Skin Penetration Enhancement by Natural Oils for Dihydroquercetin Delivery
Abstract
1. Introduction
2. Results
2.1. Fatty Acid Composition Analysis of Natural Oils
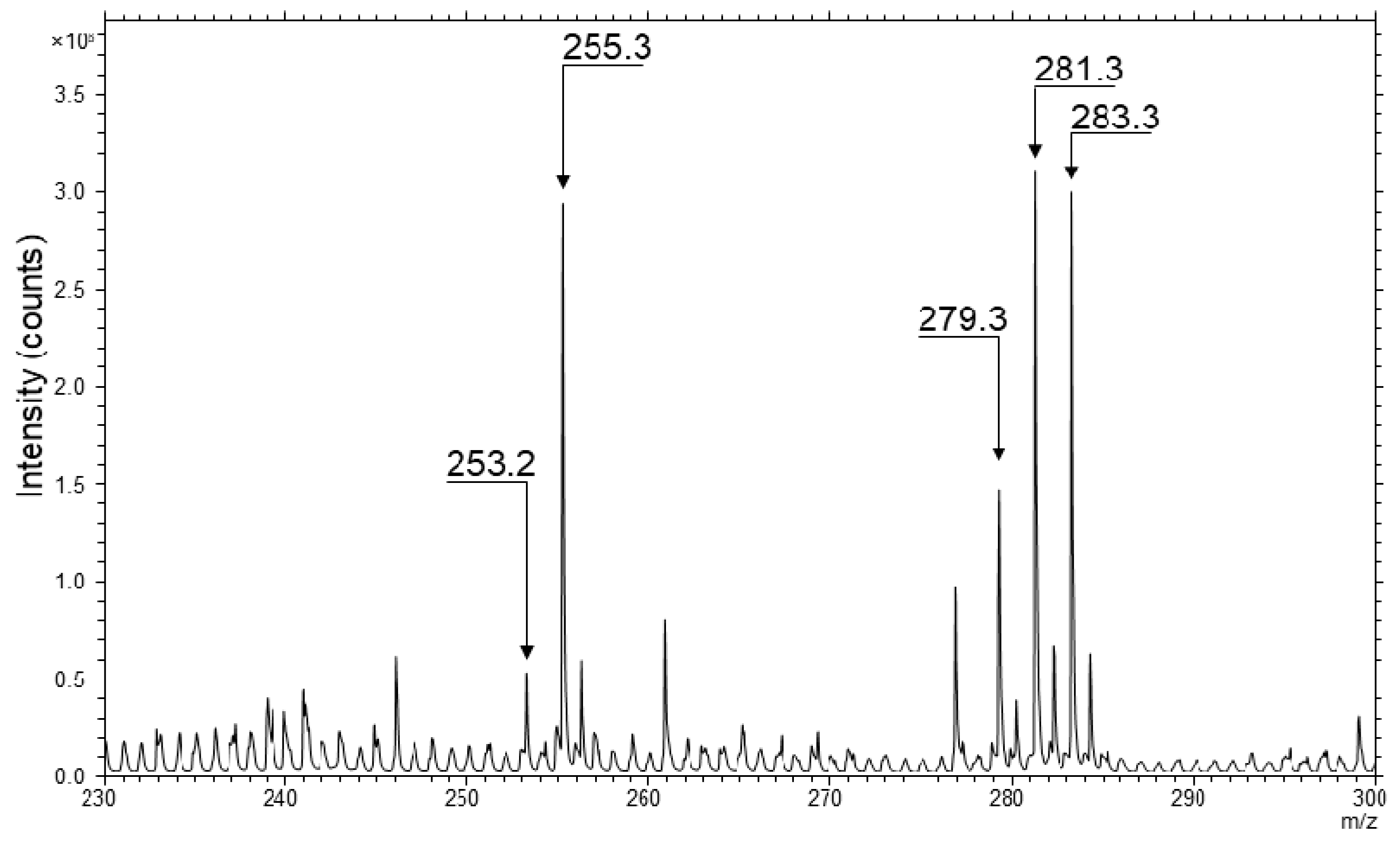
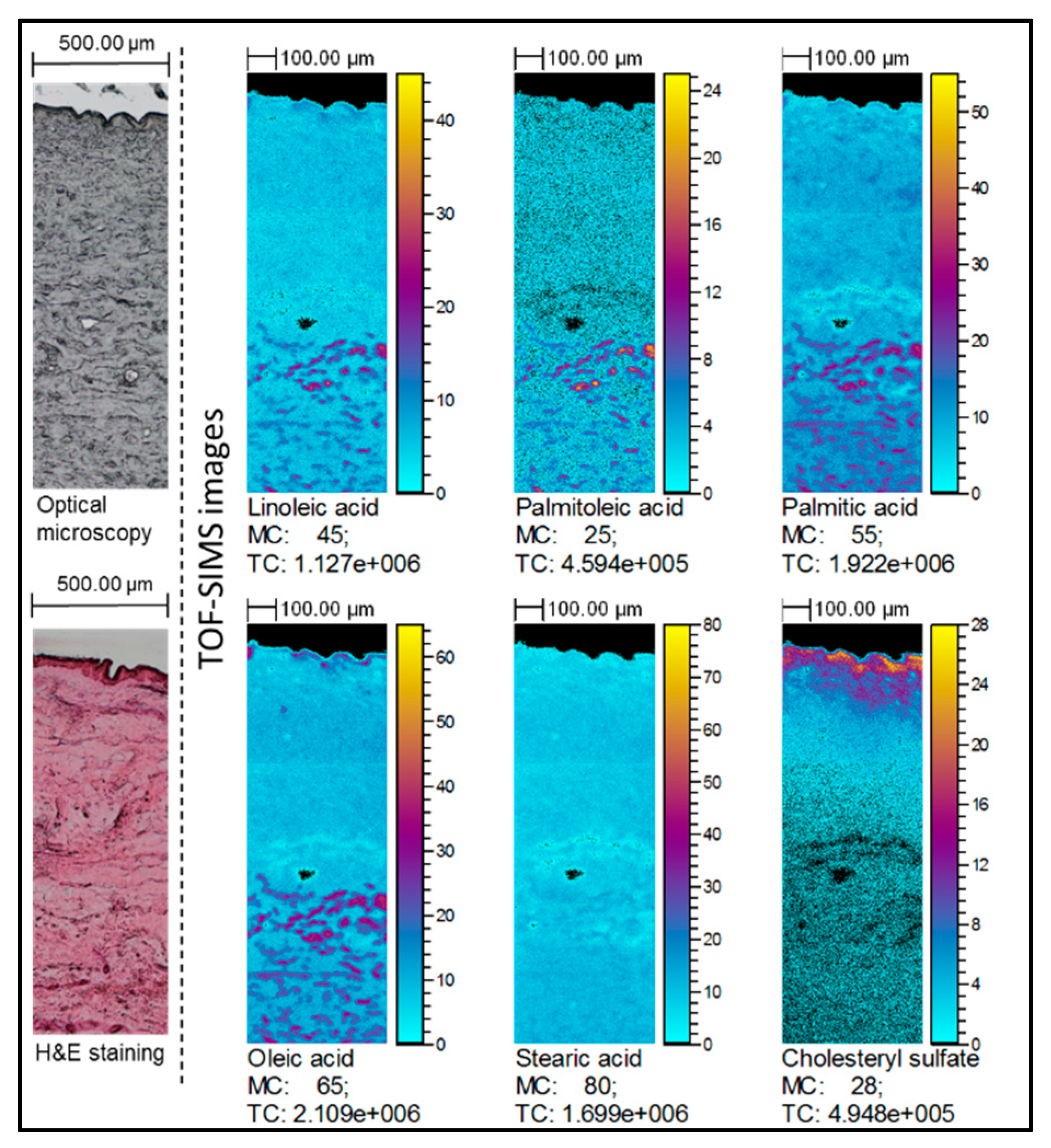
2.2. TOF-SIMS Imaging of Skin Samples Treated with Natural Oils
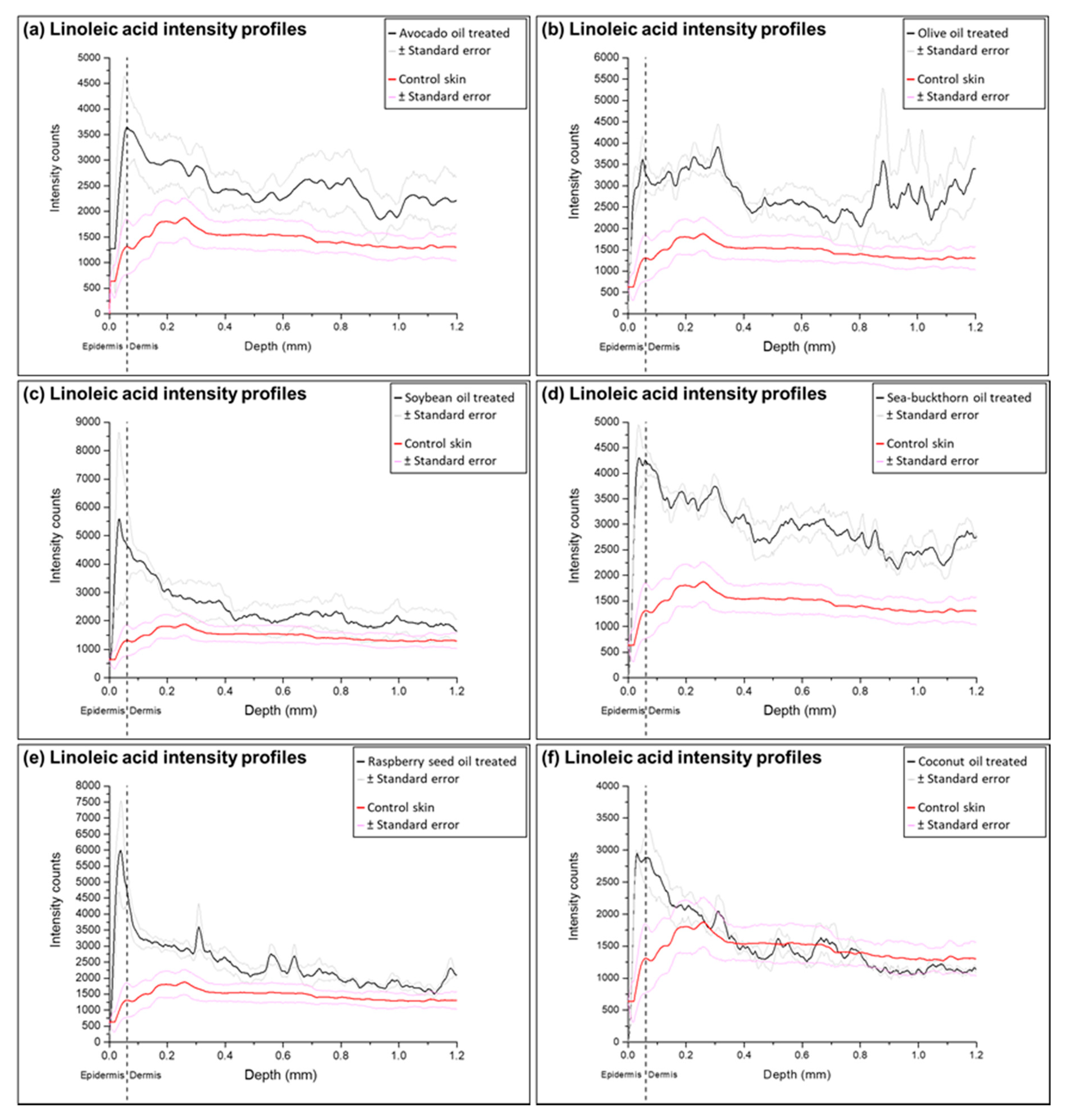
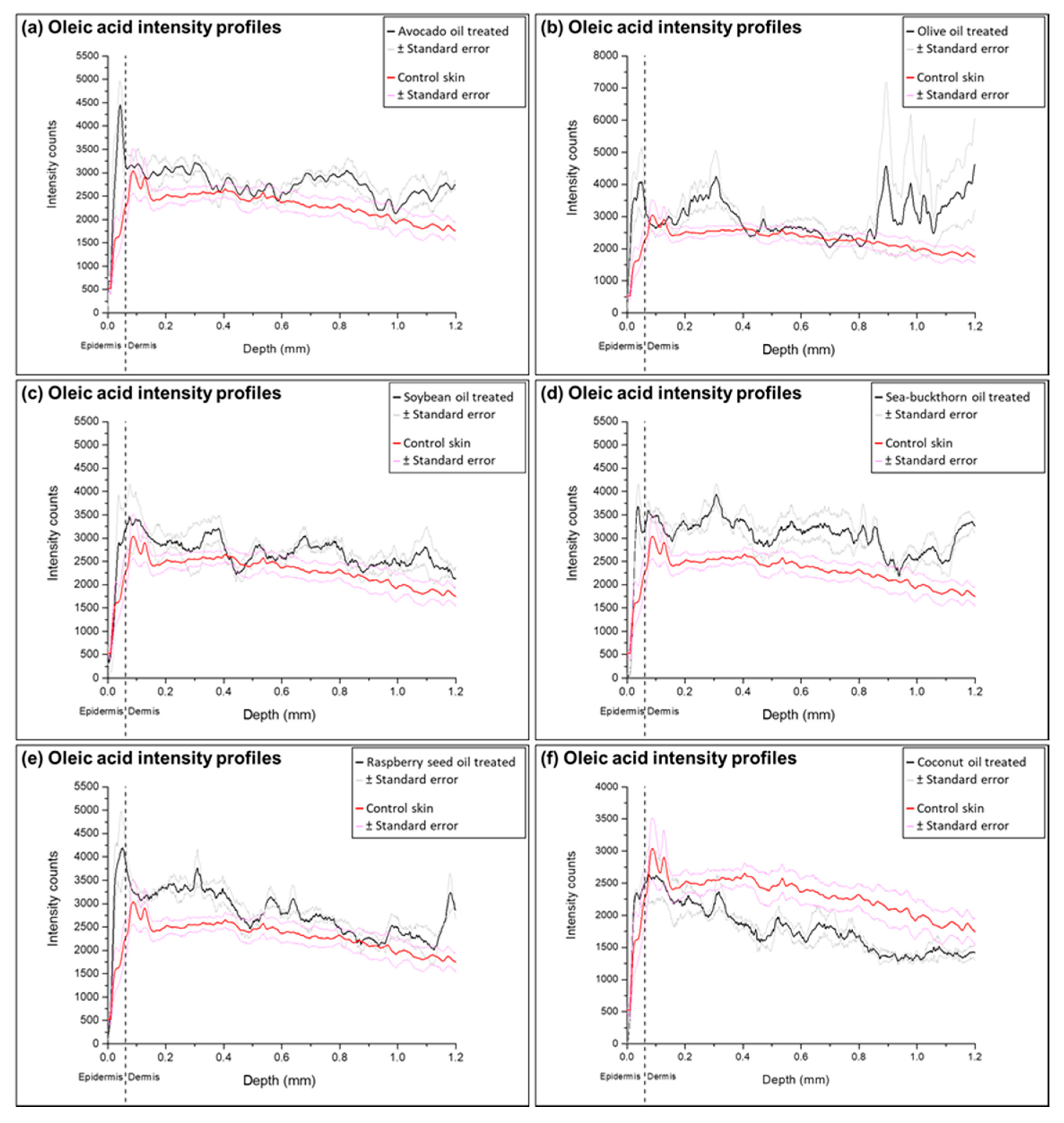
2.3. Analysis of Ion Intensity Profiles of the Samples Treated with Natural Oils
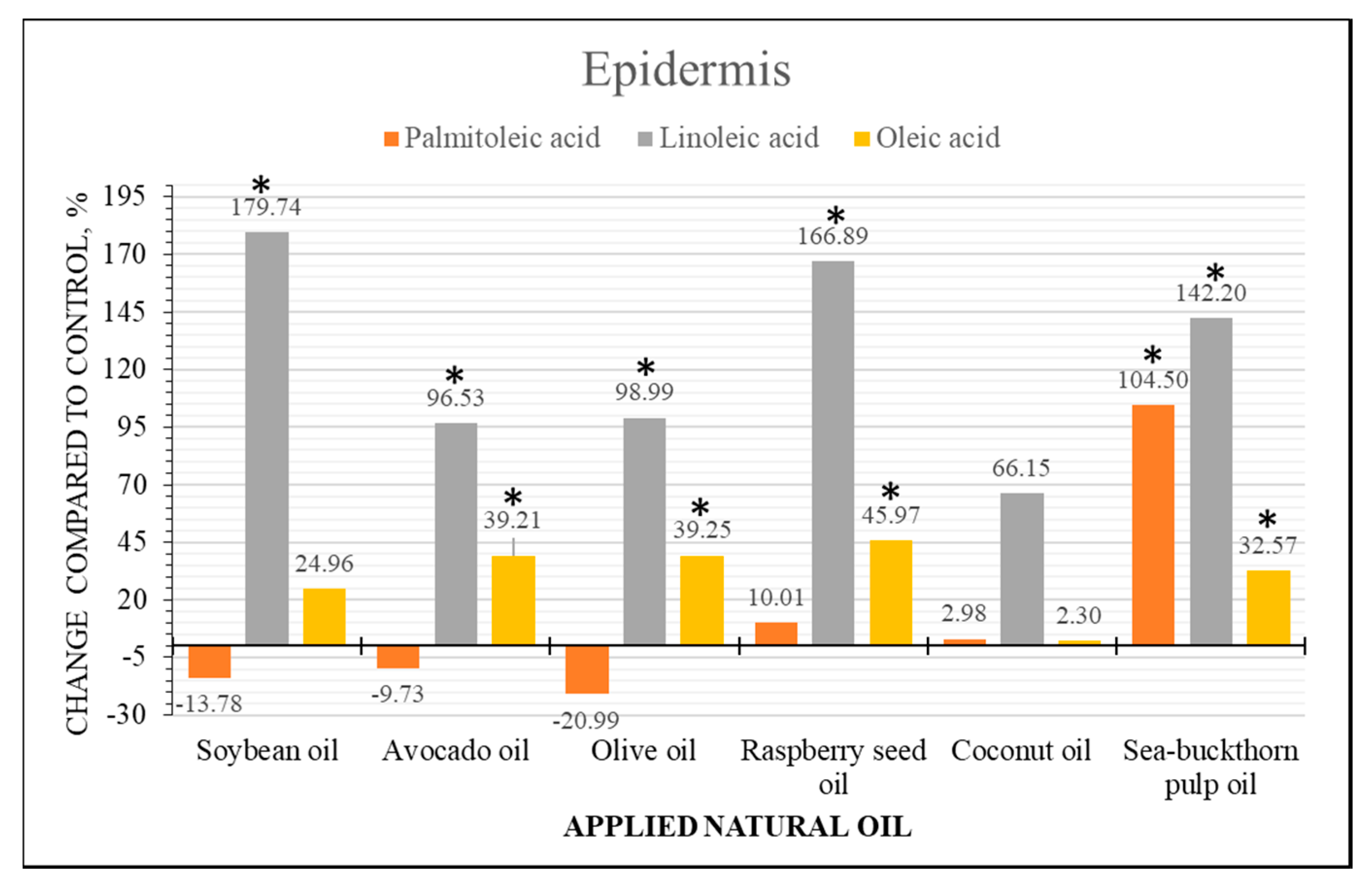
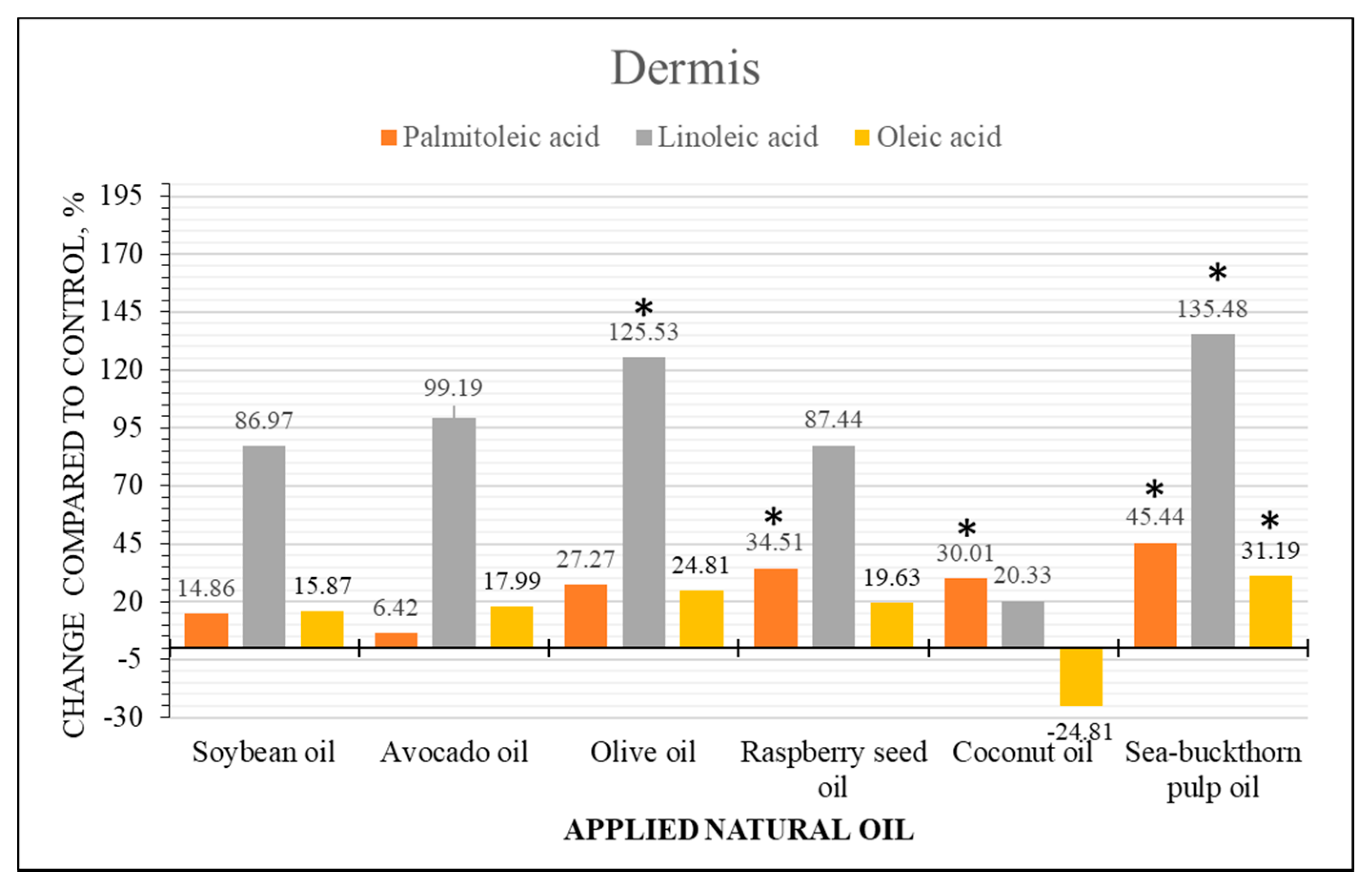
2.4. Semi-Quantitative Analysis of Fatty Acid Content Changes
2.5. Effect of Natural Oils on Dihydroquercetin Skin Distribution
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Analysis of Fatty Acid Composition in Natural Oils
4.3. Human Skin Distribution Experiments
4.4. Quantitative Analysis of Dihydroquercetin in the Skin Samples
4.5. Fatty Acids Distribution Analysis in the Skin Samples
4.6. Statistical Analysis of Data
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fox, L.T.; Gerber, M.; Du Plessis, J.; Hamman, J.H. Transdermal drug delivery enhancement by compounds of natural origin. Molecules 2011, 16, 10507–10540. [Google Scholar] [CrossRef]
- Manconi, M.; Marongiu, F.; Manca, M.L.; Caddeo, C.; Sarais, G.; Cencetti, C.; Pucci, L.; Longo, V.; Bacchetta, G.; Fadda, A.M. Nanoincorporation of bioactive compounds from red grape pomaces: In vitro and ex vivo evaluation of antioxidant activity. Int. J. Pharm. 2017, 523, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, J.M.; Cowley, A.; du Preez, J.; Gerber, M.; du Plessis, J. Penetration enhancing effects of selected natural oils utilized in topical dosage forms. Drug Dev. Ind. Pharm. 2015, 41, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Gunstone, F.D. Vegetable Oils in Food Technology: Composition, Properties and Uses; Blackwell Publishing Ltd.: Oxford, UK, 2002; ISBN 9781444339925. [Google Scholar]
- Barry, B.W. Mode of action of penetration enhancers in human skin. J. Control Release 1987, 6, 85–97. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Hussain, A.; Khan, G.M.; Jan, S.U.; Shah, S.U.; Shah, K.; Akhlaq, M.; Rahim, N.; Nawaz, A.; Wahab, A. Effect of olive oil on transdermal penetration of flurbiprofen from topical gel as enhancer. Pak. J. Pharm. Sci. 2012, 25, 365–369. [Google Scholar] [PubMed]
- Patzelt, A.; Lademann, J.; Richter, H.; Darvin, M.E.; Schanzer, S.; Thiede, G.; Sterry, W.; Vergou, T.; Hauser, M. In vivo investigations on the penetration of various oils and their influence on the skin barrier. Ski. Res. Technol. 2012, 18, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Songkro, S. An overview of skin penetration enhancers: Penetration enhancing activity, skin irritation potential and mechanism of action. Songklanakarin J. Sci. Technol. 2009, 31, 299–321. [Google Scholar]
- Leefmann, T.; Heim, C.; Siljeström, S.; Blumenberg, M.; Sjövall, P.; Thiel, V. Spectral characterization of ten cyclic lipids using time-of-flight secondary ion mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Leefmann, T.; Heim, C.; Kryvenda, A.; Siljeström, S.; Sjövall, P.; Thiel, V. Biomarker imaging of single diatom cells in a microbial mat using time-of-flight secondary ion mass spectrometry (ToF-SIMS). Org. Geochem. 2013, 57, 23–33. [Google Scholar] [CrossRef]
- Lee, P.-L.; Chen, B.-C.; Gollavelli, G.; Shen, S.-Y.; Yin, Y.-S.; Lei, S.-L.; Jhang, C.-L.; Lee, W.-R.; Ling, Y.-C. Development and validation of TOF-SIMS and CLSM imaging method for cytotoxicity study of ZnO nanoparticles in HaCaT cells. J. Hazard. Mater. 2014, 277, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.L.; Klitzing, H.A. Imaging lipids with secondary ion mass spectrometry. Biochim. Biophys. Acta 2014, 1841, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Debois, D.; Bralet, M.; Naour, L.; Brunelle, A.; Lapre, O. In situ lipidomic analysis of nonalcoholic fatty liver by cluster TOF-SIMS imaging. Anal. Chem. 2009, 81, 2823–2831. [Google Scholar] [CrossRef] [PubMed]
- Bich, C.; Touboul, D.; Brunelle, A. Study of experimental variability in TOF-SIMS mass spectrometry imaging of biological samples. Int. J. Mass Spectrom. 2013, 337, 43–49. [Google Scholar] [CrossRef]
- Kadar, H.; Pham, H.; Touboul, D.; Brunelle, A.; Baud, O. Impact of inhaled nitric oxide on the sulfatide profile of neonatal rat brain studied by TOF-SIMS imaging. Int. J. Mol. Sci. 2014, 15, 5233–5245. [Google Scholar] [CrossRef] [PubMed]
- Čižinauskas, V.; Elie, N.; Brunelle, A.; Briedis, V. Fatty acids penetration into human skin ex vivo : A TOF-SIMS analysis approach. Biointerphases 2017, 12, 11003. [Google Scholar] [CrossRef] [PubMed]
- Kezutyte, T.; Desbenoit, N.; Brunelle, A.; Briedis, V. Studying the penetration of fatty acids into human skin by ex vivo TOF-SIMS imaging. Biointerphases 2013, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Park, N.H.; Kim, J.W.; Um, B.H.; Shpatov, A.V.; Shults, E.E.; Sorokina, I.V.; Popov, S.A. Study of skin anti-ageing and anti-inflammatory effects of dihydroquercetin, natural triterpenoinds, and their synthetic derivatives. Russ. J. Bioorganic Chem. 2012, 38, 328–334. [Google Scholar] [CrossRef]
- Wu, C.; Feng, D.; Ma, H.; Xie, H.; Wang, H.; Wang, J. Effect of Pinus massoniana bark extract on IFN-γ-induced ICAM-1 expression in HaCaT human keratinocytes. J. Ethnopharmacol. 2009, 122, 48–53. [Google Scholar] [CrossRef] [PubMed]
- An, S.M.; Kim, H.J.; Kim, J.-E.; Boo, Y.C. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phyther. Res. 2008, 22, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Pantouris, G.; Mowat, C.G. Antitumour agents as inhibitors of tryptophan 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2014, 443, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Manigandan, K.; Jayaraj, R.L.; Jagatheesh, K.; Elangovan, N. Taxifolin mitigates oxidative DNA damage in vitro and protects zebrafish (Danio rerio) embryos against cadmium toxicity. Environ. Toxicol. Pharmacol. 2015, 39, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Oi, N.; Chen, H.; Kim, M.O.; Lubet, R.A.; Bode, A.M.; Dong, Z. Taxifolin suppresses UV-induced skin carcinogenesis by targeting EGFR and PI3K. Cancer Prev. Res. 2012, 5, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, O.S.; Ha, S.; Kim, J.H.; Park, G.; Kim, J.K.; Oh, C.H. In vivo assessment of the effect of taxifolin glycoside on atopic dermatitis-like skin lesions using biomedical tools in NC/Nga mice. Clin. Exp. Dermatol. 2015, 40, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Shubina, V.S.; Shatalin, Y.V. Skin regeneration after chemical burn under the effect of taxifolin-based preparations. Bull. Exp. Biol. Med. 2012, 154, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Shubina, V.S.; Shatalin, Y.V. Effect of the liposomal form of flavonoid-metal complexes on skin regeneration after chemical burn. Tsitologiya 2012, 54, 251–260. [Google Scholar] [CrossRef]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Barry, B.W. Action of skin penetration enhancers-the lipid protein partitioning theory. Int. J. Cosmet. Sci. 1988, 10, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Tanojo, H.; Bouwstra, J.A.; Junginger, H.E.; Bodde, H.E. In vitro human skin barrier modulation by fatty acids: skin permeation and thermal analysis studies. Pharm. Res. 1997, 14, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Doh, H.-J.; Choi, M.-K.; Chung, S.-J.; Shim, C.-K.; Kim, D.-D.; Kim, J.S.; Yong, C.-S.; Choi, H.-G. Skin permeation enhancement of diclofenac by fatty acids. Drug Deliv. 2008, 15, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Li, S.K. Efficiency of fatty acids as chemical penetration enhancers: Mechanisms and structure enhancement relationship. Pharm. Res. 2010, 27, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Jabeur, H.; Zribi, A.; Abdelhedi, R.; Bouaziz, M. Effect of olive storage conditions on Chemlali olive oil quality and the effective role of fatty acids alkyl esters in checking olive oils authenticity. Food Chem. 2015, 169, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Fregapane, G.; Salvador, M.D. Production of superior quality extra virgin olive oil modulating the content and profile of its minor components. Food Res. Int. 2013, 54, 1907–1914. [Google Scholar] [CrossRef]
- Brkić Bubola, K.; Lukić, M.; Mofardin, I.; Butumović, A.; Koprivnjak, O. Filtered vs. naturally sedimented and decanted virgin olive oil during storage: Effect on quality and composition. LWT-Food Sci. Technol. 2017, 84, 370–377. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Quality of extra virgin olive oils produced in an emerging olive growing area in north-western Spain. Food Chem. 2014, 164, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Karande, P.; Jain, A.; Mitragotri, S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat. Biotechnol. 2004, 22, 192–197. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds: dihydroquercetin, polyethylene glycol 400, olive oil, soybean oil, coconut oil, avocado oil, sea buckthorn pulp oil, raspberry seed oil are available from the authors. |

| Fatty Acid | Soybean Oil | Olive Oil | Avocado Oil | Sea-Buckthorn Pulp Oil | Raspberry Seed Oil | Coconut Oil |
|---|---|---|---|---|---|---|
| C6:0 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.285 |
| C8:0 | 0.000 | 0.019 | 0.000 | 0.016 | 0.000 | 5.582 |
| C10:0 | 0.000 | 0.000 | 0.000 | 0.032 | 0.000 | 5.216 |
| C12:0 | 0.000 | 0.000 | 0.000 | 0.363 | 0.000 | 45.943 |
| C14:0 | 0.067 | 0.011 | 0.042 | 0.402 | 0.044 | 17.983 |
| C14:1 | 0.000 | 0.000 | 0.000 | 0.097 | 0.000 | 0.000 |
| C15:0 | 0.015 | 0.000 | 0.000 | 0.068 | 0.000 | 0.010 |
| C16:0 | 10.478 | 10.037 | 8.252 | 33.294 | 3.705 | 9.212 |
| C16:1 | 0.100 | 0.859 | 1.974 | 30.540 | 0.122 | 0.024 |
| C17:0 | 0.094 | 0.056 | 0.041 | 0.157 | 0.055 | 0.039 |
| C17:1 | 0.052 | 0.075 | 0.065 | 0.102 | 0.046 | 0.000 |
| C18:0 | 3.702 | 3.475 | 2.300 | 0.814 | 1.812 | 2.774 |
| C18:1 | 23.033 | 79.585 | 71.820 | 29.126 | 14.320 | 7.754 |
| C18:2 | 53.909 | 3.959 | 14.596 | 3.547 | 45.929 | 4.565 |
| C18:3 | 7.251 | 0.580 | 0.133 | 0.898 | 28.530 | 0.356 |
| C20:0 | 0.286 | 0.345 | 0.133 | 0.140 | 0.719 | 0.098 |
| C20:1 | 0.354 | 0.218 | 0.167 | 0.124 | 2.939 | 0.065 |
| C20:2 | 0.088 | 0.010 | 0.174 | 0.000 | 0.276 | 0.000 |
| C20:3 | 0.000 | 0.574 | 0.029 | 0.000 | 0.000 | 0.000 |
| C20:4 | 0.036 | 0.037 | 0.000 | 0.000 | 0.038 | 0.000 |
| C20:5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.112 | 0.000 |
| C21:0 | 0.055 | 0.013 | 0.094 | 0.061 | 0.117 | 0.000 |
| C22:0 | 0.323 | 0.095 | 0.108 | 0.032 | 0.148 | 0.035 |
| C22:1 | 0.000 | 0.000 | 0.000 | 0.023 | 0.423 | 0.000 |
| C24:0 | 0.102 | 0.043 | 0.044 | 0.046 | 0.055 | 0.034 |
| C24:1 | 0.016 | 0.000 | 0.000 | 0.019 | 0.037 | 0.000 |
| Unidentified | 0.041 | 0.012 | 0.031 | 0.105 | 0.576 | 0.029 |
| Donor Phase | DHQ Flux Into Epidermis | ER Epidermis | DHQ Flux Into Dermis | ER Dermis |
|---|---|---|---|---|
| Control solution | 0.109 ± 0.070 | - | 0.095 ± 0.056 | - |
| Soybean oil (10%) | 1.577 ± 1.011 | 14.451 * | 0.457 ± 0.273 | 4.810 * |
| Olive oil (10%) | 0.104 ± 0.085 | 0.950 | 0.352 ± 0.261 | 3.708 * |
| Avocado oil (10%) | 0.139 ± 0.038 | 1.276 | 0.106 ± 0.035 | 1.116 |
| Sea buckthorn oil (10%) | 0.099 ± 0.061 | 0.909 | 0.171 ± 0.117 | 1.802 |
| Raspberry seed oil (10%) | 0.036 ± 0.018 | 0.330 | 0.147 ± 0.061 | 1.549 |
| Coconut oil (10%) | 0.022 ± 0.011 | 0.201 | 0.025 ± 0.016 | 0.259 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čižinauskas, V.; Elie, N.; Brunelle, A.; Briedis, V. Skin Penetration Enhancement by Natural Oils for Dihydroquercetin Delivery. Molecules 2017, 22, 1536. https://doi.org/10.3390/molecules22091536
Čižinauskas V, Elie N, Brunelle A, Briedis V. Skin Penetration Enhancement by Natural Oils for Dihydroquercetin Delivery. Molecules. 2017; 22(9):1536. https://doi.org/10.3390/molecules22091536
Chicago/Turabian StyleČižinauskas, Vytis, Nicolas Elie, Alain Brunelle, and Vitalis Briedis. 2017. "Skin Penetration Enhancement by Natural Oils for Dihydroquercetin Delivery" Molecules 22, no. 9: 1536. https://doi.org/10.3390/molecules22091536
APA StyleČižinauskas, V., Elie, N., Brunelle, A., & Briedis, V. (2017). Skin Penetration Enhancement by Natural Oils for Dihydroquercetin Delivery. Molecules, 22(9), 1536. https://doi.org/10.3390/molecules22091536





