The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Results
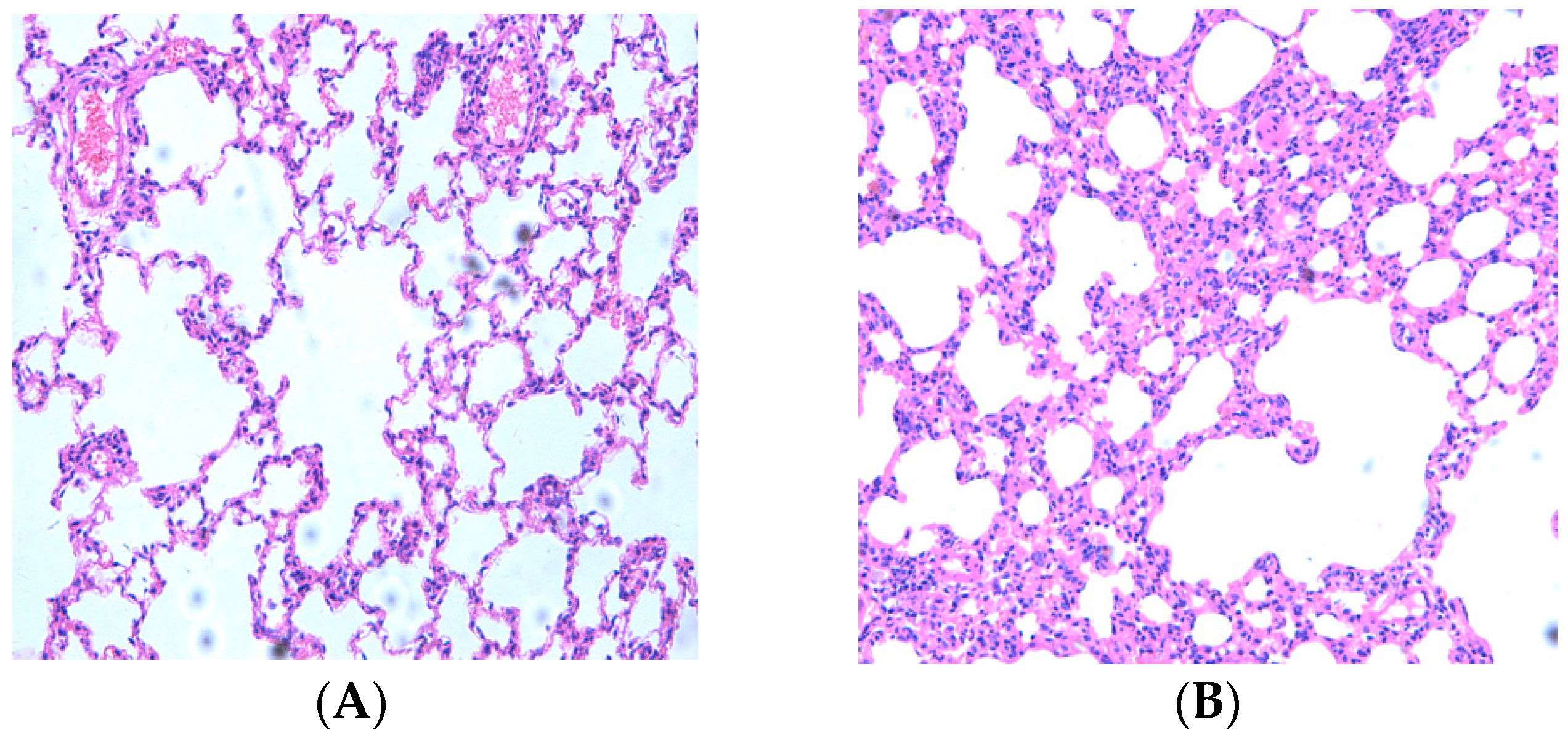
2.1. Resveratrol Reduced Lung Inflammatory Response
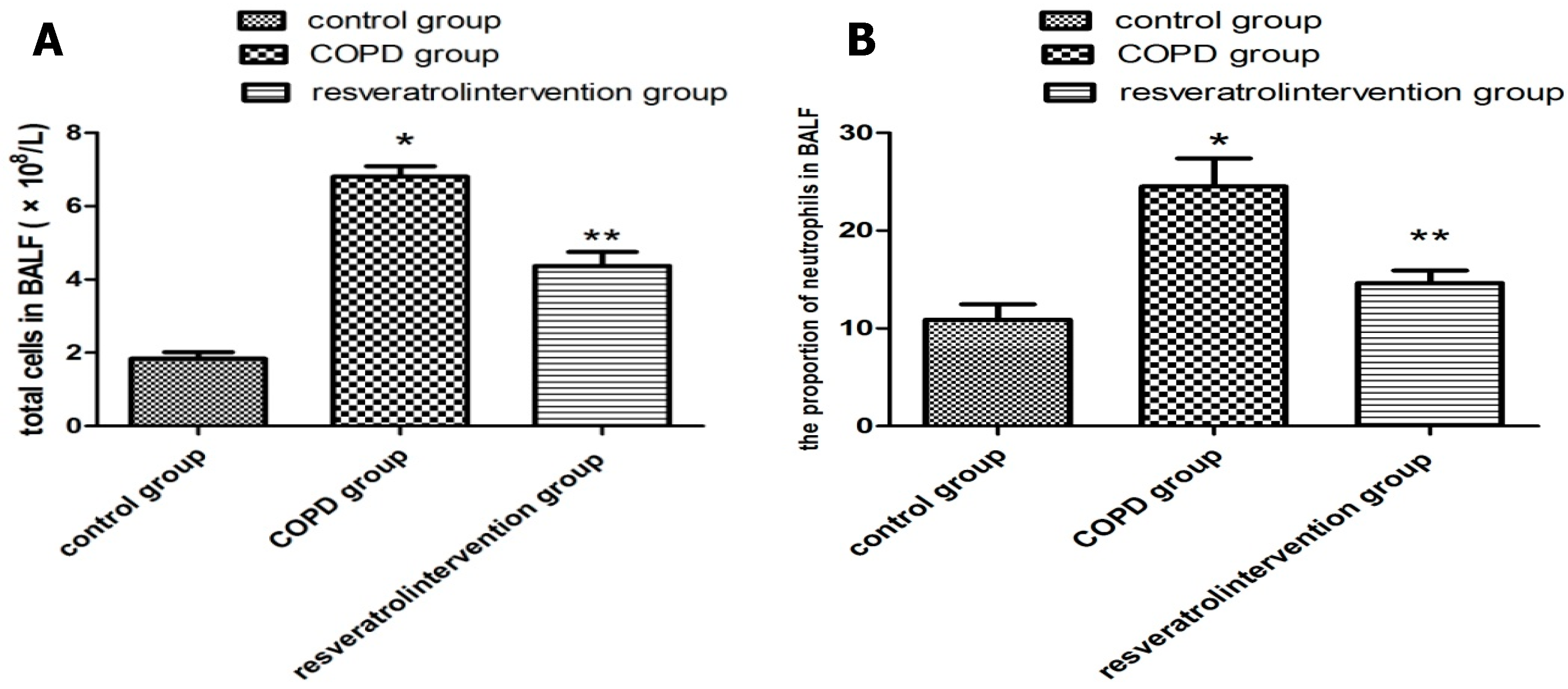
2.2. Resveratrol Decreased the Number of Inflammatory Cells inBronchial Alveolar Lavage Fluid (BALF)
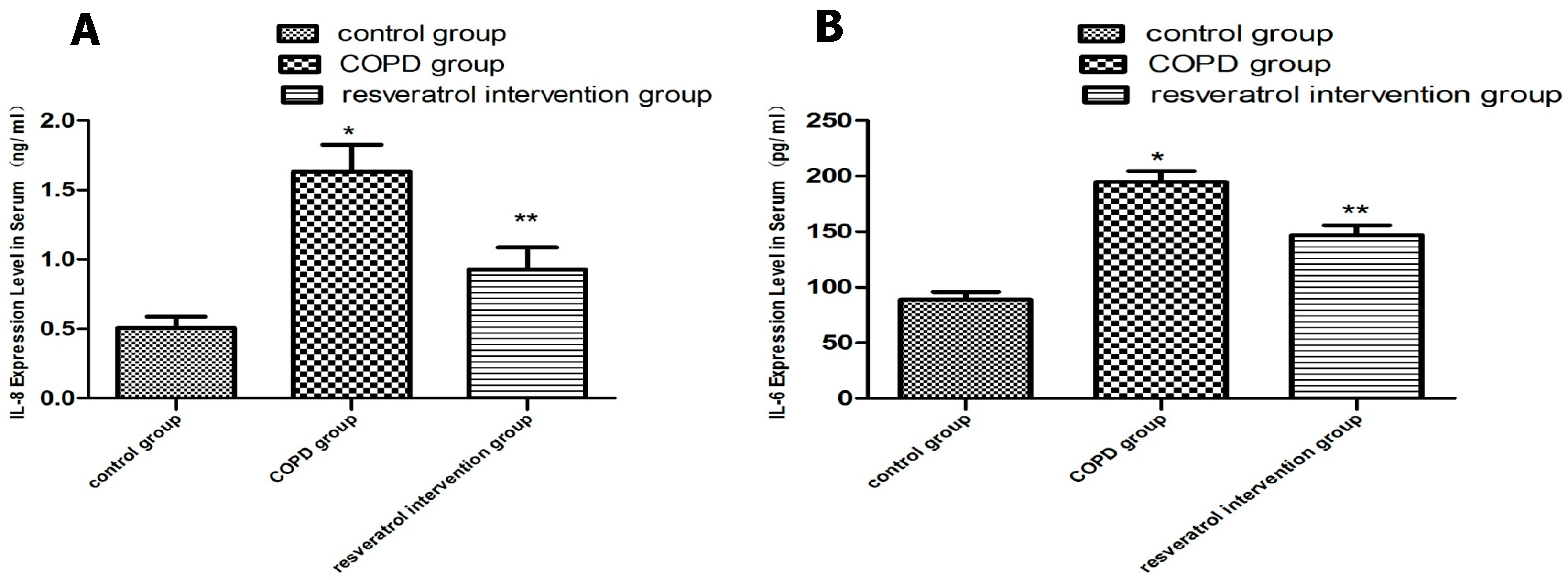
2.3. Resveratrol Decreased the Expression of IL-6 and IL-8 in Serums
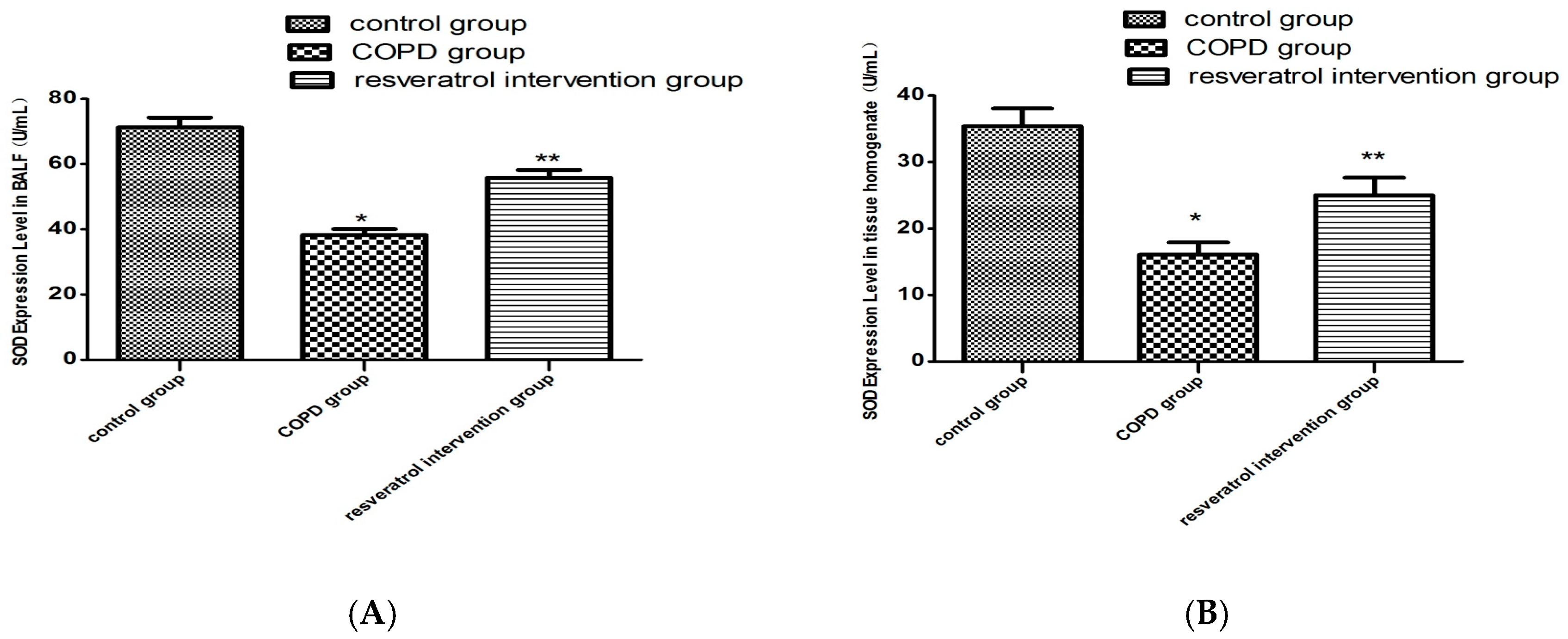
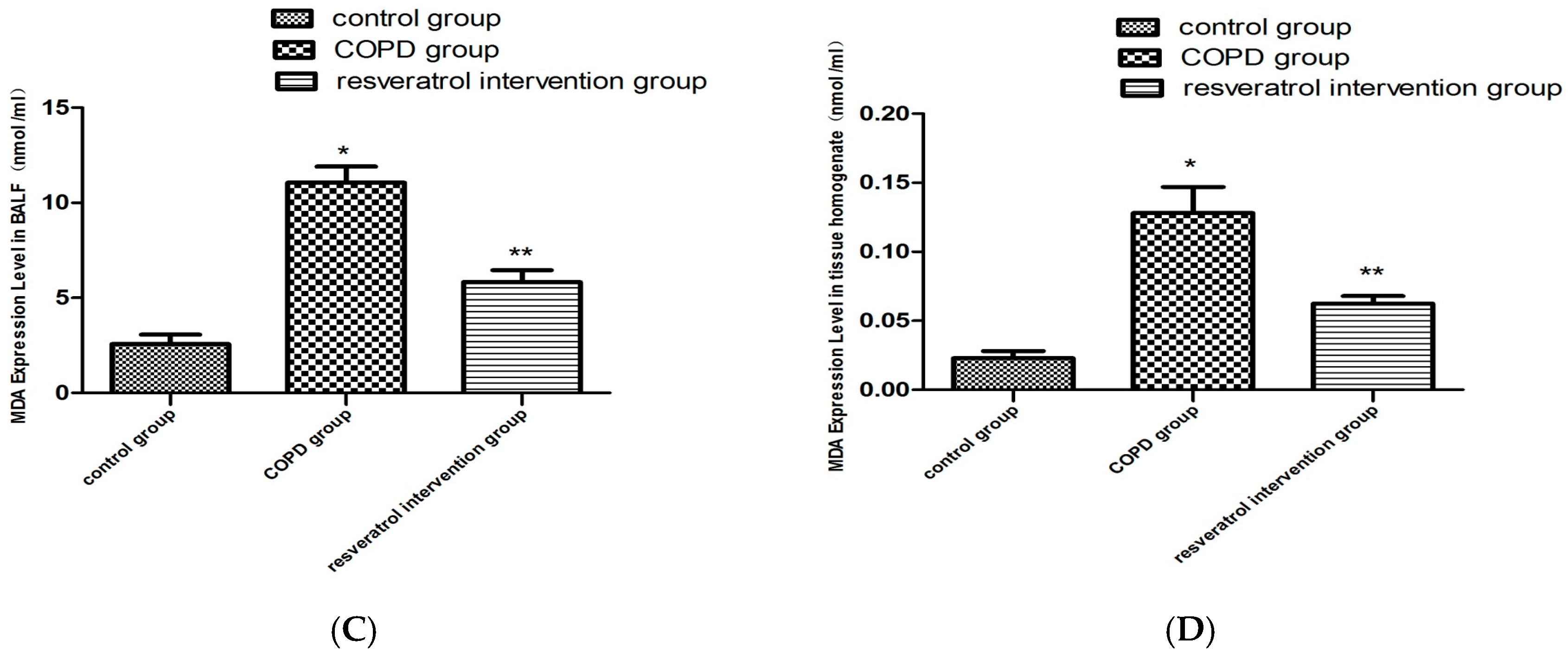
2.4. Resveratrol Decreased the Activity of MDA and Increased the Activity of SOD
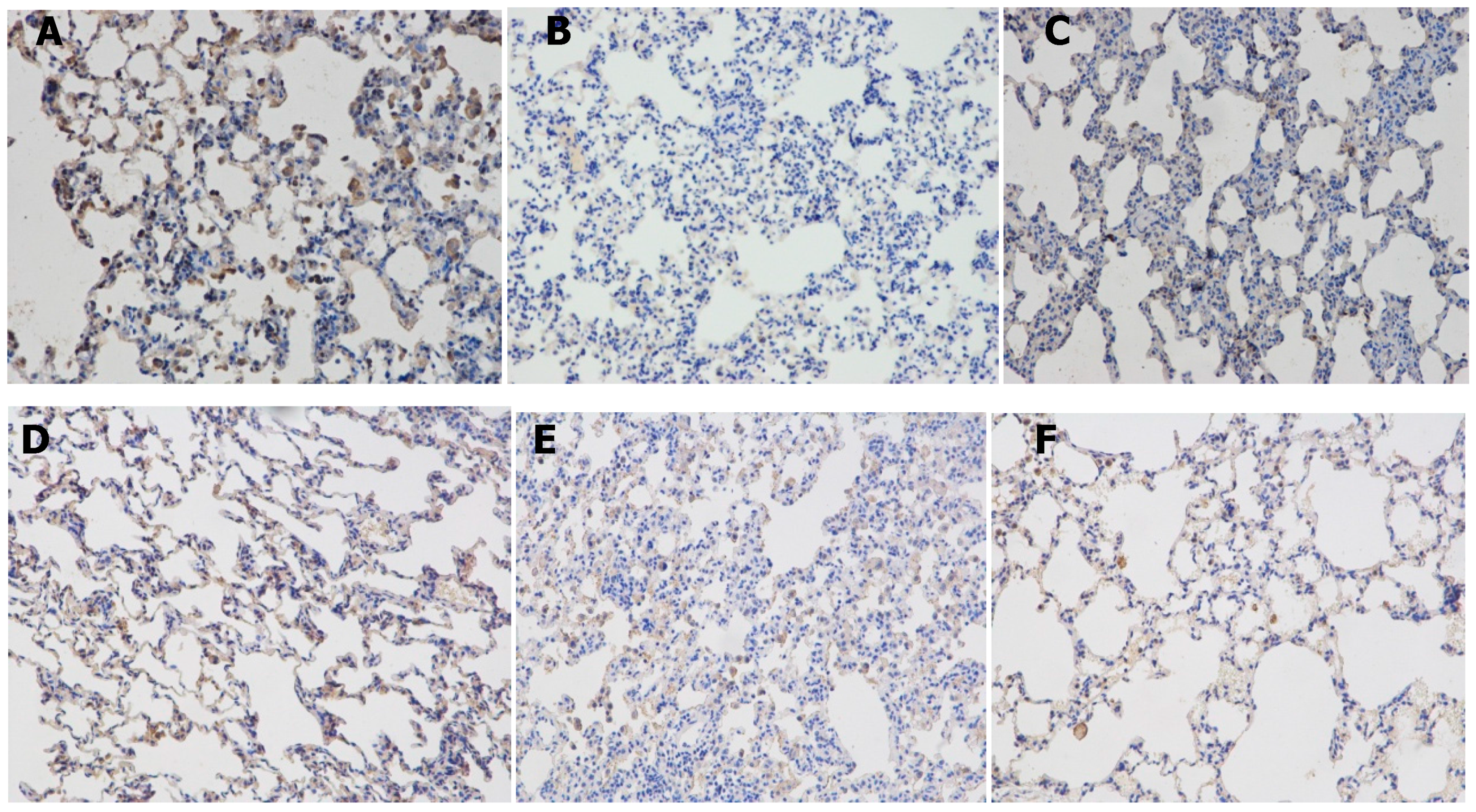
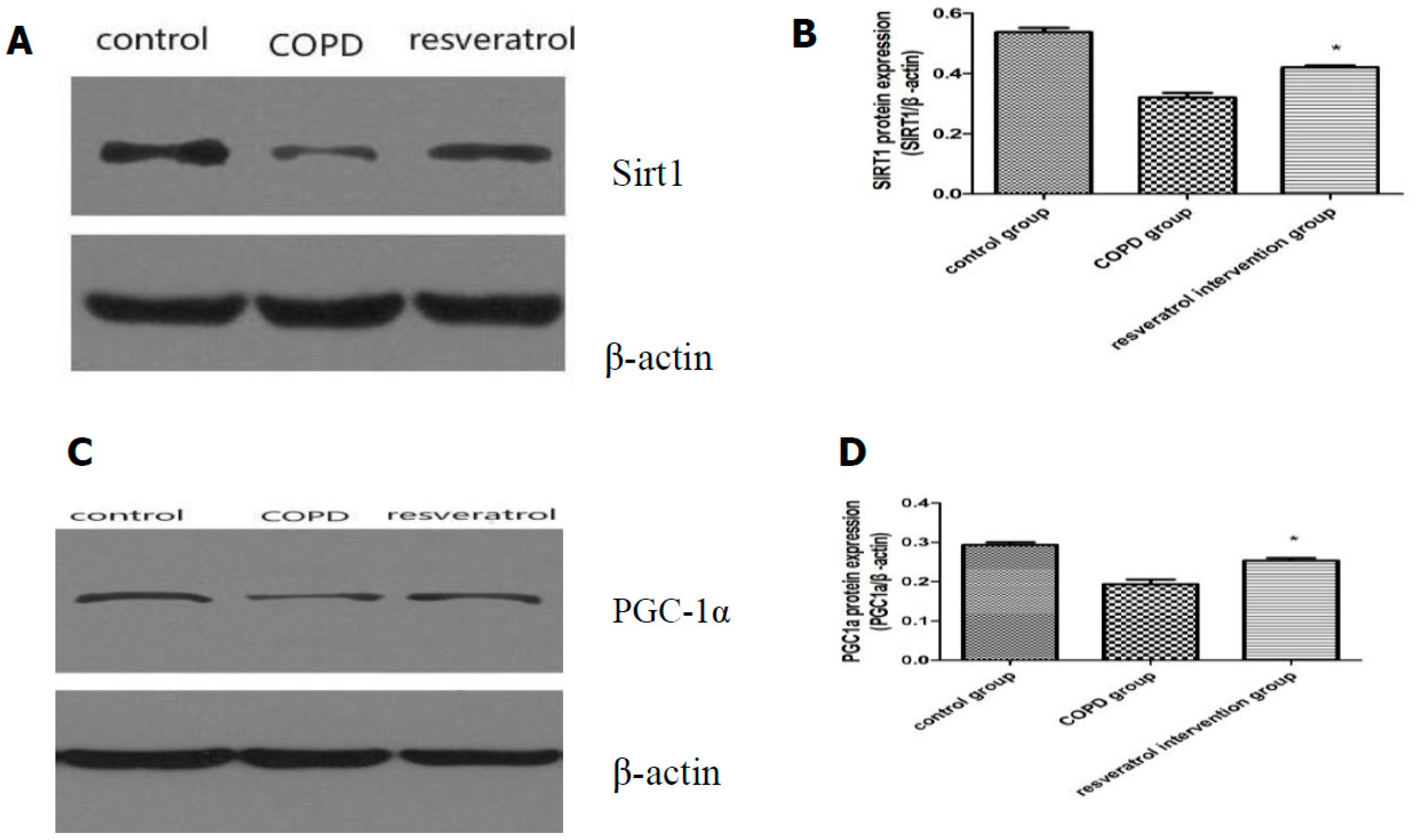
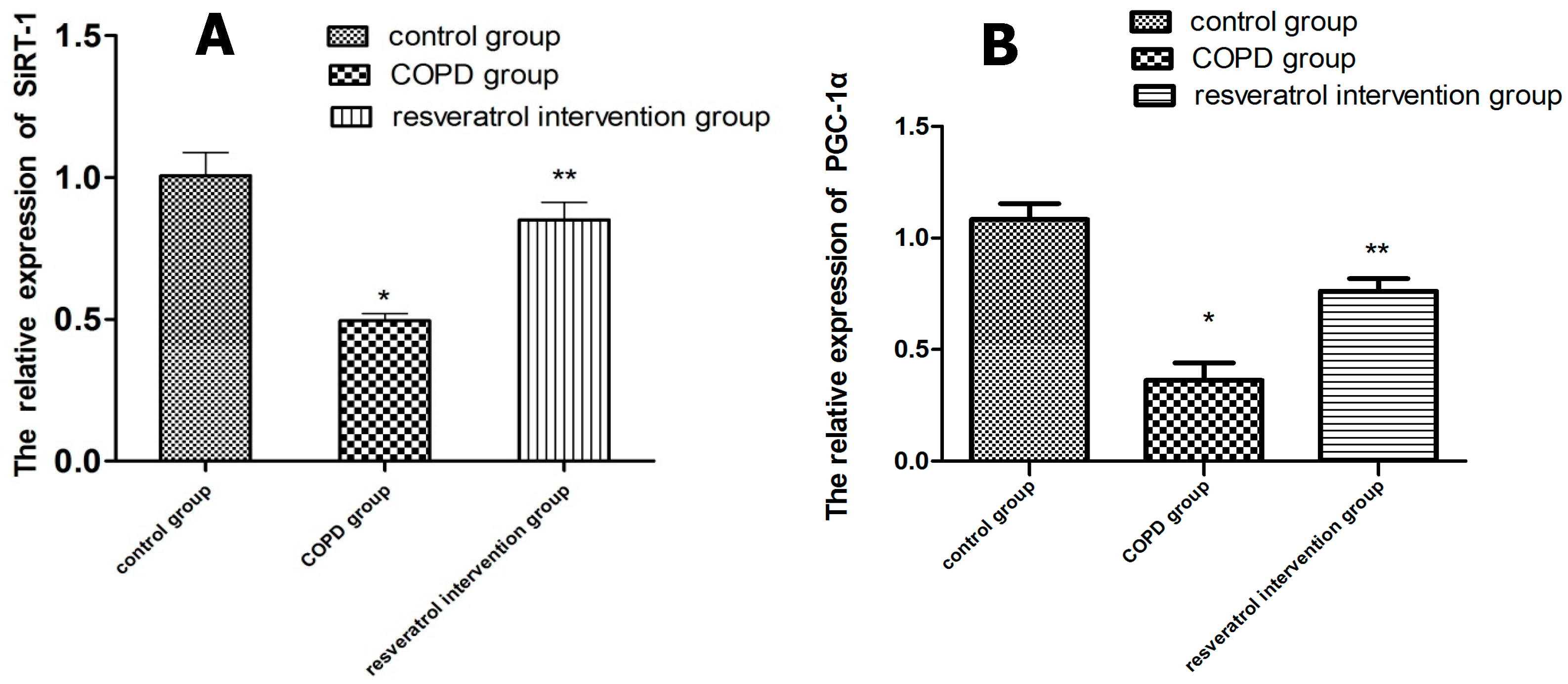
2.5. Effects of Resveratrolon the Expression of SIRT1 and PGC-1α in Lung Tissues
3. Discussion
4. Materials and Methods
4.1. Animals and Cigarette Smoke Exposure
4.2. Lung Histological Examination
4.3. Serum Collection
4.4. Preparation of BALF and Tissue Processing
4.5. Determination of Levels of Inflammation
4.6. Determination of Levels of Oxidative Stress
4.7. Immunohistochemical
4.8. Real-Time PCR
4.9. Western Blotting of SIRT1 and PGC-1α
4.10. Statistical Analysis
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Shergis, J.L.; Di, Y.M.; Zhang, A.L.; Vlahos, R.; Helliwell, R.; Ye, J.M.; Xue, C.C. Therapeutic potential of Panax ginsengand ginsenosides in the treatment of chronic obstructive pulmonary disease. Complement. Ther. Med. 2014, 22, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.M.; Pavlisko, E.; Voynow, J.A. Pathogenic triad in COPD: Oxidativestress, protease–antiprotease imbalance, and inflammation. Int. J. Chron. Obstruct. Pulmon. Dis. 2011, 6, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.; Han, G.; Li, D.; Tschernig, T.; Dinh, Q.T.; Beißwenger, C.; Bals, R. Combined exposure to bacteria and cigarette smoke resembles characteristic phenotypes of human COPD in a murine disease model. Exp. Toxicol. Pathol. 2015, 67, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, H.; Wang, J.; Zhang, B.; Liu, F.; Huang, J.; Li, J.; Lin, J.; Bai, J.; Liu, R. Therapeutic effects of resveratrol in a mouse model of HDM-induced allergic asthma. Int. Immunopharmacol. 2015, 25, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Milevoj, K.L.; Domijan, A.M.; Posavac, K.; Čepelak, I.; Žanić, G.T.; Rumora, L. Systemic redox imbalance in stable chronic obstructive pulmonary disease. Biomarkers 2016, 28, 1–7. [Google Scholar]
- Rahman, I.; Kinnula, V.L.; Gorbunova, V.; Yao, H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev. Med. 2012, 54, S20–S28. [Google Scholar] [CrossRef] [PubMed]
- Lavu, S.; Boss, O.; Elliott, P.J.; Lambert, P.D. Sirtuins—Novel therapeutic targets to treat age-associated diseases. Nat. Rev. Drug Discov. 2008, 7, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Said, R.S.; E-Demerdash, E.; Nada, A.S.; Kamal, M.M. Resveratrol inhibits inflammatory signaling implicated in ionizing radiation-induced premature ovarian failure through antagonistic crosstalk between silencing information regulator 1 (SIRT1) and poly (ADP-ribose) polymerase 1 (PARP-1). Biochem. Pharmacol. 2016, 103, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, J.; Wahl, C.; Feldmann, M.; Jungck, D.; Strauch, J.; Stoelben, E.; Koch, A. Resveratrol attenuates the release of inflammatory cytokines from human bronchial smooth muscle cells exposed to lipoteichoic acid in chronic obstructive pulmonary disease. Basic Clin. Pharmacol. Toxicol. 2014, 114, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Petrache, I. Pathogenesis of chronic obstructive pulmonary disease. J. Clin. Investig. 2012, 122, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Wark, P.A.; Garg, M.L. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid. Redox Signal. 2010, 13, 1535–1548. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, J.; Sibbing, B.; Jungck, D.; Lin, Y.; Urban, K.; Stoelben, E.; Strauch, J.; Koch, A. Resveratrol impairs the release of steroid-resistant inflammatory cytokines from human airway smooth muscle cells in chronic obstructive pulmonary disease. J. Pharmacol. Exp. Ther. 2010, 335, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Antus, B.; Harnasi, G.; Drozdovszky, O.; Barta, I. Monitoring oxidative stress during chronic obstructive pulmonary disease exacerbations using malondialdehyde. Respirology 2014, 19, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.X.; Cui, H.; Fan, L.; Pan, X.J.; Wu, J.H.; Shi, S.Z.; Cui, S.Y.; Wei, Z.M.; Liu, L. Resveratrol attenuates left ventricular remodeling in old rats with COPD induced by cigarette smoke exposure and LPS instillation. Can. J. Physiol. Pharmacol. 2013, 91, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Shang, Y.C.; Wang, S.; Maiese, K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert. Opin. Ther. Targets 2012, 16, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.P.; Odewale, I.; Alcendor, R.R.; Sadoshima, J. SIRT1 protects the heart from aging and stress. Biol. Chem. 2008, 389, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Fusco, S.; Maulucci, G.; Pani, G. Sirt1: Def-eatingsenescence? Cell Cycle 2012, 11, 4135–4146. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, C.; Wang, F.; Wang, H. SIRT1 negatively regulates amyloid-beta-induced inflammation via the NF-κB pathway. Braz. J. Med. Biol. Res. 2013, 46, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, S.; Fergusson, M.M.; Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 2005, 280, 16456–16460. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, N.F.; Rodrigues-Junior, V.; Santos, A.A., Jr.; Leite, C.E.; Dias, A.C.; Batista, E.L., Jr.; Basso, L.A.; Campos, M.M.; Santos, D.S.; Souto, A.A. Protective effects of resveratrol on hepatotoxicity induced by isoniazid and rifampicin via SIRT1 modulation. J. Nat. Prod. 2014, 77, 2190–2195. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jager, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Lin, T.K.; Yang, D.I.; Lee, S.Y.; Shaw, F.Z.; Liou, C.W.; Chuang, Y.C. Protective effects of peroxisome proliferator-activated receptors gamma coactivator-1α against neuronal cell death in the hippocampal CA1 subfield after transient global ischemia. J. Neurosci. Res. 2010, 88, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Yang, D.I.; Lin, T.K.; Shaw, F.Z.; Liou, C.W.; Chuang, Y.C. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesisin cerebral ischemia. Int. J. Mol. Sci. 2011, 12, 7199–7215. [Google Scholar] [CrossRef] [PubMed]
- Vladimir, L.; Matthew, B.; John, A.L.; Bernard, J.J. Resveratrol induces expression of the slow, oxidative phenotype in mdxmouse muscle together with enhanced activity of the SIRT1-PGC-1α axis. Am. J. Physiol. Cell Physiol. 2014, 307, 66–82. [Google Scholar]
- Zuo, L.; Lucas, K.; Fortuna, C.A.; Chuang, C.C.; Best, T.M. Molecular Regulation of Toll-like Receptors in Asthma and COPD. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Sadarani, B.N.; Majumdar, A.S. Resveratrol potentiates the effect of examethasone in rat model of acute lung inflammation. Int. Immunopharmacol. 2015, 28, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.C.; Wu, H.; Li, P.B.; Luo, Y.L.; Zhang, C.C.; Shen, J.G.; Su, W.W. Characteristic comparison of three ratmodels induced by cigarette smoke or combined with LPS: To establish a suitable model for study of airway mucus hypersecretion in chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2012, 25, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xie, J.; Xu, S.; Lv, H.; Lin, M.; Yuan, S.; Bai, J.; Hou, Q.; Yu, S. Novel nitric oxide-releasing derivatives of brusatol as antiinflammatory agents: Design, synthesis, biological evaluation, and nitric oxide release studies. J. Med. Chem. 2014, 57, 7600–7612. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, A.A.; Nunes, F.B.; Lunardelli, A.; Pauli, V.; Amaral, R.H.; de Oliveira, L.M.; Saciura, V.C.; da Silva, G.L.; Simoes Pires, M.G.; Fagundes Donadio, M.V.; et al. Treatment with N-methyl-D-aspartate receptor antagonist (MK-801) protects against oxidative stress in lipopolysaccharide-induced acute lung injury in the rat. Int. Immunopharmacol. 2011, 11, 706–711. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-L.; Li, T.; Li, J.-H.; Miao, S.-Y.; Xiao, X.-Z. The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease. Molecules 2017, 22, 1529. https://doi.org/10.3390/molecules22091529
Wang X-L, Li T, Li J-H, Miao S-Y, Xiao X-Z. The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease. Molecules. 2017; 22(9):1529. https://doi.org/10.3390/molecules22091529
Chicago/Turabian StyleWang, Xiao-Li, Ting Li, Ji-Hong Li, Shu-Ying Miao, and Xian-Zhong Xiao. 2017. "The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease" Molecules 22, no. 9: 1529. https://doi.org/10.3390/molecules22091529
APA StyleWang, X.-L., Li, T., Li, J.-H., Miao, S.-Y., & Xiao, X.-Z. (2017). The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease. Molecules, 22(9), 1529. https://doi.org/10.3390/molecules22091529




