Synthesis and Evaluation of the Antioxidant Activity of Lipophilic Phenethyl Trifluoroacetate Esters by In Vitro ABTS, DPPH and in Cell-Culture DCF Assays
Abstract
1. Introduction
2. Results and Discussion
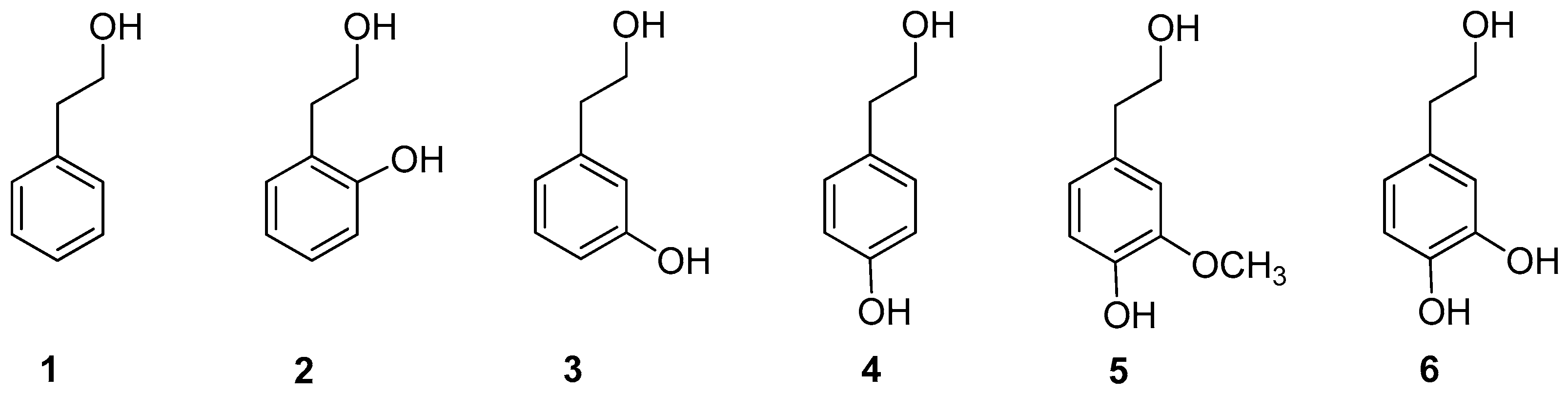
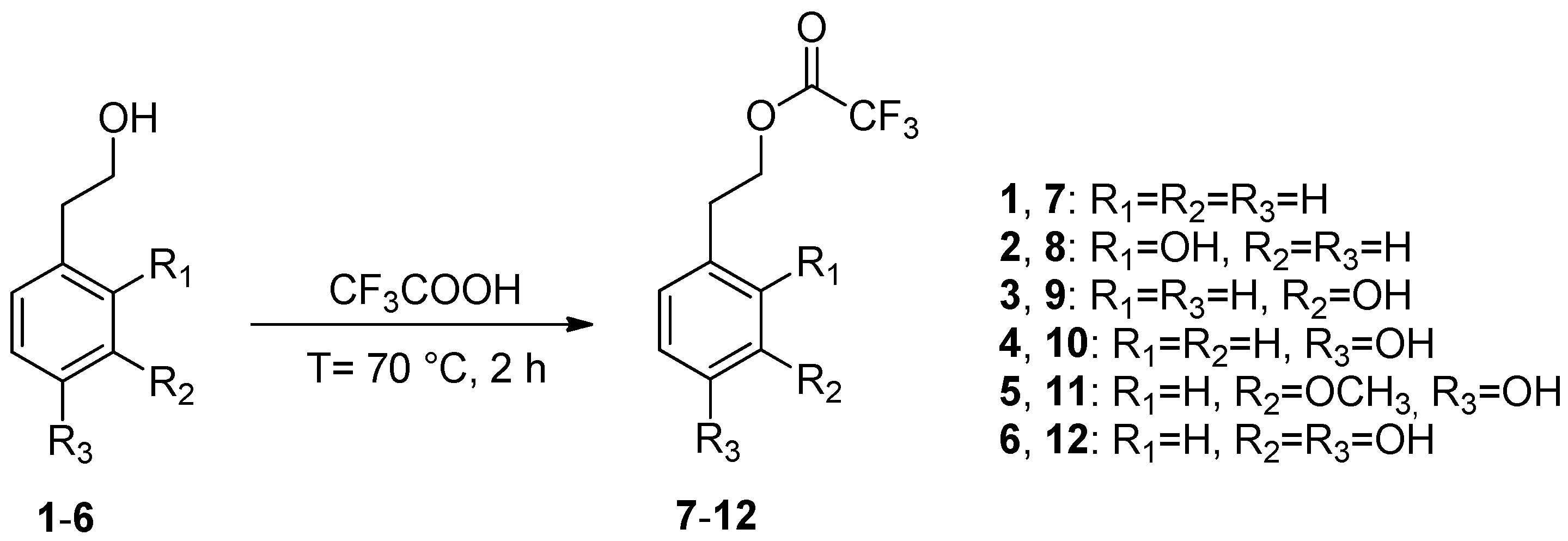
2.1. Synthesis of Phenethyl Trifluoroacetate Esters
2.2. LogP Determination
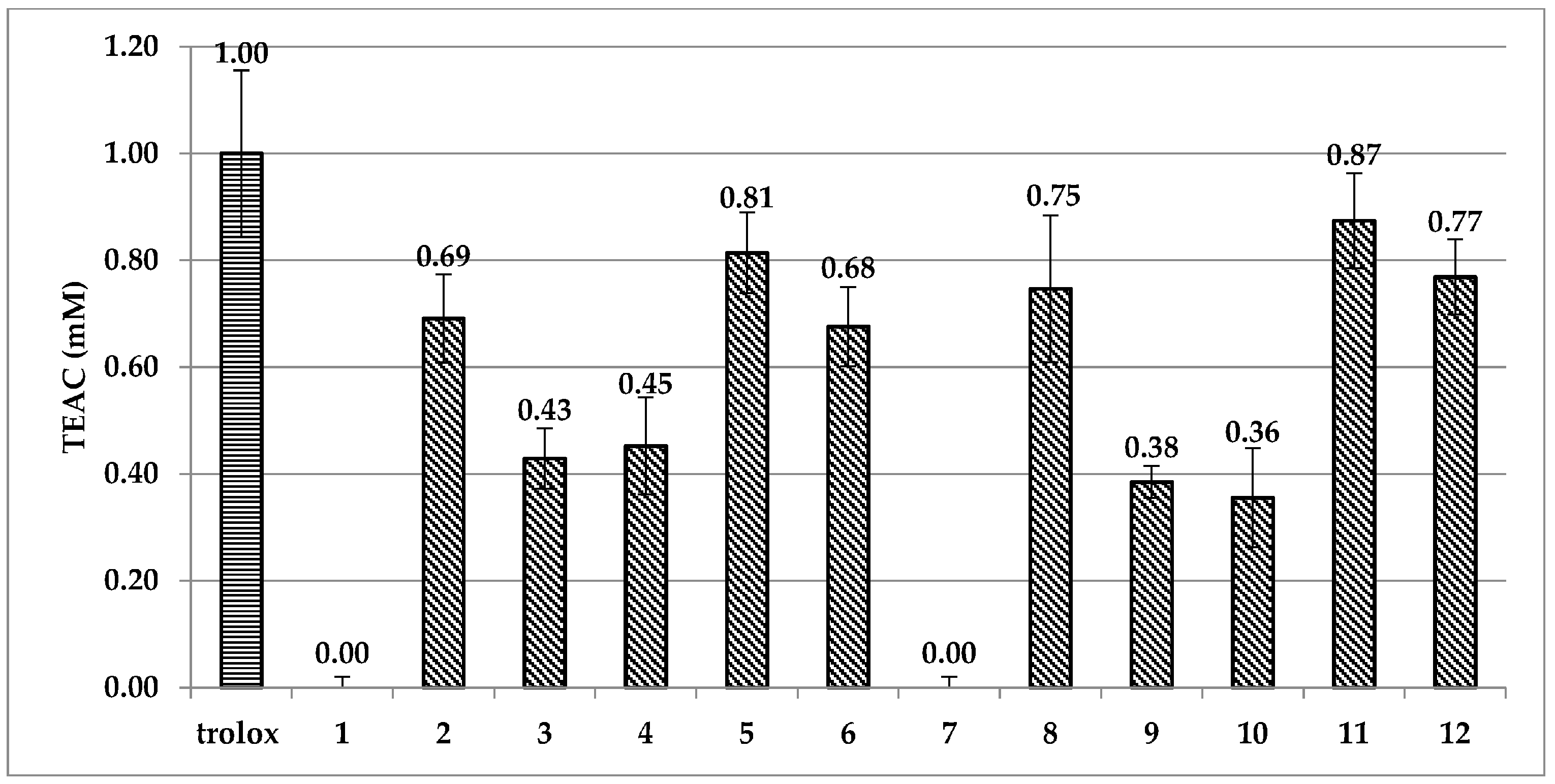
2.3. ABTS and DPPH Spectrophotometric Assays
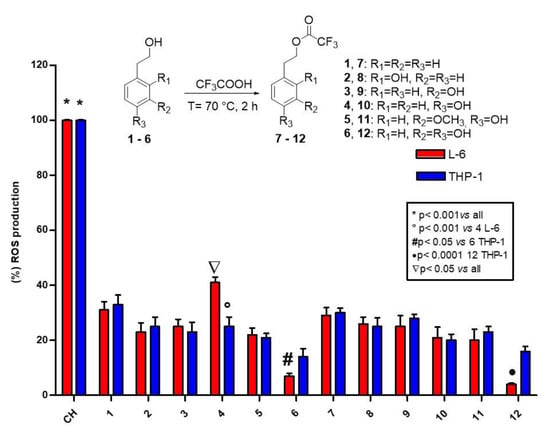
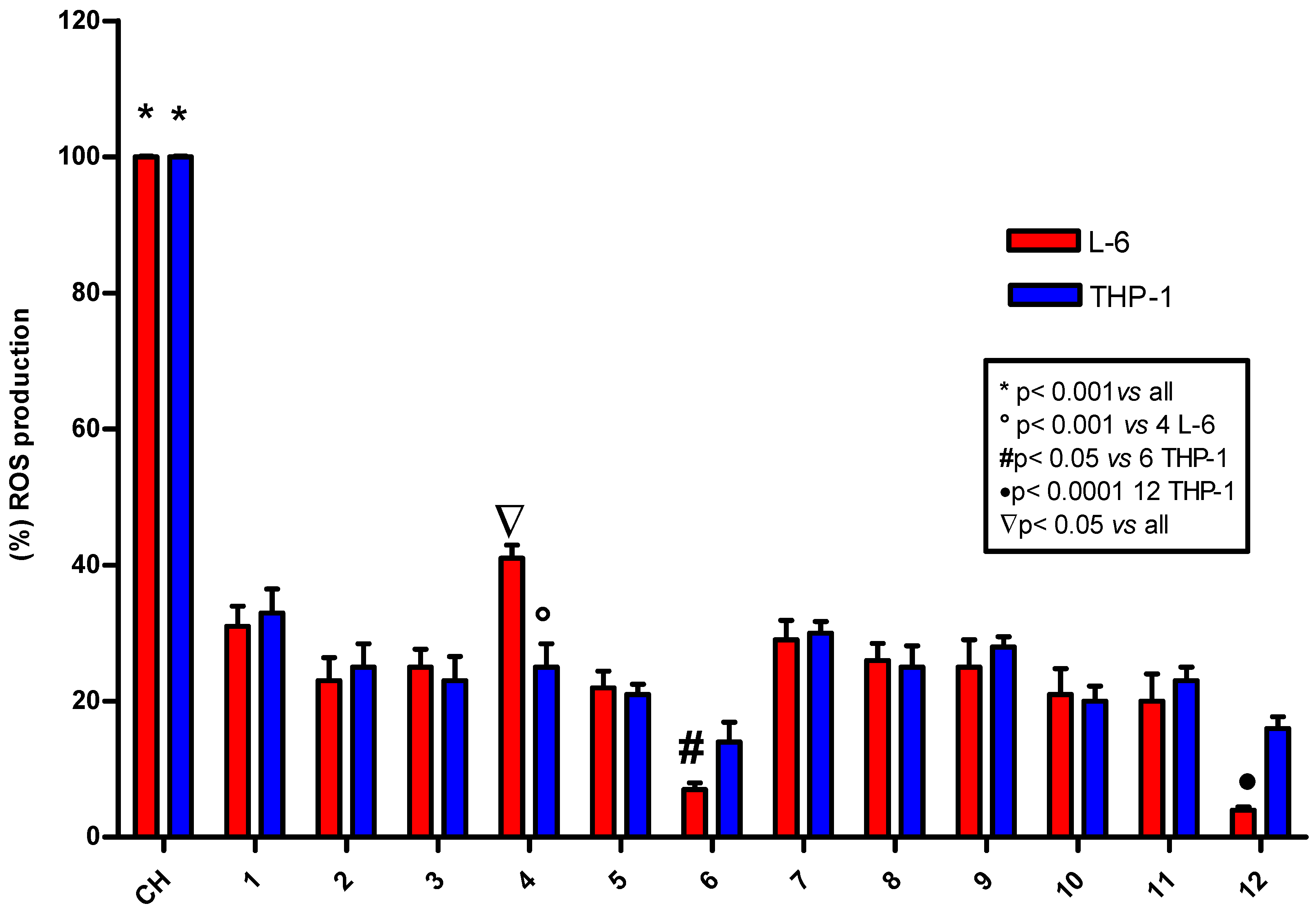
2.4. ROS Determination
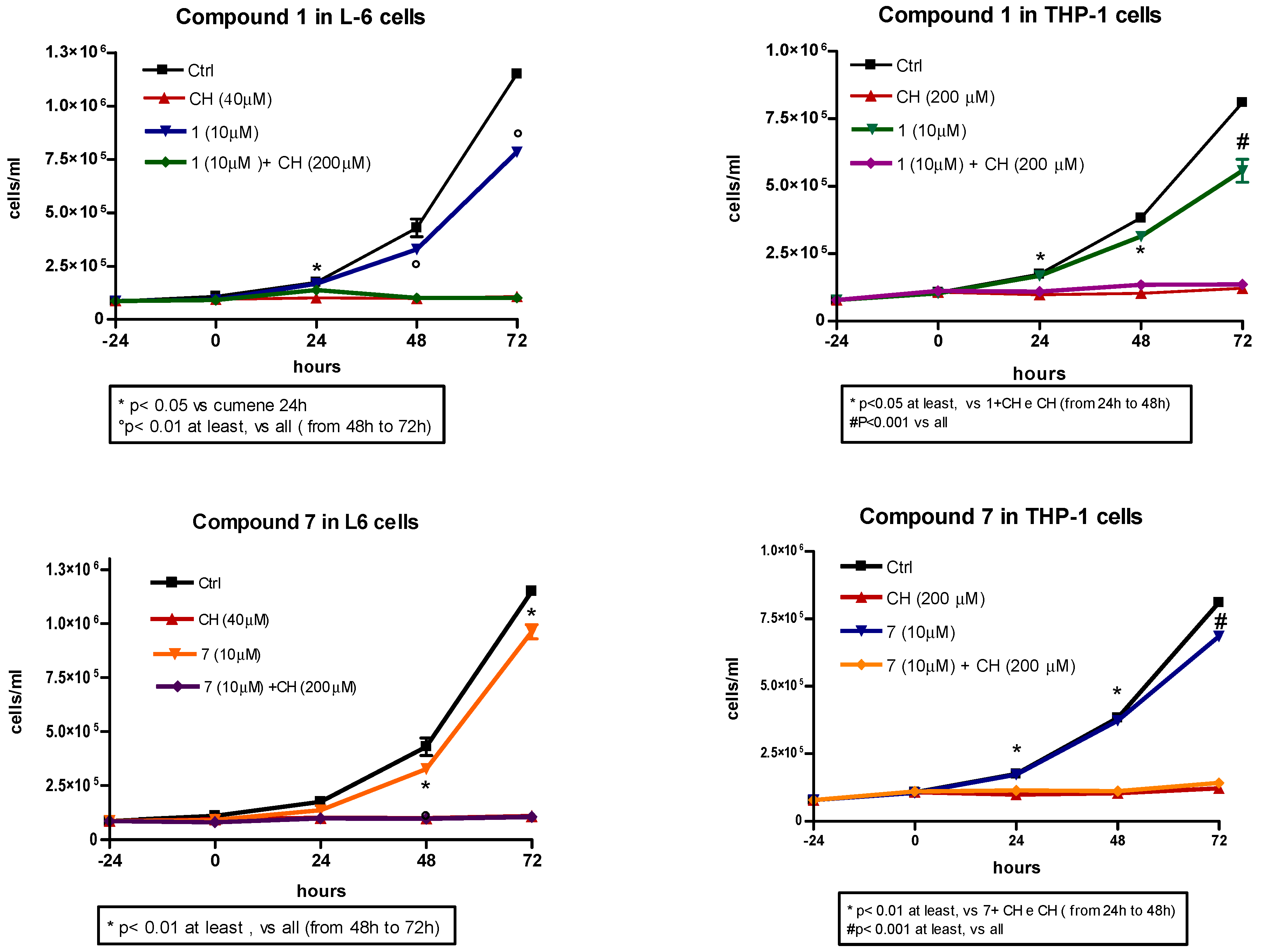
2.5. Compound Toxicity and Cell Proliferation
3. Materials and Methods
3.1. Chemicals
3.2. Instruments
3.3. Synthesis of Phenethyl Trifluoroacetate Esters: General Procedure
3.4. Measurement of Partition Coefficient (logP)
3.5. ABTS Assay
3.6. DPPH Assay
3.7. Cells in Culture
3.8. Intracellular ROS Determination
3.9. MTT Assay
3.10. Proliferation Curves
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Pollio, A.; Zarrelli, A.; Romanucci, V.; Di Mauro, A.; Barra, F.; Pinto, G.; Crescenzi, E.; Roscetto, E.; Palumbo, G. Polyphenolic profile and targeted bioactivity of methanolic extracts from Mediterranean ethnomedicinal plants on human cancer cell lines. Molecules 2016, 21, 395. [Google Scholar] [CrossRef] [PubMed]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Zarrelli, A. Low molecular weight phenols from the bioactive aqueous fraction of Cestrum parqui. J. Agric. Food Chem. 2004, 52, 4101–4108. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Bernini, R.; Mincione, E.; Ciafrino, R. A new and efficient synthesis of 8-hydroxypurine derivatives by dimethyldioxirane oxidation. Tetrahedron Lett. 1995, 36, 2665–2668. [Google Scholar] [CrossRef]
- Saladino, R.; Bernini, R.; Crestini, C.; Mincione, E.; Bergamini, A.; Marini, S.; Palamara, A.T. Studies on the chemistry of pyrimidine derivatives with dimethyldioxirane: Synthesis, cytotoxic effects and antiviral activity of new 5,6-oxiranyl-5,5-dihydro and 5-hydroxy-5,6-dihydro-6-substitued uracil derivatives and pyrimidine nucleosides. Tetrahedron 1995, 51, 7561–7578. [Google Scholar] [CrossRef]
- Shibutami, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Bouallagui, Z.; Bouaziz, M.; Lassoued, S.; Engasser, J.M.; Ghoul, M.; Sayadi, S. Hydroxytyrosol acyl esters: Biosynthesis and activities. Appl. Biochem. Biotechnol. 2011, 163, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Tofani, D.; Balducci, V.; Gasperi, T.; Incerpi, S.; Gambacorta, A. Fatty acid hydroxytyrosyl esters: Structure/antioxidant activity relationship by ABTS and in cell-culture DCF assays. J. Agric. Food Chem. 2010, 58, 5292–5299. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Crisante, F.; Barontini, M.; Tofani, D.; Balducci, V.; Gambacorta, A. Synthesis and structure/antioxidant activity relationship of novel catecholic antioxidant structural analogues to hydroxytyrosol and its lipophilic esters. J. Agric. Food Chem. 2012, 60, 7408–7416. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of hydroxytyrosol-enriched fractions from Olea europaea L. by-products and evaluation of the in vitro effects on a model of colorectal cancer cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Gilardini Montani, M.S.; Merendino, N.; Romani, A.; Velotti, F. Hydroxytyrosol-derived compounds: A basis for the creation of new pharmacological agents for cancer prevention and therapy. J. Med. Chem. 2015, 58, 9089–9107. [Google Scholar] [CrossRef] [PubMed]
- Madrona, A.; Pereira-Caro, G.; Mateos, R.; Rodriguez, G.; Trujillo, M.; Fernadez-Bolanos, J.; Espartero, J.L. Synthesis of hydroxytyrosyl alkyl ethers from olive oil waste waters. Molecules 2009, 14, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Gerebtzoff, G.; Li-Blatter, X.; Fischer, H.; Frentzel, A.; Seelig, A. Halogenation of drugs enhances membrane binding and permeation. Chembiochem 2004, 5, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.M.D. Important fluorinated drugs in experimental and clinical use. J. Fluor. Chem. 2002, 118, 27–33. [Google Scholar] [CrossRef]
- Bovicelli, P.; Mincione, E.; Antonioletti, R.; Bernini, R.; Colombari, M. Selective halogenation of aromatics by dimethyldioxirane and halogen ions. Synth. Commun. 2001, 31, 2955–2963. [Google Scholar] [CrossRef]
- Bovicelli, P.; Bernini, R.; Antonioletti, R.; Mincione, E. Selective halogenation of flavanones. Tetrahedron Lett. 2002, 43, 5563–5567. [Google Scholar] [CrossRef]
- Bernini, R.; Pasqualetti, M.; Provenzano, G.; Tempesta, S. Ecofriendly synthesis of halogenated flavonoids and evaluation of their antifungal activity. New J. Chem. 2015, 39, 2980–2987. [Google Scholar] [CrossRef]
- Strunekà, A.; Patočka, J.; Connet, P. Fluorine in medicine. J. Appl. Biomed. 2004, 2, 141–150. [Google Scholar]
- Zaldini Hernandes, M.; Cavalcanti, S.M.T.; Moreira, D.R.M.; Filgueira de Azevedo Junior, W.; Leite, A.C.L. Halogen atoms in the modern medicinal chemistry: Hints for the drug design. Curr. Drug Targets 2010, 11, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Barontini, M.; Bernini, R.; Carastro, I.; Gentili, P.; Romani, A. Synthesis and DPPH radical scavenging activity of novel compounds obtained from tyrosol and cinnamic acid derivatives. New J. Chem. 2014, 38, 809–816. [Google Scholar] [CrossRef]
- Bernini, R.; Crisante, F.; D’Acunzo, F.; Gentili, P.; Ussia, E. Oxidative cleavage of 1-aryl-isochroman derivatives by the Trametes villosa laccase/1-hydroxybenzotroazole system. New J. Chem. 2016, 40, 3314–3322. [Google Scholar] [CrossRef]
- Loizo, M.; Bonesi, M.; Di Lecce, G.; Boselli, E.; Tundis, R.; Pugliese, A.; Menichini, F.; Frega, N.G. Phenolics, aroma profile, and in vitro antioxidant activity of Italian dessert passito wine from Saracena (Italy). J. Food Sci. 2013, 78, C703–C708. [Google Scholar] [CrossRef] [PubMed]
- Cosmeticinfo.org. Available online: http://www.cosmeticsinfo.org/ingredient/phenethyl-alcohol-0 (accessed on 18 December 2017).
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/default.htm (accessed on 18 December 2017).
- Zhu, Y.J.; Zhou, H.T.; Hu, Y.H.; Tang, J.Y.; Su, M.X.; Guo, Y.J.; Chen, Q.X.; Liu, B. Antityrosinase and antimicrobial activities of 2-phenylethanol, 2-phenylacetaldehyde and 2-phenylacetic acid. Food Chem. 2011, 124, 298–302. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Falke, D.; Heicke, B.; Zahn, R.K. Biological activity of 2-phenylethanol and its derivatives VIII. Influence on Herpes virus DNA-synthesis in rabbit kidney cells. Arch. Virol. 1973, 40, 205–214. [Google Scholar]
- Brossmer, R.; Bohn, B.; Schlicker, H. Influence of 2-phenylethanol and 1,1′-dimethylphenyl-ethanol on metabolic activity and cell membrane function in Ehrlich ascites tumour cells. FEBS Lett. 1973, 35, 191–194. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, innovation and green chemistry in the production and valorization of phenolic extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Cacchi, S.; Fabrizi, G.; Filisti, E. 2-Arylhydroxytyrosol derivatives via Suzuki-Miyaura cross-coupling. Org. Lett. 2008, 10, 3457–3460. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Crisante, F.; Merendino, N.; Molinari, R.; Soldatelli, M.C.; Velotti, F. Synthesis of a novel ester of hydroxytyrosol and alpha-lipoic acid exhibiting an antiproliferative effect on human colon cancer HT-29 cells. Eur. J. Med. Chem. 2011, 46, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Luzi, F.; Dugo, L.; Fanali, C.; Tripodo, G.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Hydroxytyrosol as active ingredient in poly(vinyl alcohol) films for food packaging applications. J. Renew. Mater. 2017, 5, 81–95. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Dugo, L.; Fanali, C.; Tripodo, G.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Effect of hydroxytyrosol methyl carbonate on the thermal, migration and antioxidant properties of PVA based films for active food packaging. Polym. Int. 2016, 65, 872–882. [Google Scholar] [CrossRef]
- Bernini, R.; Mincione, E.; Crisante, F.; Barontini, M.; Fabrizi, G.; Gentili, P. Chemoselective and efficient carboxymethylation of the alcoholic chain of phenols by dimethyl carbonate (DMC). Tetrahedron Lett. 2007, 48, 7000–7003. [Google Scholar] [CrossRef]
- Grasso, S.; Siracusa, L.; Spatafora, C.; Renis, M.; Tringali, C. Hydroxytyrosol lipophilic analogues: Enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorg. Chem. 2007, 35, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Leone, S.; Fiore, M.; Lauro, M.G.; Pino, S.; Cornetta, T.; Cozzi, R. Resveratrol and X rays affect gap junction intercellular communications in human glioblastoma cells. Mol. Carcinog. 2008, 47, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Mincione, E.; Barontini, M.; Crisante, F. Convenient synthesis of hydroxytyrosol and its lipophilic derivatives from tyrosol or homovanillyl alcohol. J. Agric. Food Chem. 2008, 56, 8897–8904. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.; Kuo, J.-H.; Pawar, V.D.; Munot, Y.S.; Weng, S.-S.; Ku, C.-H.; Liu, C.-Y. Nucleophilic acyl substitutions of anhydrides with protic nucleophiles catalyzed by amphoteric, oxomolybdenum species. J. Org. Chem. 2005, 70, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Visioli, F.; Buratti, S.; Brighenti, F. Direct analysis of total antioxidant activity of olive oil and studies on the influence of heating. J. Agric. Food Chem. 2001, 49, 2532–2538. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar]
- D´Arezzo, S.; Incerpi, S.; Davis, F.B.; Acconcia, F.; Marino, F.; Farías, R.N.; Davis, P.J. Rapid nongenomic effects of 3,5,3′-triiodo L-thyronine on the intracellular pH of L-6 myoblasts are mediated by intracellular calcium mobilization and kinase pathways. Endocrinology 2004, 145, 5694–5703. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, E.; Sabellico, C.; Hájek, J.; Staňková, V.; Filipský, T.; Balducci, V.; De Vito, P.; Leone, S.; Bavavea, E.I.; Proietti Silvestri, I.; et al. Protection of cells against oxidative stress by nanomolar levels of hydroxyflavones indicates a new type of intracellular antioxidant mechanism. PLoS ONE 2013, 8, e60796. [Google Scholar] [CrossRef] [PubMed]
- Narasimhulu, S. New cytochrome P450 mechanisms: Implications for understanding molecular basis for drug toxicity at the level of the cytochrome. Expert Opin. Drug Metab. Toxicol. 2010, 1–15. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 7–12 are available from the authors. |


| Compound | LogP ± SD a |
|---|---|
| 1 | 1.18 ± 0.08 |
| 2 | 0.72 ± 0.07 |
| 3 | 0.66 ± 0.06 |
| 4 | 0.69 ± 0.05 |
| 5 | 0.46 ± 0.02 |
| 6 | 0.10 ± 0.01 |
| 7 | 2.38 ± 0.18 |
| 8 | 2.05 ± 0.16 |
| 9 | 1.96 ± 0.14 |
| 10 | 1.98 ± 0.14 |
| 11 | 1.90 ± 0.12 |
| 12 | 1.62 ± 0.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernini, R.; Barontini, M.; Cis, V.; Carastro, I.; Tofani, D.; Chiodo, R.A.; Lupattelli, P.; Incerpi, S. Synthesis and Evaluation of the Antioxidant Activity of Lipophilic Phenethyl Trifluoroacetate Esters by In Vitro ABTS, DPPH and in Cell-Culture DCF Assays. Molecules 2018, 23, 208. https://doi.org/10.3390/molecules23010208
Bernini R, Barontini M, Cis V, Carastro I, Tofani D, Chiodo RA, Lupattelli P, Incerpi S. Synthesis and Evaluation of the Antioxidant Activity of Lipophilic Phenethyl Trifluoroacetate Esters by In Vitro ABTS, DPPH and in Cell-Culture DCF Assays. Molecules. 2018; 23(1):208. https://doi.org/10.3390/molecules23010208
Chicago/Turabian StyleBernini, Roberta, Maurizio Barontini, Valentina Cis, Isabella Carastro, Daniela Tofani, Rosa Anna Chiodo, Paolo Lupattelli, and Sandra Incerpi. 2018. "Synthesis and Evaluation of the Antioxidant Activity of Lipophilic Phenethyl Trifluoroacetate Esters by In Vitro ABTS, DPPH and in Cell-Culture DCF Assays" Molecules 23, no. 1: 208. https://doi.org/10.3390/molecules23010208
APA StyleBernini, R., Barontini, M., Cis, V., Carastro, I., Tofani, D., Chiodo, R. A., Lupattelli, P., & Incerpi, S. (2018). Synthesis and Evaluation of the Antioxidant Activity of Lipophilic Phenethyl Trifluoroacetate Esters by In Vitro ABTS, DPPH and in Cell-Culture DCF Assays. Molecules, 23(1), 208. https://doi.org/10.3390/molecules23010208