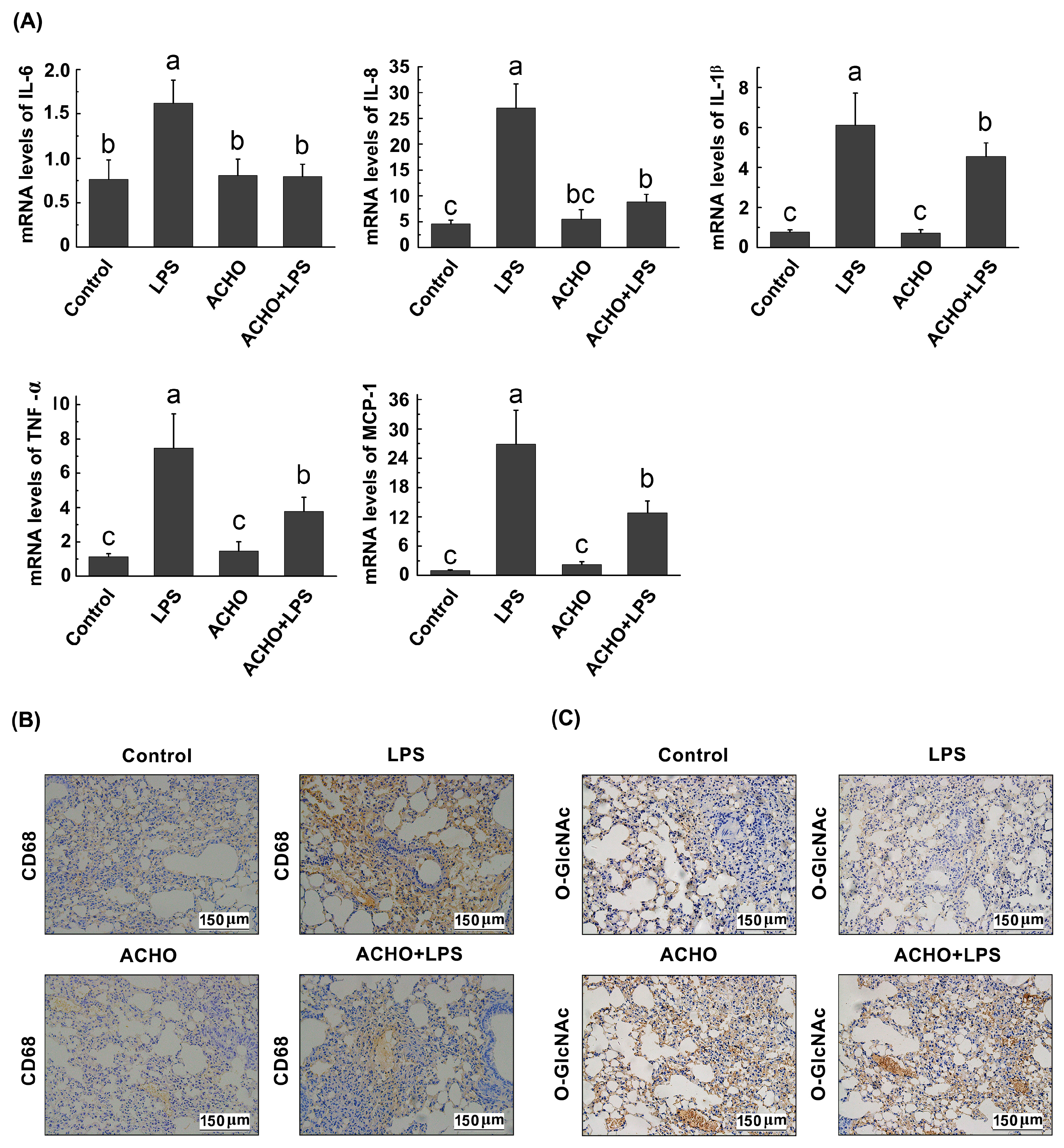
2.6. ACHO Suppressed the Inflammatory Reaction in Lung Tissues of LPS-Injected Mice
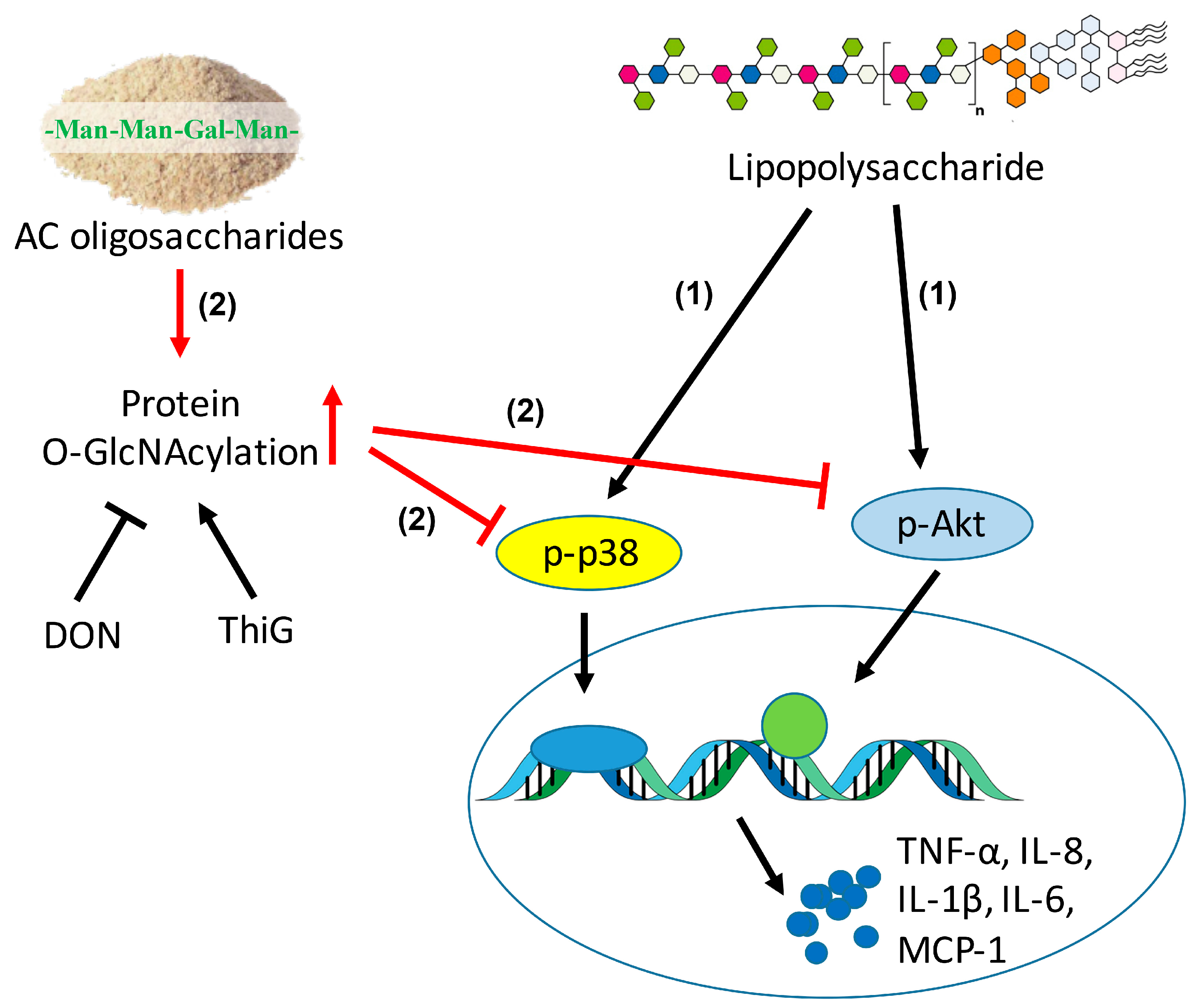
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Oligosaccharides from AC Fruit Bodies
4.3. Ultra Performance Liquid Chromatography-Mass Spectrometry (UPLC-MS) Analysis of ACCO and ACHO
4.4. Cell Culture
4.5. Animal Experiment
4.6. Isolation and Analysis of O-GlcNAcylated Proteins
4.7. Western Blotting
4.8. RNA Extraction and Real-Time PCR
4.9. Immunohistochemistry Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yue, P.Y.; Wong, Y.Y.; Chan, T.Y.; Law, C.K.; Tsoi, Y.K.; Leung, K.S. Review of biological and pharmacological activities of the endemic Taiwanese bitter medicinal mushroom, Antrodia camphorata (M. Zang et C.H. Su) Sh. H. Wu et al. (higher Basidiomycetes). Int. J. Med. Mushrooms 2012, 14, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; El-Shazly, M.; Wu, T.Y.; Du, Y.C.; Chang, T.T.; Chen, C.F.; Hsu, Y.M.; Lai, K.H.; Chiu, C.P.; Chang, F.R.; et al. Recent research and development of Antrodia cinnamomea. Pharmacol. Ther. 2013, 139, 12456. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Hirakawa, A.; Gao, J.J.; Kakuda, H.; Shiro, M.; Komatsu, Y.; Sheu, C.C.; Hattori, M. Five new maleic and succinic acid derivatives from the mycelium of Antrodia camphorata and their cytotoxic effects on LLC tumor cell line. J. Nat. Prod. 2004, 67, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Shie, P.H.; Wang, S.Y.; Lay, H.L.; Huang, G.J. 4,7-Dimethoxy-5-methyl-1,3-benzodioxole from Antrodia camphorata inhibits LPS-induced inflammation via suppression of NF-kappaB and induction HO-1 in RAW264.7 cells. Int. Immunopharmacol. 2016, 31, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-C.; Chiang, P.-C.; Lu, P.-H.; Kuo, M.-T.; Wen, W.-C.; Chen, P.; Guh, J.-H. Antroquinonol, a natural ubiquinone derivative, induces a cross talk between apoptosis, autophagy and senescence in human pancreatic carcinoma cells. J. Nutr. Biochem. 2012, 23, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Chen, C.C.; Huang, C.J.; Sung, P.J.; Huang, S.S.; Kuo, Y.H. Methanol extract of Antrodia camphorata protects against lipopolysaccharide-induced acute lung injury by suppressing NF-kappaB and MAPK pathways in mice. J. Agric. Food Chem. 2014, 62, 5321–5329. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liu, Y.W.; Ker, Y.B.; Wu, Y.Y.; Lai, E.Y.; Chyau, C.C.; Hseu, T.H.; Peng, R.Y. Chemical characterization and anti-inflammatory effect of polysaccharides fractionated from submerge-cultured Antrodia camphorata mycelia. J. Agric. Food Chem. 2007, 55, 5007–5012. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-J.; Lu, M.-K.; Lin, C.-Y.; Chang, C.-C. Characterization and functional elucidation of a fucosylated 1,6-alpha-d-mannogalactan polysaccharide from Antrodia cinnamomea. Carbohydr. Polym. 2011, 83, 545–553. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Lin, M.-T.; Duan, K.-J.; Chen, Y.-S. The hepatoprotective activity against ethanol-induced cytotoxicity by aqueous extract of Antrodia cinnamomea. J. Chin. Inst. Eng. 2008, 39, 441–447. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Chen, C.-C.; Chyau, C.-C.; Chung, S.-Y.; Liu, Y.-W. Modulation of inflammation-related genes of polysaccharides fractionated from mycelia of medicinal basidiomycete Antrodia camphorata. Acta Pharmacol. Sin. 2007, 28, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Chyau, C.; Chen, C.; Wu, Y.; Liu, Y.; Peng, R.Y. Antioxidative and anti-inflammatory activity of polysaccharides fractionated from submerge-cultured Antrodia camphorata mycelia. Free Radic. Res. 2006, 40, S104. [Google Scholar]
- Song, A.-R.; Qin, D.; Zhao, C.; Sun, X.-L.; Huang, F.; Kong, C.; Yang, S. Immunomodulatory Effect of Polysaccharides Extracted from the Medicinal Mushroom Antrodia camphorata (Higher Basidiomycetes) in Specific Pathogen-Free Chickens. Int. J. Med. Mushrooms 2014, 16, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-J.; Chang, C.-C.; Chao, C.-H.; Lu, M.-K. Characterization of fungal sulfated polysaccharides and their synergistic anticancer effects with doxorubicin. Carbohydr. Polym. 2012, 90, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Toress, C.R.; Hart, G.W. Topography and Polypeptide Distribution of Terminal N-Acetylglucosamine Residues on the Surfaces of Intact Lymphocytes. J. Biol. Chem. 1984, 259, 3308–3317. [Google Scholar]
- Haltiwanger, R.S.; Holt, G.D.; Hart, G.W. Enzymatic Addition of O-Glcnac to Nuclear and Cytoplasmic Proteins—Identification of a Uridine Diphospho-N-Acetylglucosamine-Peptide Beta-N-Acetylglucosaminyltransferase. J. Biol. Chem. 1990, 265, 2563–2568. [Google Scholar] [PubMed]
- Dong, D.L.; Hart, G.W. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-d-glucosaminidase from rat spleen cytosol. J. Biol. Chem. 1994, 269, 19321–19330. [Google Scholar] [PubMed]
- Bond, M.R.; Hanover, J.A. O-GlcNAc cycling: A link between metabolism and chronic disease. Annu. Rev. Nutr. 2013, 33, 205–229. [Google Scholar] [CrossRef] [PubMed]
- Naseem, S.; Parrino, S.M.; Buenten, D.M.; Konopka, J.B. Novel roles for GlcNAc in cell signaling. Commun. Integr. Biol. 2012, 5, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, L.; Issad, T. O-GlcNAcylation and Inflammation: A Vast Territory to Explore. Front. Endocrinol. 2015, 5, 235. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Xu, Q.S.; Du, Y.G.; Xu, J. Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-kappaB and endothelial inflammatory response. Carbohydr. Polym. 2014, 99, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Not, L.G.; Brocks, C.A.; Vamhidy, L.; Marchase, R.B.; Chatham, J.C. Increased O-linked beta-N-acetylglucosamine levels on proteins improves survival, reduces inflammation and organ damage 24 hours after trauma-hemorrhage in rats. Crit. Care Med. 2010, 38, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, A.G.; Ming, X.F.; Carvas, J.M.; Yang, Z. O-linked beta-N-acetylglucosamine during hyperglycemia exerts both anti-inflammatory and pro-oxidative properties in the endothelial system. Oxid. Med. Cell. Longev. 2009, 2, 172–175. [Google Scholar] [CrossRef] [PubMed]
- McCranie, E.K.; Bachmann, B.O. Bioactive oligosaccharide natural products. Nat. Prod. Rep. 2014, 31, 1026–1042. [Google Scholar] [CrossRef] [PubMed]
- Hilgers, R.H.; Xing, D.; Gong, K.; Chen, Y.F.; Chatham, J.C.; Oparil, S. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H513–H522. [Google Scholar] [CrossRef] [PubMed]
- Siddhanta, A.K.; Goswami, A.M.; Ramavat, B.K.; Mody, K.H.; Mairh, O.P. Water soluble polysaccharides of marine algal species of Ulva (Ulvales, Chlorophyta) of Indian waters. Indian J. Mar. Sci. 2001, 30, 166–172. [Google Scholar]
- Chen, P.H.; Weng, Y.M.; Yu, Z.R.; Koo, M.; Wang, B.J. Extraction temperature affects the activities of antioxidation, carbohydrate-digestion enzymes, and angiotensin-converting enzyme of Pleurotus citrinopileatus extract. J. Food Drug Anal. 2016, 24, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cha, J.D.; Choi, K.M.; Lee, K.Y.; Han, K.M.; Jang, Y.S. Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo. Int. Immunopharmacol. 2017, 43, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, H.; Zhou, Y.; Zhang, J.; Long, C.; Li, S.; Chen, S.; Zhou, J.M.; Shao, F. The phosphothreonine lyase activity of a bacterial type III effector family. Science 2007, 315, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Chou, P.Y.; Chien, Y.C.; Wu, C.H.; Wu, T.S.; Sheu, M.J. Ethanol extracts of fruiting bodies of Antrodia cinnamomea exhibit anti-migration action in human adenocarcinoma CL1-0 cells through the MAPK and PI3K/AKT signaling pathways. Phytomedicine 2012, 19, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Liu, F.C.; Chou, P.Y.; Chien, Y.C.; Chang, W.S.; Huang, G.J.; Wu, C.H.; Sheu, M.J. Ethanol extracts of fruiting bodies of Antrodia cinnamomea suppress CL1-5 human lung adenocarcinoma cells migration by inhibiting matrix metalloproteinase-2/9 through ERK, JNK, p38, and PI3K/Akt signaling pathways. Evid.-Based Complement. Altern. Med. 2012, 2012, 378415. [Google Scholar] [CrossRef]
- Lu, M.K.; Lin, T.Y.; Chao, C.H.; Hu, C.H.; Hsu, H.Y. Molecular mechanism of Antrodia cinnamomea sulfated polysaccharide on the suppression of lung cancer cell growth and migration via induction of transforming growth factor beta receptor degradation. Int. J. Biol. Macromol. 2017, 95, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, S.; Andrali, S.S.; Cantrell, J.E. Modulation of transcription factor function by O-GlcNAc modification. Biochim. Biophys. Acta 2010, 1799, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, H.; Whiteside, C.; Fantus, I.G. O-Linked beta-N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E713–E726. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gu, J.H.; Dai, C.L.; Gu, J.; Jin, X.; Sun, J.; Iqbal, K.; Liu, F.; Gong, C.X. O-GlcNAcylation regulates ischemia-induced neuronal apoptosis through AKT signaling. Sci. Rep. 2015, 5, 14500. [Google Scholar] [CrossRef] [PubMed]
- Fulop, N.; Zhang, Z.; Marchase, R.B.; Chatham, J.C. Glucosamine cardioprotection in perfused rat hearts associated with increased O-linked N-acetylglucosamine protein modification and altered p38 activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2227–H2236. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.S.; Han, D.; Park, J.; Kwak, T.K.; Oh, M.A.; Lee, S.A.; Choi, S.; Park, Z.Y.; Kim, Y.; Lee, J.W. O-GlcNAc modulation at Akt1 Ser473 correlates with apoptosis of murine pancreatic beta cells. Exp. Cell Res. 2008, 314, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Vosseller, K.; Wells, L.; Lane, M.D.; Hart, G.W. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 5313–5318. [Google Scholar] [CrossRef] [PubMed]
- Sevag, M.G.; Lackman, D.B.; Smolens, J. The isolation of the components of streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 1938, 124, 0425–0436. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Zachara, N.E.; Vosseller, K.; Hart, G.W. Detection and Analysis of Proteins Modified by O-linked N-acetylglucosamine. In Current Protocols in Protein Science; John Wiley & Sons: New York, NY, USA, 2011; Chapter 12; pp. 12.8.1–12.8.33. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds including ACCO and ACHO are available from the authors. |
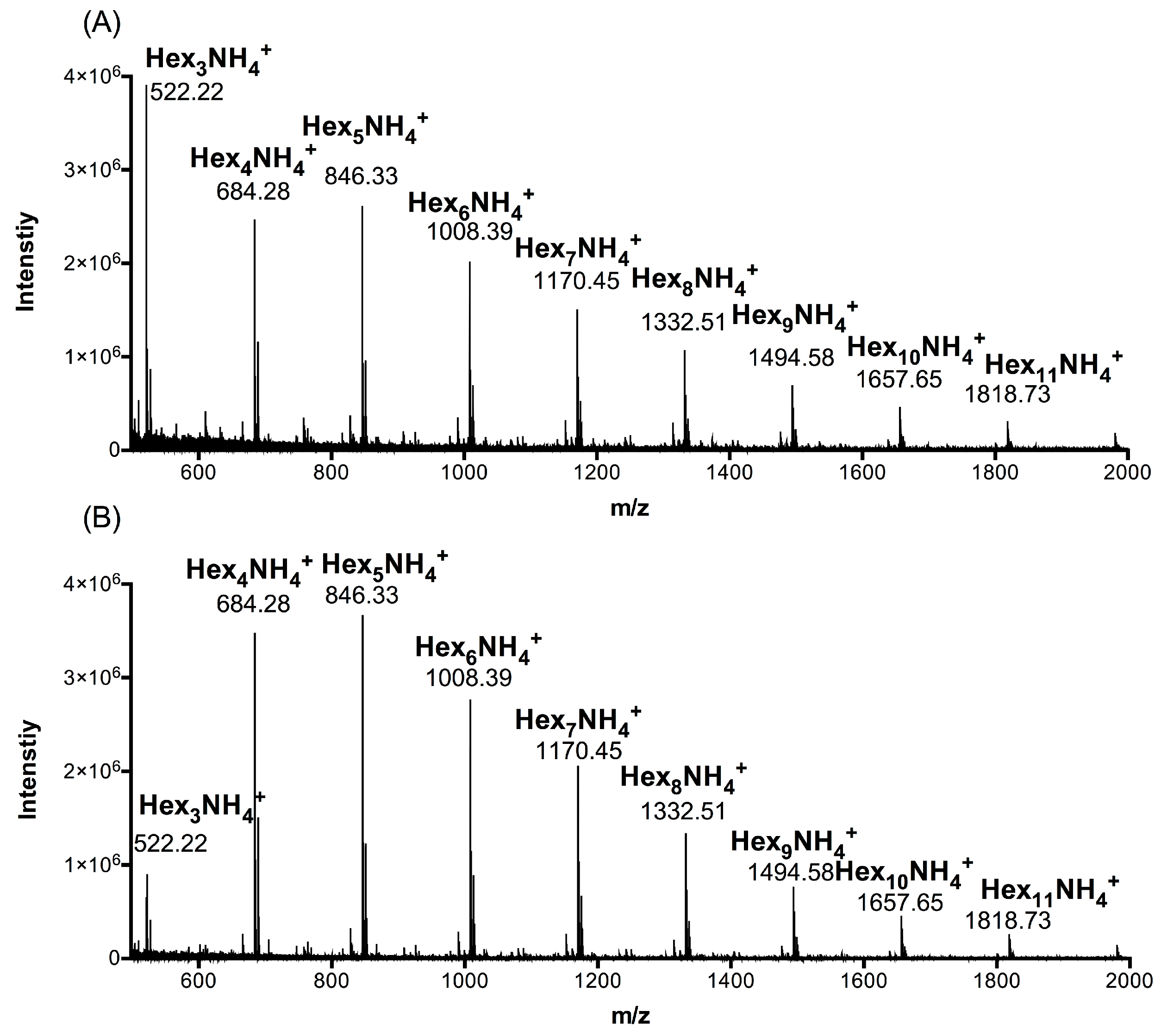

| Group | Hex3 1 | Hex4 | Hex5 | Hex6 | Hex7 | Hex8 | Hex9 | Hex10 | Hex11 |
|---|---|---|---|---|---|---|---|---|---|
| ACCO | 27.25 | 20.76 | 17.49 | 11.62 | 10.94 | 5.11 | 3.63 | 1.68 | 1.52 |
| ACHO | 7.12 | 28.88 | 25.02 | 14.95 | 14.05 | 5.77 | 2.68 | 0.87 | 0.66 |
| Group | Composition | Ara 1 | Glu 2 | Fuc 3 | Gal 4 | Man 5 |
|---|---|---|---|---|---|---|
| ACCO | mg/mL | 0.06 | 0.23 | 0.01 | 1.21 | 3.49 |
| % | 1.18 | 4.56 | 0.17 | 24.26 | 69.83 | |
| ACHO | mg/mL | 0.02 | 0.22 | 0.03 | 1.22 | 3.51 |
| % | 0.45 | 4.45 | 0.53 | 24.42 | 70.09 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Jiao, S.; Li, Q.; Jia, P.; Yin, H.; Zhao, X.; Du, Y.; Liu, H. Antrodia cinnamomea Oligosaccharides Suppress Lipopolysaccharide-Induced Inflammation through Promoting O-GlcNAcylation and Repressing p38/Akt Phosphorylation. Molecules 2018, 23, 51. https://doi.org/10.3390/molecules23010051
Zheng J, Jiao S, Li Q, Jia P, Yin H, Zhao X, Du Y, Liu H. Antrodia cinnamomea Oligosaccharides Suppress Lipopolysaccharide-Induced Inflammation through Promoting O-GlcNAcylation and Repressing p38/Akt Phosphorylation. Molecules. 2018; 23(1):51. https://doi.org/10.3390/molecules23010051
Chicago/Turabian StyleZheng, Junping, Siming Jiao, Qiongyu Li, Peiyuan Jia, Heng Yin, Xiaoming Zhao, Yuguang Du, and Hongtao Liu. 2018. "Antrodia cinnamomea Oligosaccharides Suppress Lipopolysaccharide-Induced Inflammation through Promoting O-GlcNAcylation and Repressing p38/Akt Phosphorylation" Molecules 23, no. 1: 51. https://doi.org/10.3390/molecules23010051
APA StyleZheng, J., Jiao, S., Li, Q., Jia, P., Yin, H., Zhao, X., Du, Y., & Liu, H. (2018). Antrodia cinnamomea Oligosaccharides Suppress Lipopolysaccharide-Induced Inflammation through Promoting O-GlcNAcylation and Repressing p38/Akt Phosphorylation. Molecules, 23(1), 51. https://doi.org/10.3390/molecules23010051





