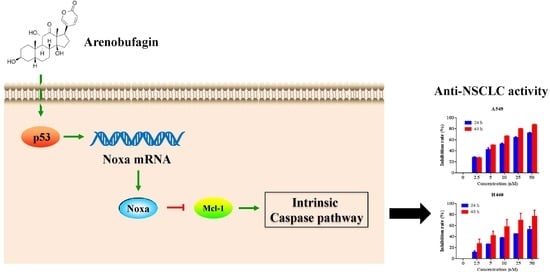
Arenobufagin Induces Apoptotic Cell Death in Human Non-Small-Cell Lung Cancer Cells via the Noxa-Related Pathway
Abstract
:1. Introduction
2. Results
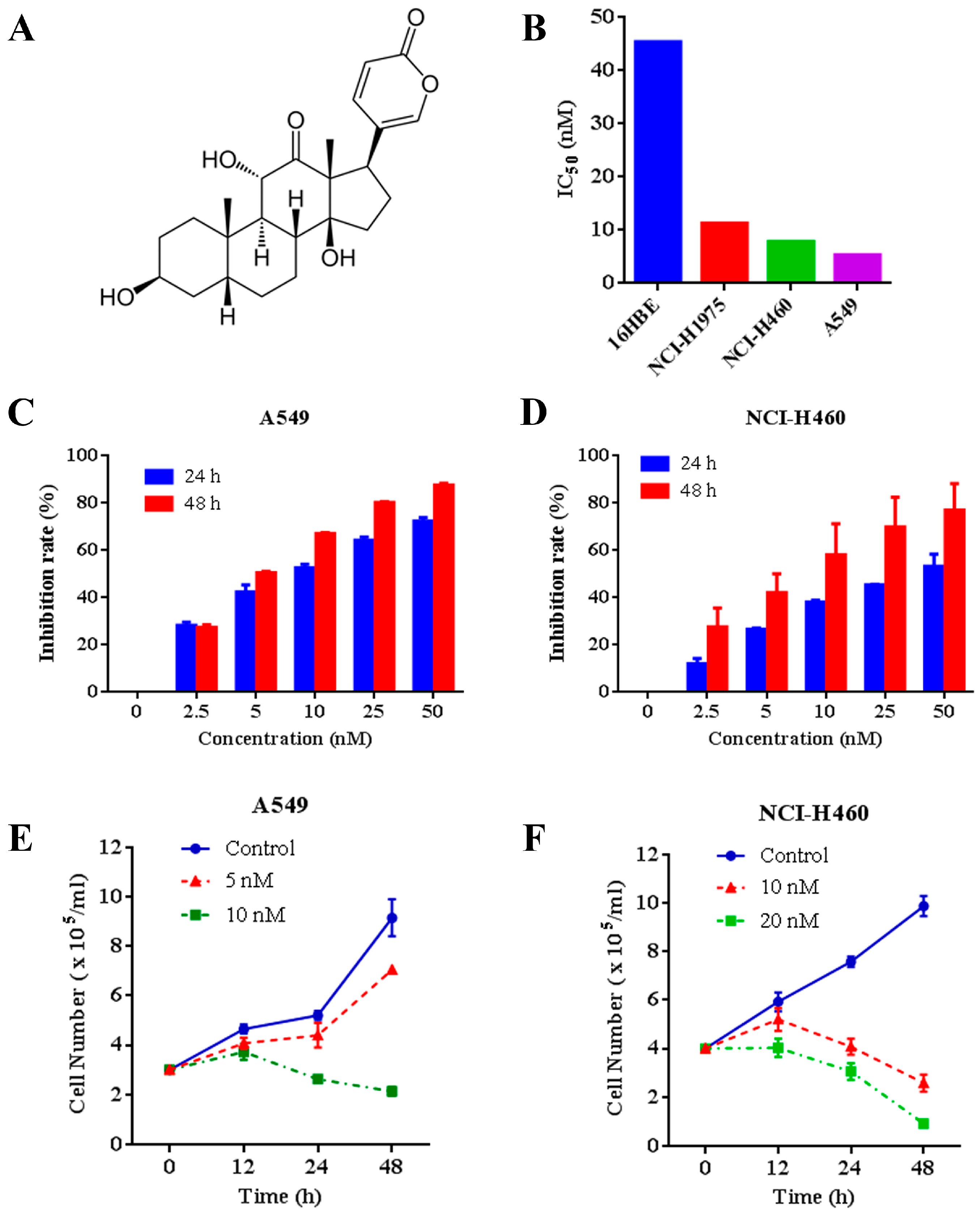
2.1. Effects of Arenobufagin on NSCLC Cells
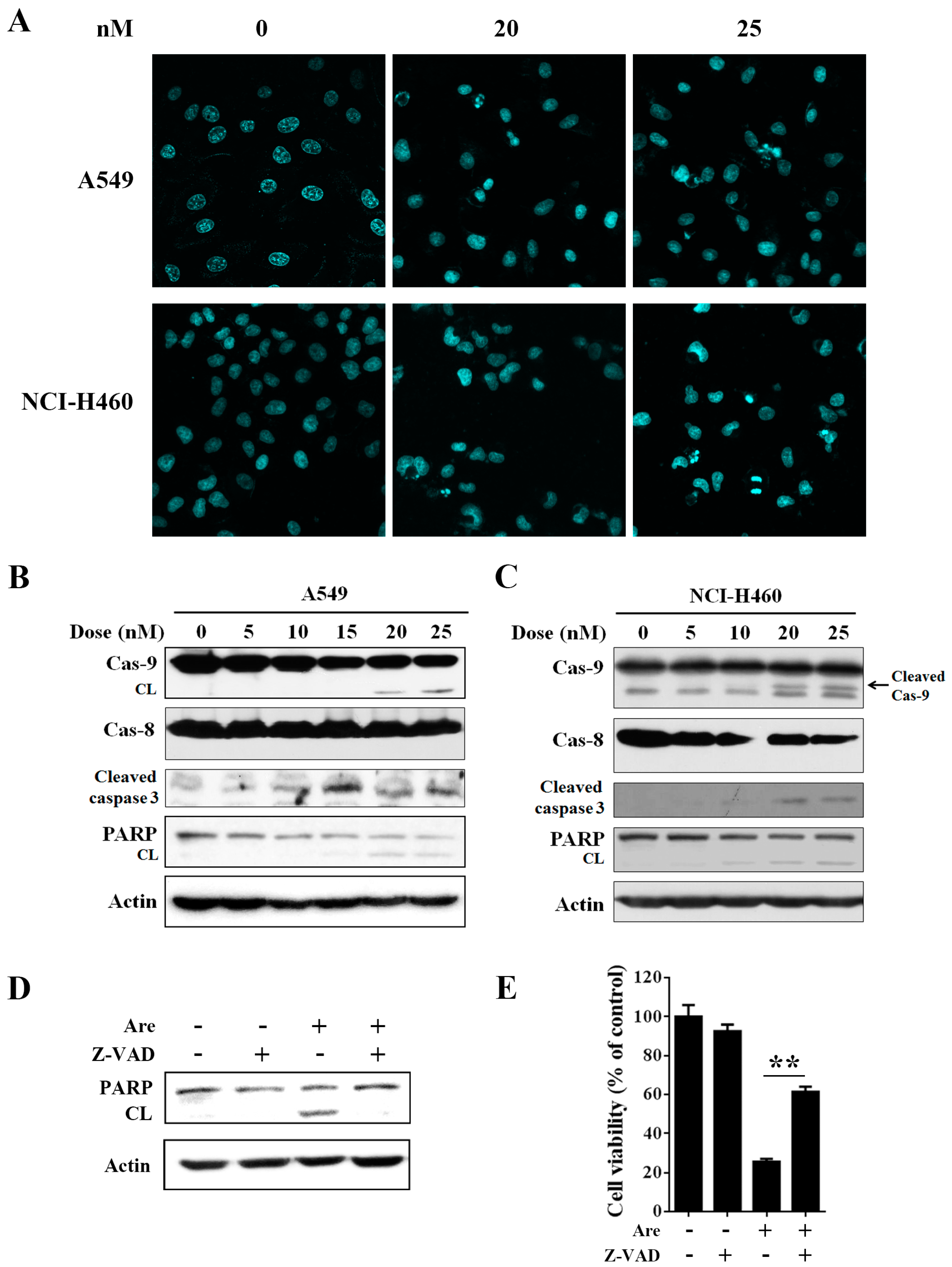
2.2. Arenobufagin Provokes NSCLC Cell Apoptosis
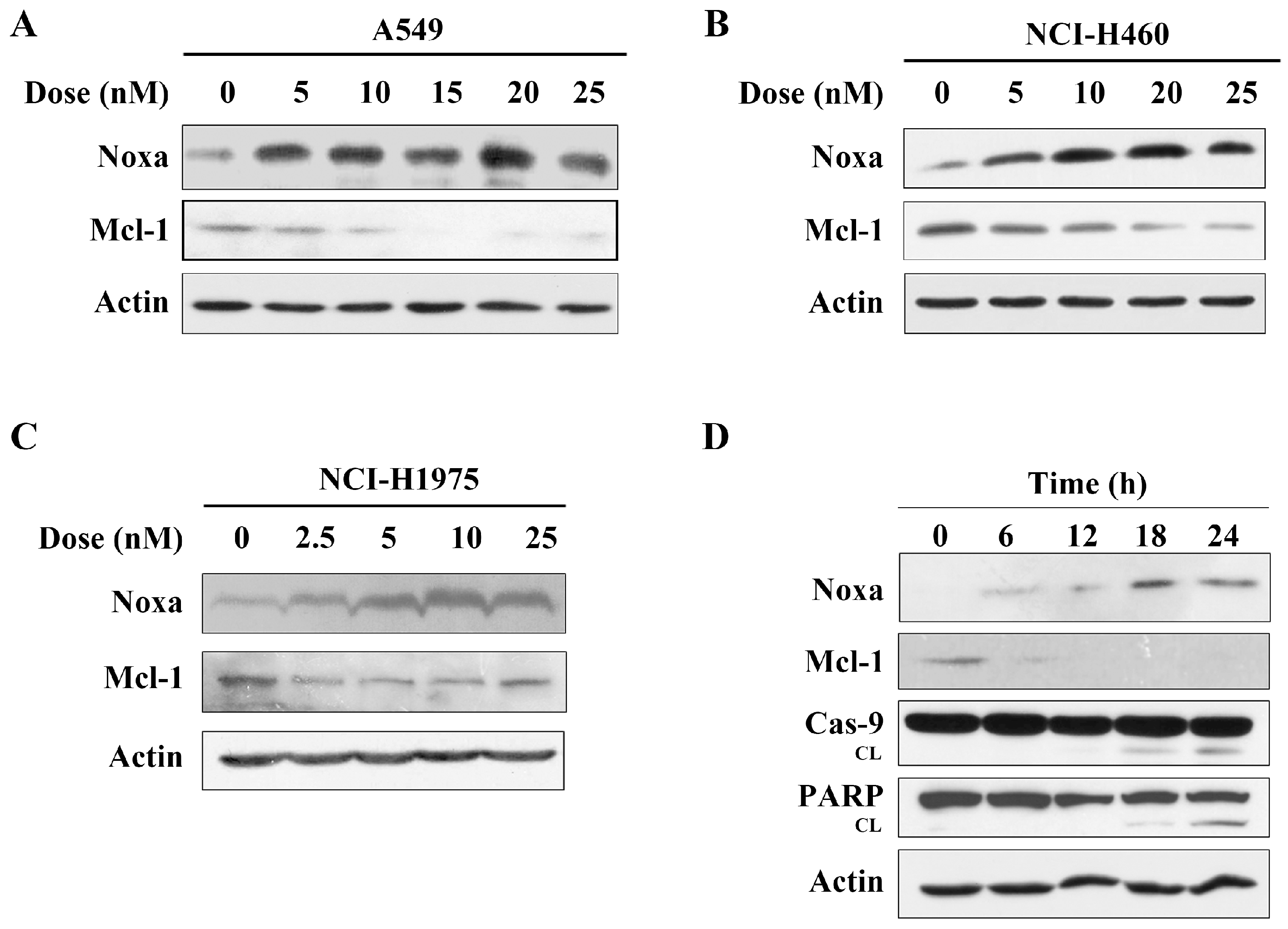
2.3. Arenobufagin Regulates Noxa and Mcl-1 in NSCLC Cells
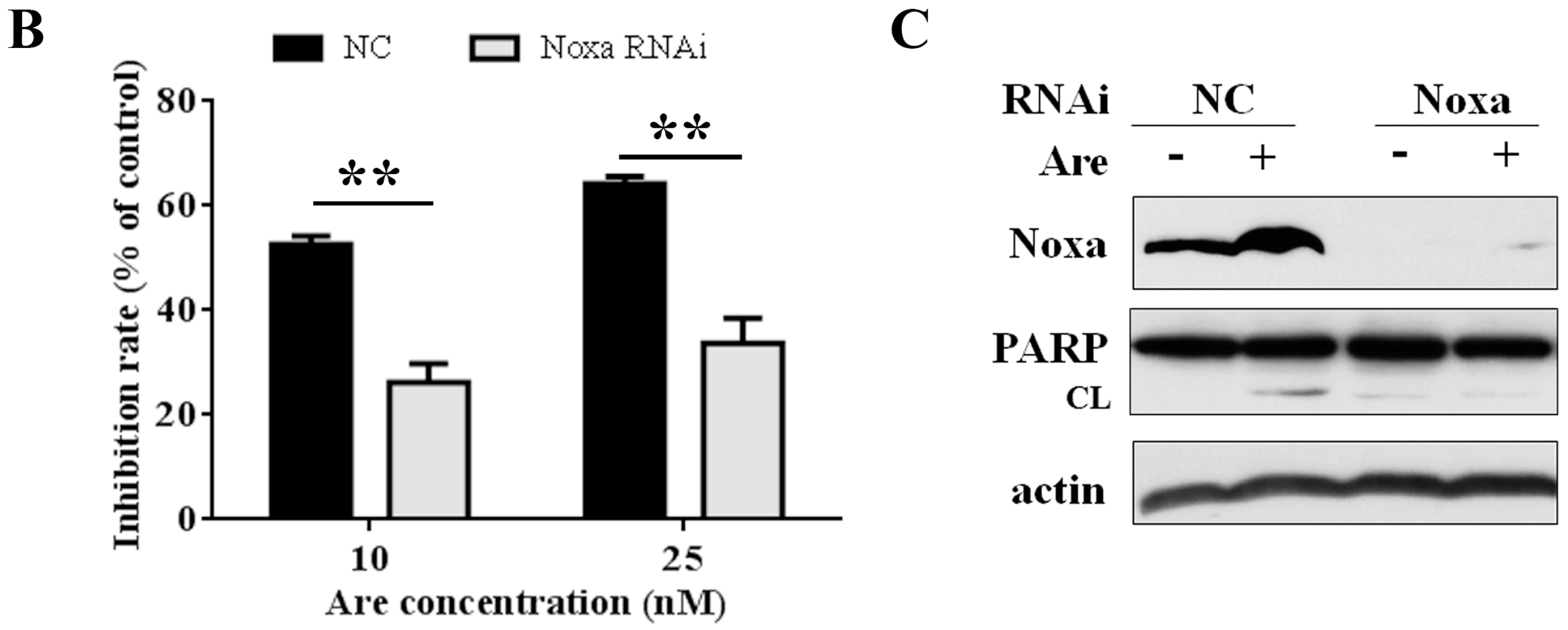
2.4. Noxa is Required for the Inhibition Effect of Arenobufagin on NSCLC Cells
2.5. p53 is Involved in Arenobufagin-Induced Upregulation of Noxa
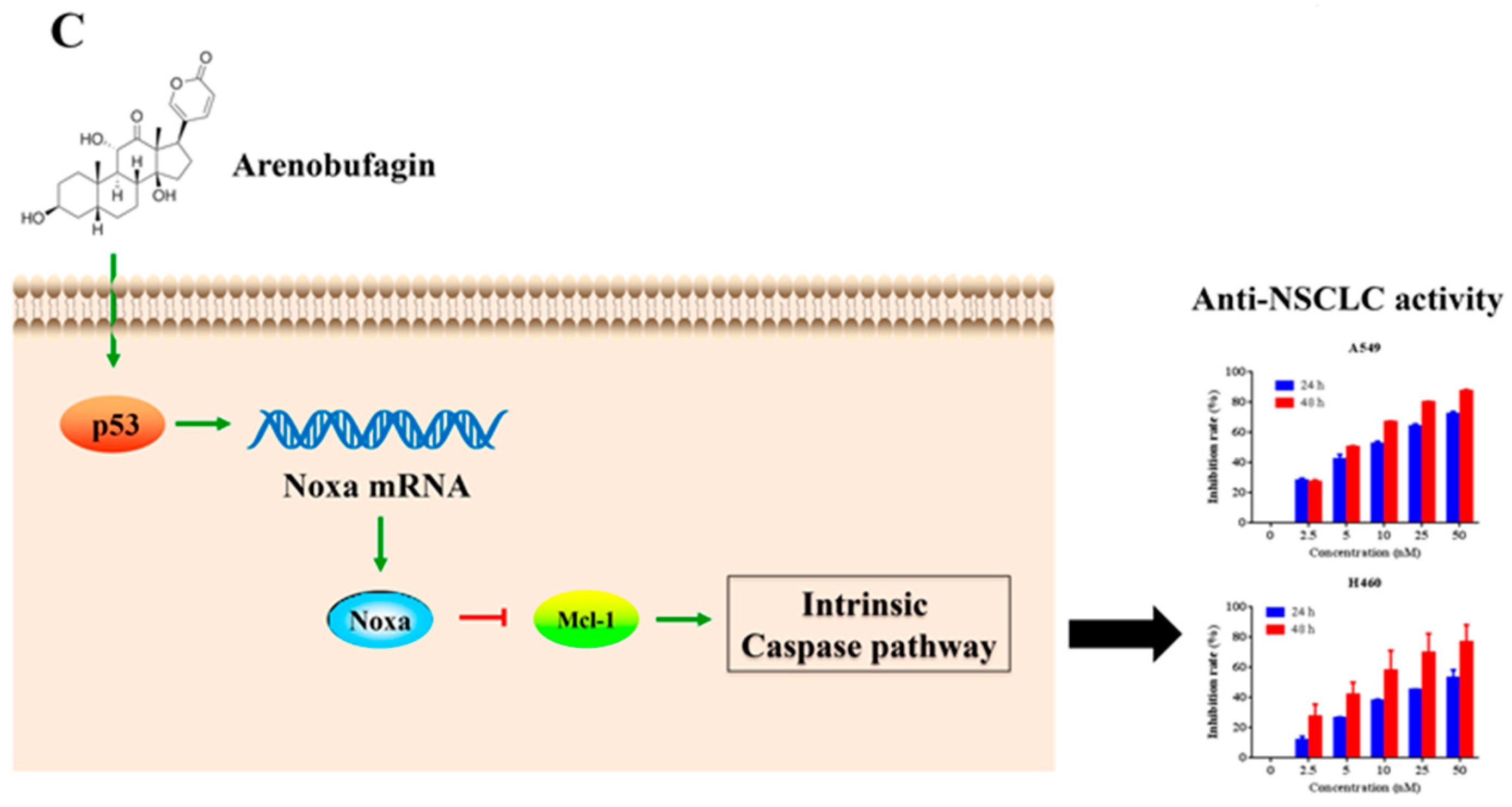
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Reagents and Antibodies
4.3. Cell Viability Assay
4.4. Hoechst 33258 Staining
4.5. Western Blot Analysis
4.6. RNA Interference
4.7. RNA Extraction and Reverse-Transcription PCR
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Bai, L.; Wang, S. Targeting apoptosis pathways for new cancer therapeutics. Annu. Rev. Med. 2014, 65, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Huang, D.C.; Adams, J.M. The Bcl-2 family: Roles in cell survival and oncogenesis. Oncogene 2003, 22, 8590–8607. [Google Scholar] [CrossRef] [PubMed]
- Oda, E.; Ohki, R.; Murasawa, H.; Nemoto, J.; Shibue, T.; Yamashita, T.; Tokino, T.; Taniguchi, T.; Tanaka, N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 2000, 288, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Simonin, K.; N'Diaye, M.; Lheureux, S.; Loussouarn, C.; Dutoit, S.; Briand, M.; Giffard, F.; Brotin, E.; Blanc-Fournier, C.; Poulain, L. Platinum compounds sensitize ovarian carcinoma cells to ABT-737 by modulation of the Mcl-1/Noxa axis. Apoptosis 2013, 18, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.M.; Mohana-Kumaran, N.; Lau, D.; Zhang, X.D.; Hersey, P.; Huang, D.C.; Weninger, W.; Haass, N.K.; Allen, J.D. Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin. Cancer Res. 2012, 18, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.C.; Brinkmann, K.; Kashkar, H. Noxa and cancer therapy: Tuning up the mitochondrial death machinery in response to chemotherapy. Mol. Cell. Oncol. 2014, 1, e29906. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhong, N.; Liu, G.; Chen, K.; Liu, X.; Su, L.; Singhal, S. Usp9x- and Noxa-mediated Mcl-1 downregulation contributes to pemetrexed-induced apoptosis in human non-small-cell lung cancer cells. Cell Death Dis. 2014, 5, e1316. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Willis, S.N.; Wei, A.; Smith, B.J.; Fletcher, J.I.; Hinds, M.G.; Colman, P.M.; Day, C.L.; Adams, J.M.; Huang, D.C. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol. Cell 2005, 17, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Bougie, P.; Menoret, E.; Juin, P.; Dousset, C.; Pellat-Deceunynck, C.; Amiot, M. Noxa controls Mule-dependent Mcl-1 ubiquitination through the regulation of the Mcl-1/USP9X interaction. Biochem. Biophys. Res. Commun. 2011, 413, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.N.; Chen, L.; Dewson, G.; Wei, A.; Naik, E.; Fletcher, J.I.; Adams, J.M.; Huang, D.C. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005, 19, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, L.; Zhou, G.B. The main anticancer bullets of the Chinese medicinal herb, thunder god vine. Molecules 2011, 16, 5283–5297. [Google Scholar] [CrossRef] [PubMed]
- Garraffo, H.M.; Gros, E.G. Biosynthesis of bufadienolides in toads. VI. Experiments with [1,H]cholesterol, [24C]coprostanol, and 5 beta-[24C]pregnanolone in the toad Bufo arenarum. Steroids 1986, 48, 251–257. [Google Scholar] [CrossRef]
- Li, M.; Wu, S.; Liu, Z.; Zhang, W.; Xu, J.; Wang, Y.; Liu, J.; Zhang, D.; Tian, H.; Li, Y.; Ye, W. Arenobufagin, a bufadienolide compound from toad venom, inhibits VEGF-mediated angiogenesis through suppression of VEGFR-2 signaling pathway. Biochem. Pharmacol. 2012, 83, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Cruz Jdos, S.; Matsuda, H. Arenobufagin, a compound in toad venom, blocks Na(+)-K+ pump current in cardiac myocytes. Eur. J. Pharmacol. 1993, 239, 223–226. [Google Scholar] [CrossRef]
- Cruz Jdos, S.; Matsuda, H. Depressive effects of arenobufagin on the delayed rectifier K+ current of guinea-pig cardiac myocytes. Eur. J. Pharmacol. 1994, 266, 317–325. [Google Scholar] [CrossRef]
- Zhang, D.M.; Liu, J.S.; Deng, L.J.; Chen, M.F.; Yiu, A.; Cao, H.H.; Tian, H.Y.; Fung, K.P.; Kurihara, H.; Pan, J.X.; et al. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis 2013, 34, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.J.; Peng, Q.L.; Wang, L.H.; Xu, J.; Liu, J.S.; Li, Y.J.; Zhuo, Z.J.; Bai, L.L.; Hu, L.P.; Chen, W.M.; et al. Arenobufagin intercalates with DNA leading to G2 cell cycle arrest via ATM/ATR pathway. Oncotarget 2015, 6, 34258–34275. [Google Scholar] [PubMed]
- Yue, Q.; Zhen, H.; Huang, M.; Zheng, X.; Feng, L.; Jiang, B.; Yang, M.; Wu, W.; Liu, X.; Guo, D. Proteasome Inhibition Contributed to the Cytotoxicity of Arenobufagin after Its Binding with Na, K-ATPase in Human Cervical Carcinoma HeLa Cells. Plos One 2016, 11, e0159034. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Lin, S.; Peng, P.; Cai, C.; Deng, J.; Wang, M.; Li, X.; Lin, R.; Lin, Y.; Fang, A.; et al. Arenobufagin activates p53 to trigger esophageal squamous cell carcinoma cell apoptosis in vitro and in vivo. Onco Targets Ther. 2017, 10, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Tromp, J.M.; Geest, C.R.; Breij, E.C.; Elias, J.A.; van Laar, J.; Luijks, D.M.; Kater, A.P.; Beaumont, T.; van Oers, M.H.; Eldering, E. Tipping the Noxa/Mcl-1 balance overcomes ABT-737 resistance in chronic lymphocytic leukemia. Clin. Cancer Res. 2012, 18, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Hershko, T.; Ginsberg, D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J. Biol. Chem. 2004, 279, 8627–8634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiao, J.; Liu, Y.; Guo, L.X.; Zhou, B.; Li, G.Q.; Yao, Z.J.; Zhou, G.B. Gefitinib analogue V1801 induces apoptosis of T790M EGFR-harboring lung cancer cells by up-regulation of the BH-3 only protein Noxa. PLoS ONE 2012, 7, e48748. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Yang, P.; Shen, Y.; Bei, W.; Zhang, Y.; Ge, Y.; Newman, R.A.; Cohen, L.; Liu, L.; Thornton, B.; et al. Pilot study of huachansu in patients with hepatocellular carcinoma, nonsmall-cell lung cancer, or pancreatic cancer. Cancer 2009, 115, 5309–5318. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Li, A.; Inagaki, Y.; Kokudo, N.; Tamura, S.; Nakata, M.; Tang, W. Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Int. Immunopharmacol. 2011, 11, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Takai, N.; Ueda, T.; Nishida, M.; Nasu, K.; Narahara, H. Bufalin induces growth inhibition, cell cycle arrest and apoptosis in human endometrial and ovarian cancer cells. Int. J. Mol. Med. 2008, 21, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Ploner, C.; Kofler, R.; Villunger, A. Noxa: At the tip of the balance between life and death. Oncogene 2008, 27 (Suppl. 1), S84–S92. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, W.; Sharma, K.; Lee, J.Y.; Maxim, N.T.; Hicks, M.A.; Vu, T.T.; Luu, A.; Yeudall, W.A.; Tanaka, N.; Harada, H. DNA damaging agent-induced apoptosis is regulated by MCL-1 phosphorylation and degradation mediated by the Noxa/MCL-1/CDK2 complex. Oncotarget 2016, 7, 36353–36365. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Pearl, L.H.; Schierz, A.C.; Ward, S.E.; Al-Lazikani, B.; Pearl, F.M. Therapeutic opportunities within the DNA damage response. Nat. Rev. Cancer 2015, 15, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wen, Z.S.; Liu, Z.; Hu, Z.; Ma, J.; Chen, X.Q.; Liu, Y.Q.; Pu, J.X.; Xiao, W.L.; Sun, H.D.; et al. Overexpression and small molecule-triggered downregulation of CIP2A in lung cancer. PLoS ONE 2011, 6, e20159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, L.; Wen, Z.S.; Hu, Z.; Wu, F.Q.; Li, W.; Liu, J.; Zhou, G.B. Cancerous inhibitor of PP2A is targeted by natural compound celastrol for degradation in non-small-cell lung cancer. Carcinogenesis 2014, 35, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Dai, H.; Correia, C.; Takahashi, R.; Lee, S.H.; Schmitz, I.; Kaufmann, S.H. Noxa/Bcl-2 protein interactions contribute to bortezomib resistance in human lymphoid cells. J. Biol. Chem. 2011, 286, 17682–17692. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Zhu, Y.; Fang, S.; Long, H.; Liu, X.; Liu, Z. Arenobufagin Induces Apoptotic Cell Death in Human Non-Small-Cell Lung Cancer Cells via the Noxa-Related Pathway. Molecules 2017, 22, 1525. https://doi.org/10.3390/molecules22091525
Ma L, Zhu Y, Fang S, Long H, Liu X, Liu Z. Arenobufagin Induces Apoptotic Cell Death in Human Non-Small-Cell Lung Cancer Cells via the Noxa-Related Pathway. Molecules. 2017; 22(9):1525. https://doi.org/10.3390/molecules22091525
Chicago/Turabian StyleMa, Liang, Yindi Zhu, Sheng Fang, Hongyan Long, Xiang Liu, and Zi Liu. 2017. "Arenobufagin Induces Apoptotic Cell Death in Human Non-Small-Cell Lung Cancer Cells via the Noxa-Related Pathway" Molecules 22, no. 9: 1525. https://doi.org/10.3390/molecules22091525
APA StyleMa, L., Zhu, Y., Fang, S., Long, H., Liu, X., & Liu, Z. (2017). Arenobufagin Induces Apoptotic Cell Death in Human Non-Small-Cell Lung Cancer Cells via the Noxa-Related Pathway. Molecules, 22(9), 1525. https://doi.org/10.3390/molecules22091525