1. Introduction
Nutritional and pharmacological factors may play a role in the prevention and treatment of several diseases [
1]. The discovery of the anticancer properties of a natural diet supplement can be used to improve the chemotherapy efficacy and thus reduce the side effects of high dose therapy. Recent studies have proved the in vitro potential anticancer activity of plants and wild fruits [
2,
3,
4]. The interest in plant phenolic extracts derives from the evidence of their potent antioxidant activity and their wide range of pharmacological properties, including anticancer activity. This led to our hypothesis of
Prunus spinosa as an interesting compound.
Prunus spinosa Trigno ecotype (PsT) drupe extract with a nutraceutical activator complex (NAC) made of amino acids, vitamins and mineral salt blends, has been chemically prepared for evaluating the drug mechanisms of action at cellular levels. The aim of this work is to show that (PsT + NAC)® is cytotoxic for cancer cells but non-toxic for normal cells and to identify the intracellular mechanisms involved in the cytotoxic behavior.
Prunus spinosa L. (blackthorn) belongs to the rose family (Rosaceae). It is a perennial deciduous plant growing as a shrub on wild uncultivated areas; although native of Italy, it can be also found in other European countries and in temperate regions of Asia. Despite being widespread in Italy, its ethnobotanical use is not well known as in other countries, where branch infusions are used in the treatment of hypertension and its macerated fruits for gastrointestinal disturbances [
5]. The active compounds of
Prunus spinosa mainly contain phenolic acids, flavonoids and anthocyanins [
6]. Phenolic compounds are common constituents of fruits and vegetables and are considered an important class of antioxidant natural substances [
6,
7]. The remarkable diversity of their structures is the reason for their biological properties, such as bioavailability, antioxidant activity, specific interactions with cell receptors and enzymes [
8]. Flavonoids have been reported to exert many biological activities in mammals, such as antibacterial, antiviral, analgesic, anti-allergic, hepatoprotective, cytostatic, apoptotic, estrogen and anti-estrogen functions [
9,
10]. Anthocyanins, from the flavonoids family, are found mainly in berries and have high antioxidant activity, which plays a vital role in the prevention of neuronal and cardiovascular illnesses, diabetes and cancer [
11]. The present work is the first study dealing with the cytotoxic and apoptotic effects of a modified extract of
Prunus spinosa. This study will show the antitumor activity of the PsT Trigno ecotype extract, supplemented with NAC, that has been patented by us [
12].
3. Discussion
The antioxidant activity of berries and drupes, like those of the
Prunus spinosa Trigno ecotype, is well known also for their use in the field of foodstuffs [
6,
14]. The chemical composition has been identified and quantified by liquid chromatography coupled to mass spectrometry. The plant extract (PsT) is characterized by the presence of active compounds as phenolic acids, flavonoids and anthocyanins. In particular, higher quantities are found of the phenolic acid group (3-
O-Caffeoylquinic and the 4-
O-Caffeoylquinic acids), the flavonoid group (quercetins and Kaempferol 3-
O-rutinoside), and the anthocyanins group (cyanidins and peonidins) (
Table 1). The extract has proven to be particularly effective for its high presence and special distribution of flavones, flavonols, phenolic acids and anthocyanins, all active components known for their antioxidant and antiproliferative activities [
6,
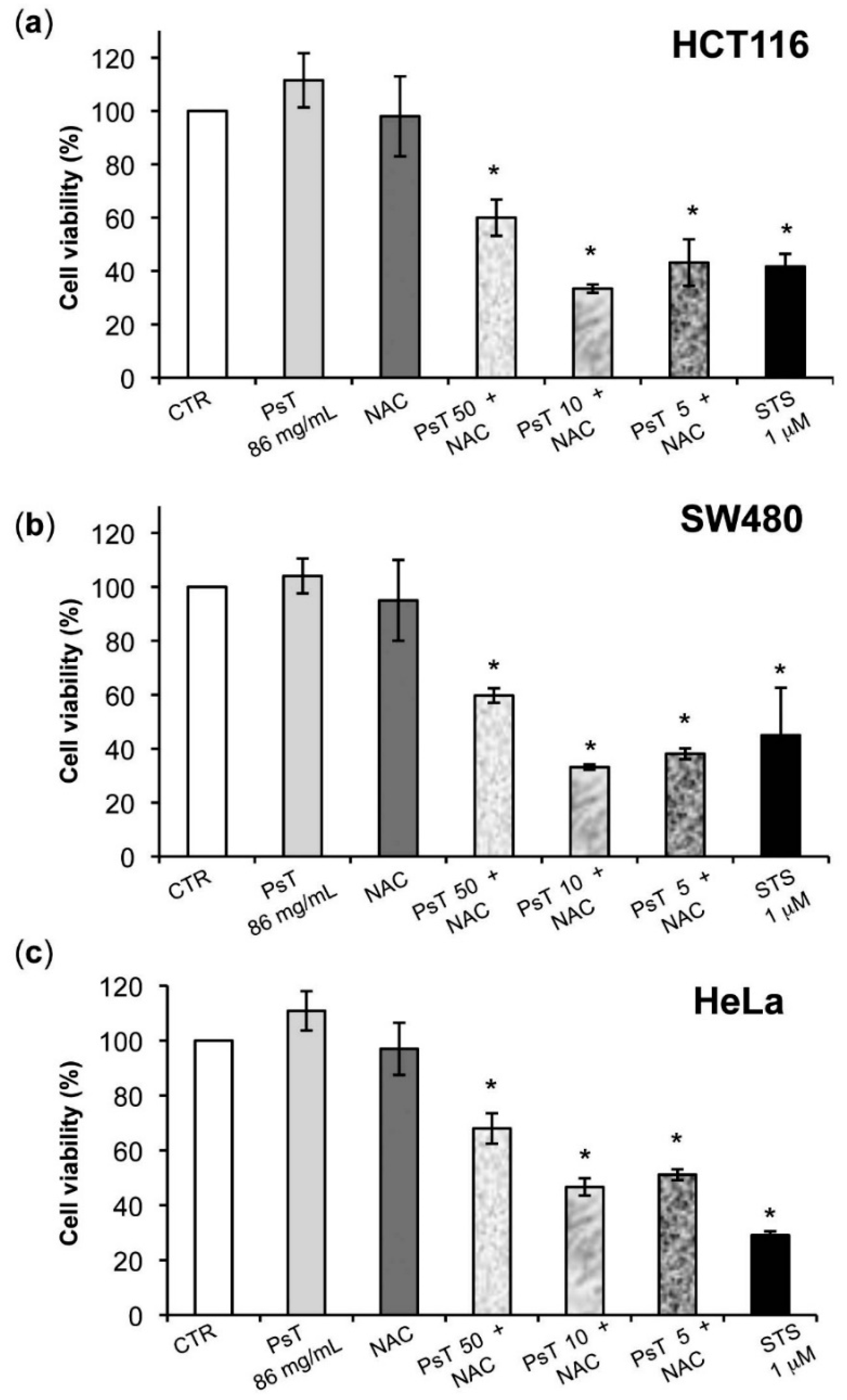
7]. Initially our study aimed at the assessment of the cytotoxic effects of this compound on histologically-different cell lines as the human colon, cervix and lung carcinoma. The analysis of the cellular vitality of tumor lines has shown that both
Prunus spinosa and NAC “alone” do not show any toxicity to the above human cancer cells. However, when
Prunus spinosa is diluted with NAC, there is a noticeable cytotoxicity effect in all cancer cells.
The cytotoxicity phenomenon observed may be ascribed to the induction of apoptosis, as the positive control used in all experiments has shown (
Figure 1).
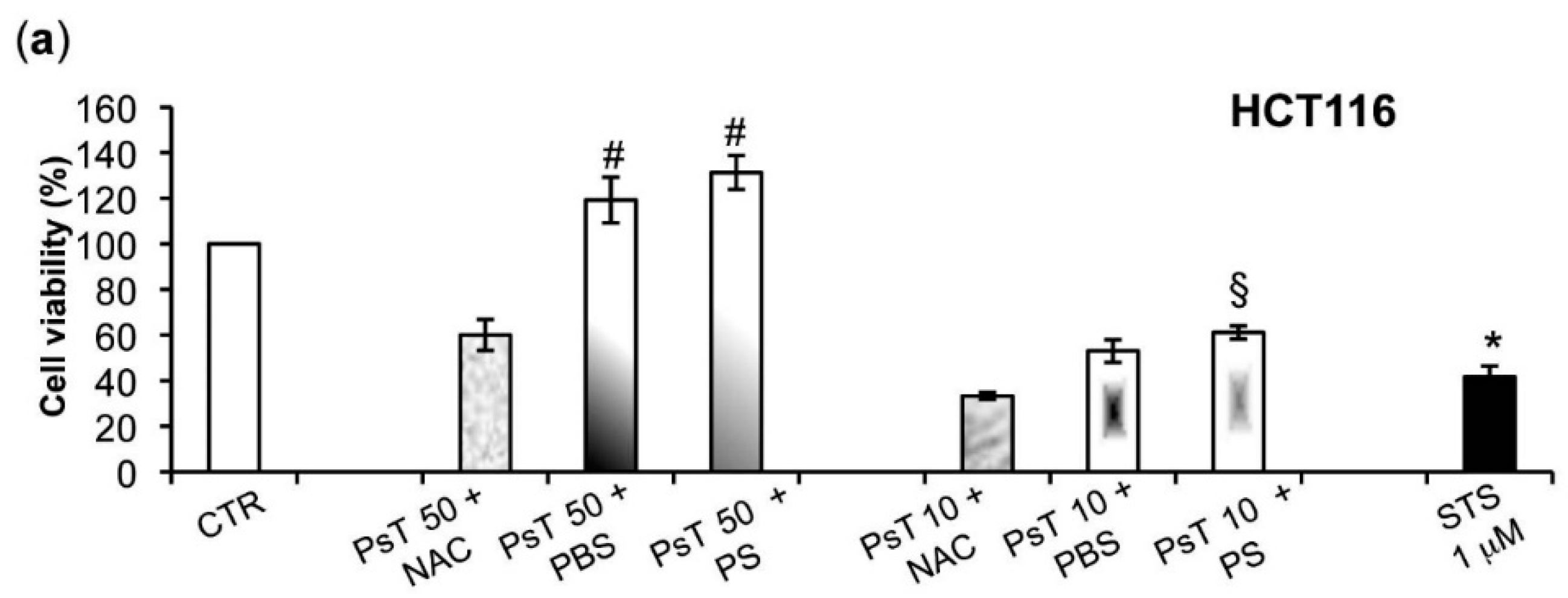
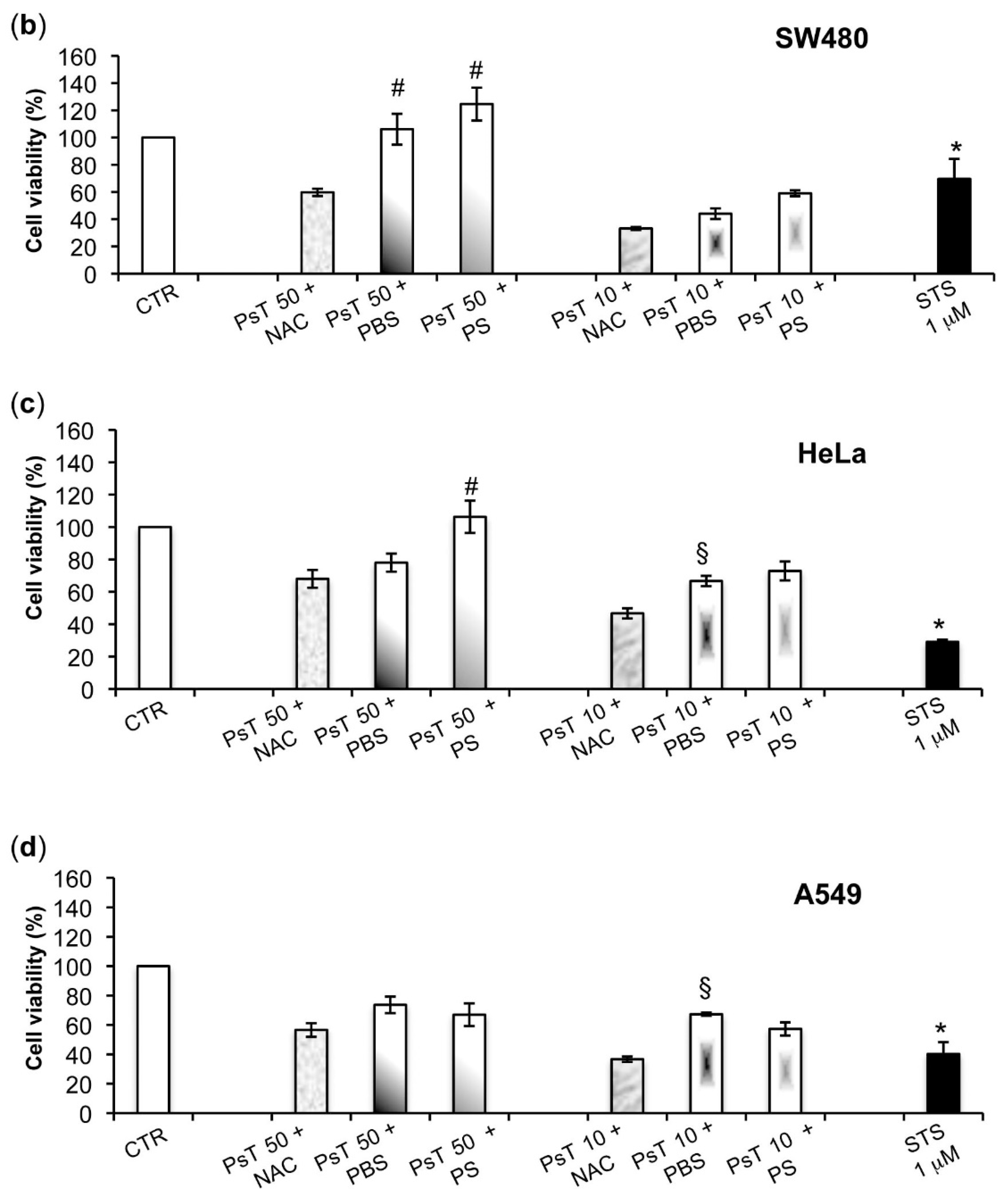
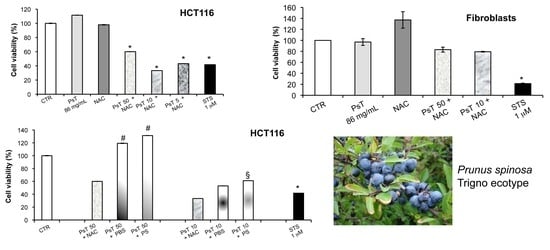
To verify whether or not the NAC is responsible for the observed cytotoxicity in the cancer cells, we experimented by using other vehicles, namely both PBS and PS, to dilute Prunus spinosa (PsT 86 mg/mL).
We found that the (PsT + NAC)
® combination effectively reduced tumor cell survival, and, conversely, that the PsT + PBS and PsT + PS, do not (
Figure 2).
Our study continued using two genetically different human colon carcinoma lines (HCT116 and SW480). This decision was taken because the ingestion of flavonoid sugar moieties, which are cleaved from the phenolic backbone in the small intestine, are adsorbed here, while only a small part of ingested anthocyanins are absorbed at the small intestine level and large amounts of these latter compounds enter the colon, where they are de-glycosylated by gut microbiota [
15].
The investigation of the anti-tumor effect of the flavonoids and anthocyanins that are found in high amounts in the
Prunus food supplement on human colon carcinoma cells seemed to us very promising. The MTT test evaluation showed that mitochondria could be directly connected in the action mechanism of these complexes. The test involves chemical reactions with NAD (P) H-dependent cellular oxidoreductase enzymes, which provide the number of viable cells in the whole population. The result shows a reduction in the mitochondrial activity [
16].
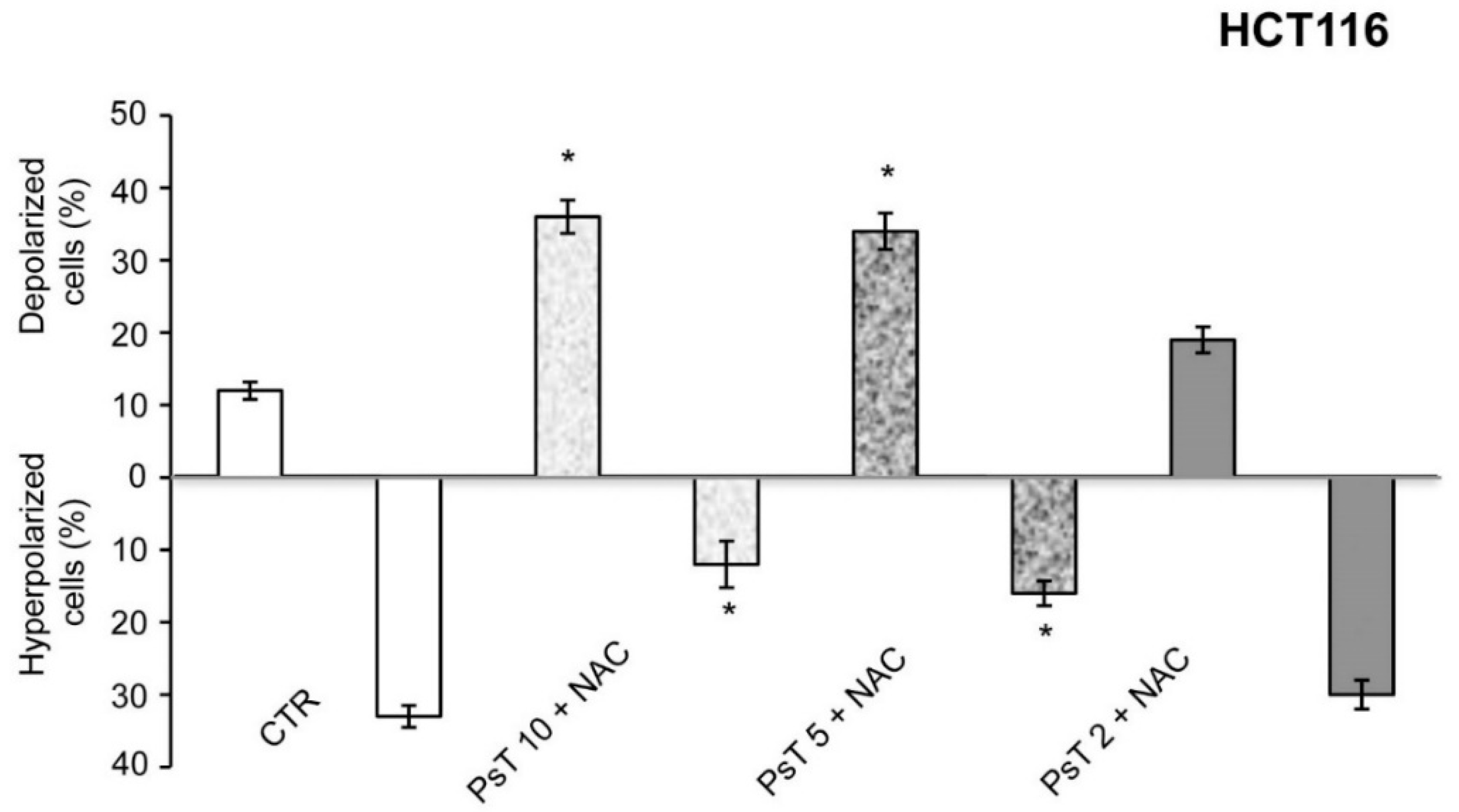
Cancer cells have a more hyperpolarized mitochondrial membrane potential (ΨIM) than normal cells; ΨIM in cancer cells is about 220 mV, whereas in normal cells it is about 140 mV [
17]. Owing to the difference in polarization between normal and tumor cells, and since this compound has the characteristic of depolarizing mitochondrial membrane, it may be selectively effective on cancer cells.
To prove the correctness of our hypothesis, we quantified the percentage of cells with depolarized and hyperpolarized mitochondria by using cytofluorimetry quantitative analysis with (PsT + NAC)
®. The result shows that it induces mitochondrial membrane depolarization of colon carcinoma cells (
Figure 3) and hence that (PsT + NAC)
® has a targeted cytotoxic action.
Our results are strengthened by the presence of high amounts of active ingredient in flavonoids as shown in
Table 1, where the drupe composition of PsT is reported. Therefore, C2=C3 double bonds/3-OH groups, in conjugation with the 4-oxo function of the C-ring in the flavonoid structure, favor the interaction of these compounds with the mitochondrial membrane, decreasing its fluidity either by inhibiting the respiratory chain of mitochondria or by causing uncoupling [
18].
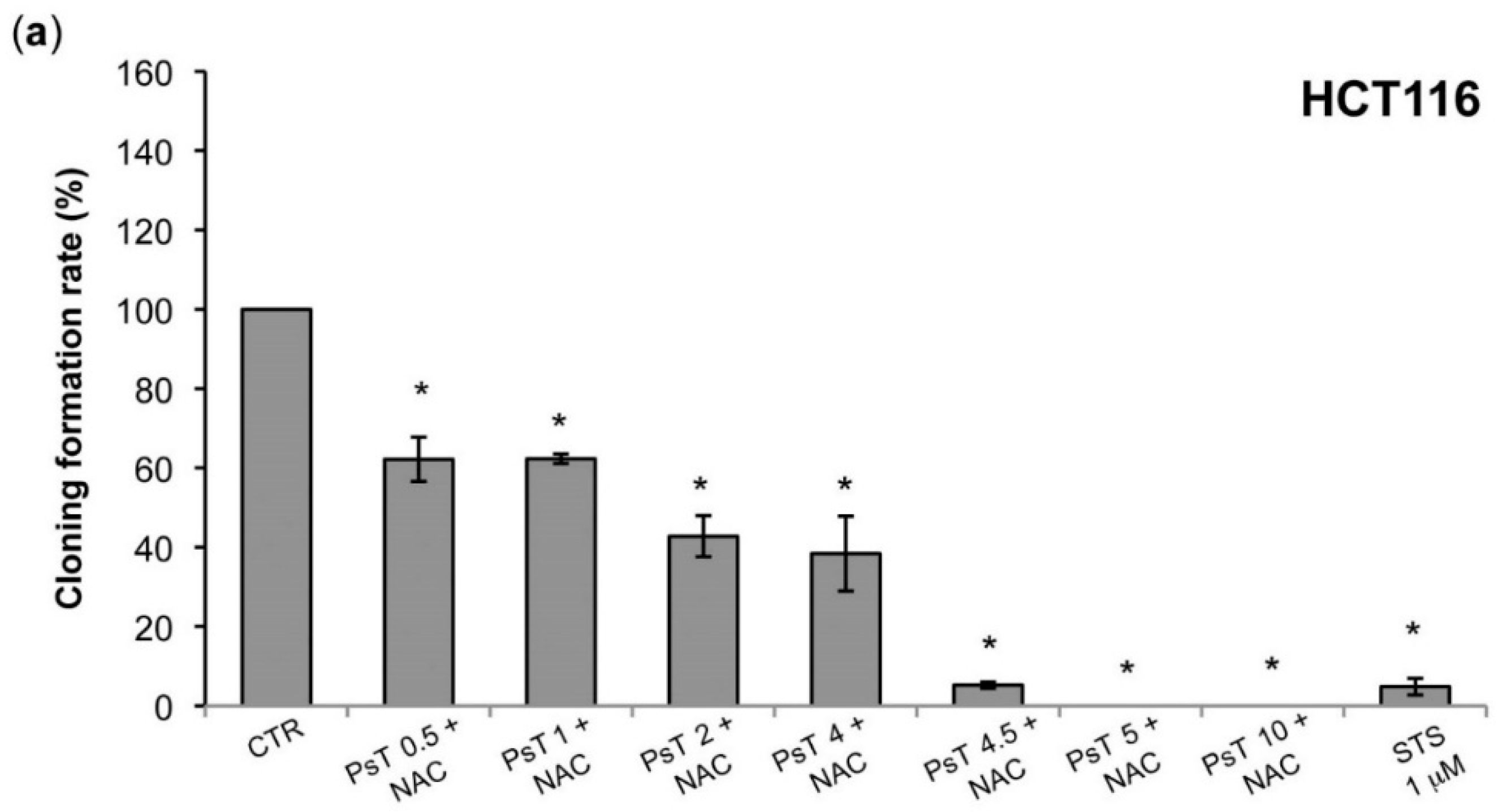
A clonogenic analysis was performed, which confirmed the cytotoxic effect, evaluated by the enzymatic test (
Figure 1 and
Figure 2). At 10 mg/mL the treatment was effective on both HCT116 and SW480 lines, while at a lower dose, from 0.5 mg/mL to 2 mg/mL, only the SW480 was resistant (
Figure 4). Although these cell lines were both colorectal cancer, they have different epigenetic and genetic features [
19]. In particular, the SW480 cell line was characterized by TP53 mutations, which made it resistant to apoptosis induction and, consequently, delayed the apoptotic effect of combined treatment in comparison with HCT116 cells.
The cytotoxic activity of (PsT + NAC)
® might be related to its peculiar constituents and the NAC vehicle, that might together increase the bioavailability of some compounds, such as quercetin [
20], present in large amounts in our ecotype (
Table 1).
Unfortunately, the clinical use of pure quercetin is limited due to its low water solubility and instability in physiological media that lead to low intestinal absorption [
21]. Recent studies have shown that a high amount of “found-food quercetin” can be easily absorbed from the intestine and subsequently converted into the active constituents [
22], showing cancer prevention and therapeutic effects in vitro as well as in vivo [
21].
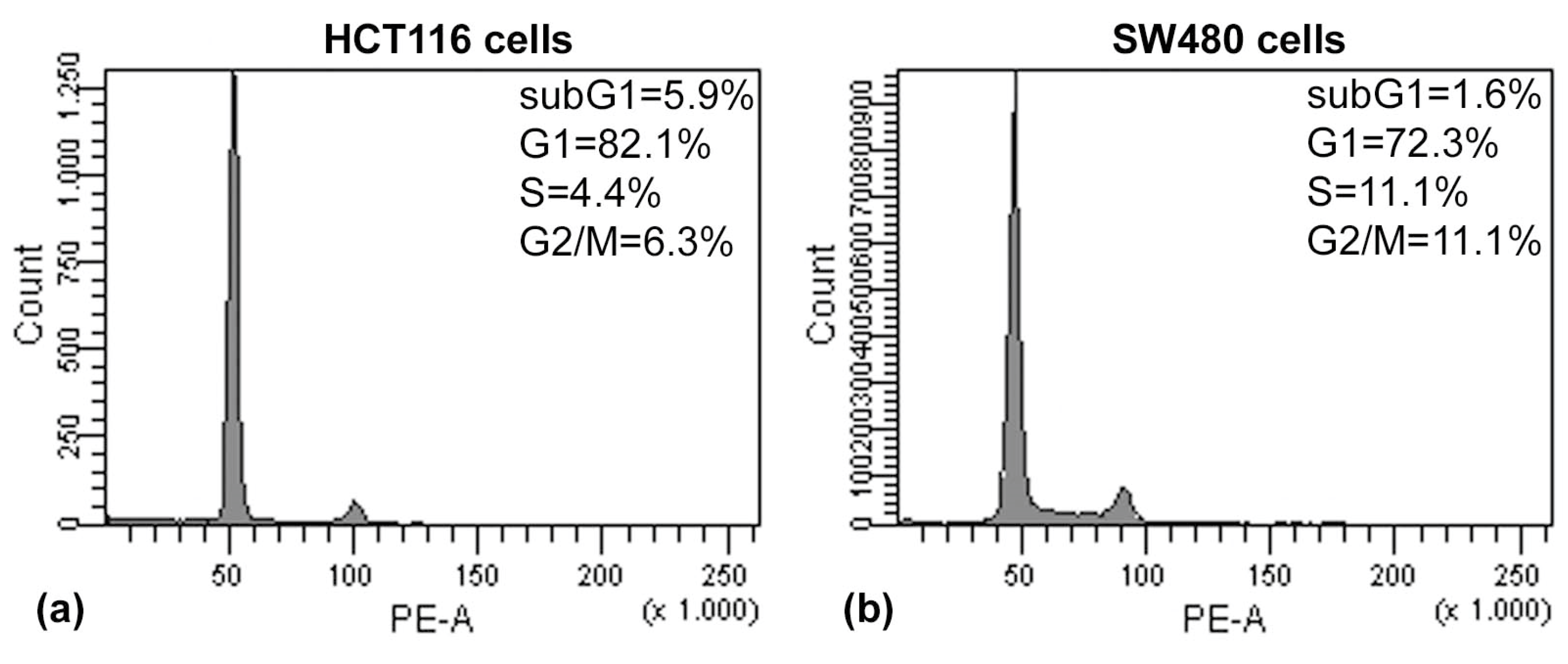
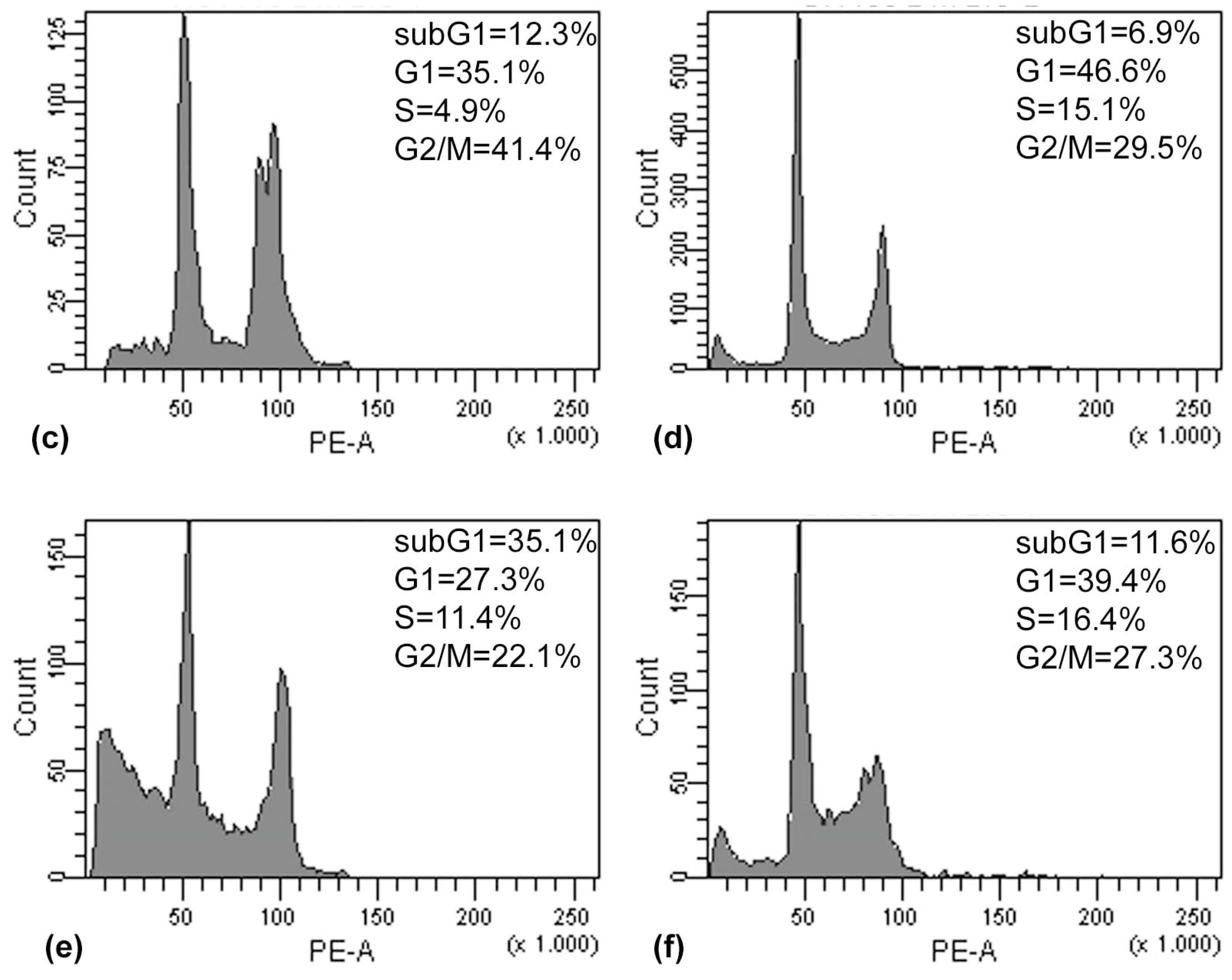
The results of the Annexin V/PI assay and cell cycle analysis, shown in
Figure 5 and
Figure 6, demonstrated that (PsT + NAC)
® combined treatment-induced apoptotic cell death in HCT116 and SW480 cells. However, HCT116 cells were more sensitive than SW480 cells, as demonstrated by the evident arrest of the G2/M phase after (PsT 5 mg/mL + NAC)
® combination, and by the remarkable subG1 peak after (PsT 10 mg/mL + NAC)
® combination.
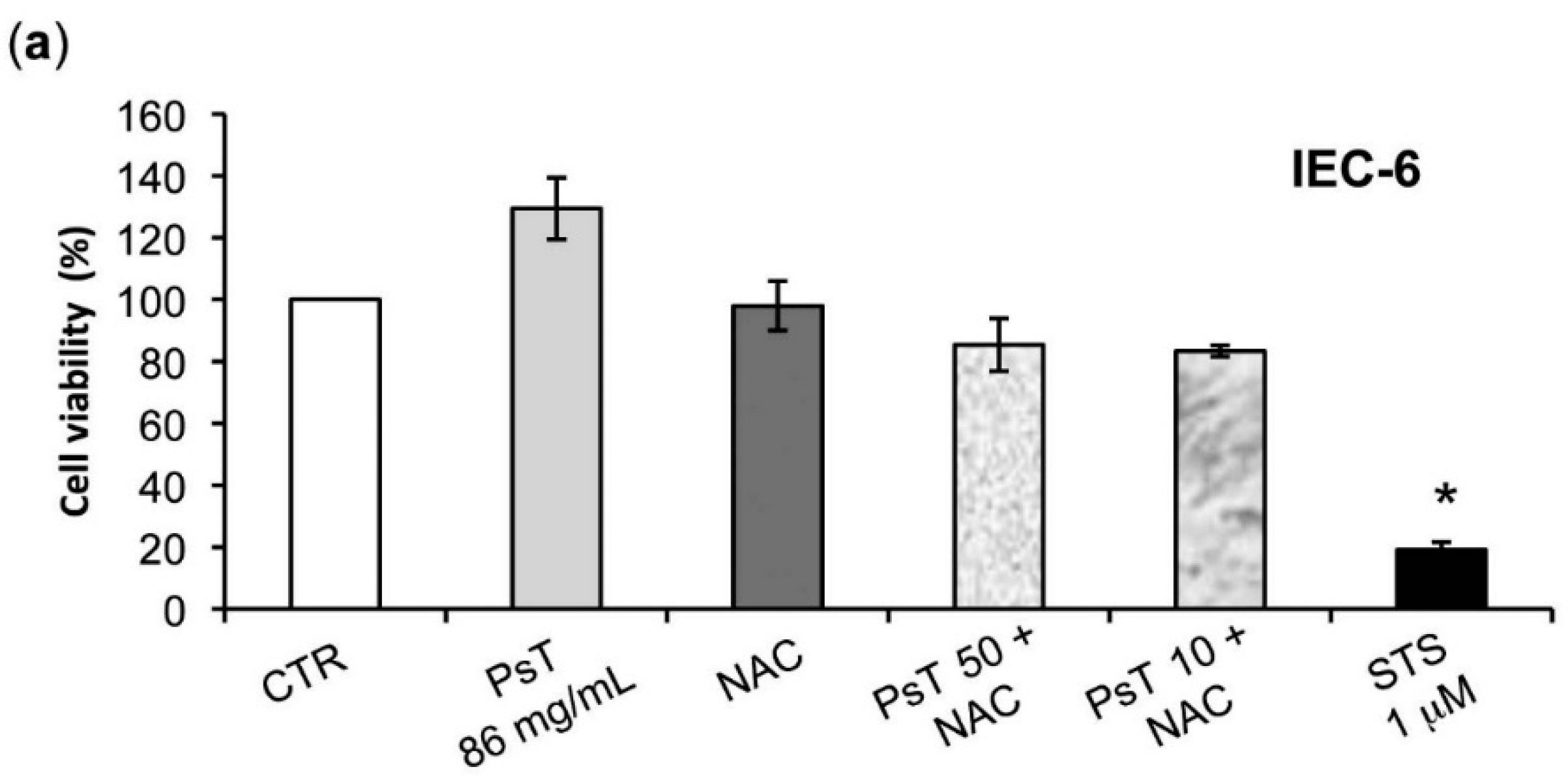
The analysis of
Prunus spinosa + NAC treatments on normal human cell lines showed that, at the same concentrations, this compound is not cytotoxic, giving a greater importance to the obtained results (
Figure 7).
In conclusion, the diversity of the bioactive compounds of the PsT extract show that it is a good source of phytochemicals, and until now has been considered a diet supplement only, due to its healthy nutritional profile. Its great efficacy as an antiproliferative compound on cancer cell lines, could be attributed to the special nature and distribution in the plant complex characterized by the enrichment in flavones, flavonols, phenolic acids and anthocyanins. However, it was found that PsT extract alone did not modify the survival of cancer cells. Only the PsT extract in combination with NAC reduced cell viability significantly, also at low doses.
Further in vivo animal model studies are in progress to investigate the use of (PsT + NAC)® together with proven chemotherapeutic agents, aiming at the improvement of efficacy, to reduce the drugs doses and their undesired collateral effects as a short-term overall benefit.
To go forward from in vivo to clinic, we will have to identify which of the (PsT + NAC)® chemical components are the factors triggering cytotoxicity and apoptosis in cancerous cells, as this is an important step for the future synthesis of the active principles or molecules to perform specific pharmacokinetic and pharmacodynamic studies.
We hope that this will lead from a “food supplement” to a real anticancer drug, as has happened with other “plant derived” chemotherapy.
4. Materials and Methods
4.1. Plant Material
Fully mature blackthorn fruits of the
Prunus spinosa Trigno ecotype (PsT) were collected in late October 2014 by hand picking in the district of Bagnoli del Trigno, which is about 35 km north east of Isernia (Molise Region, Italy, latitude 41°42′ N, longitude 14°27′ E, altitude 650 m a.s.l.). The Molise region is positioned on the eastern side of the Apennines watershed, and has the typical Mediterranean climate of south-central Italy. The area has an average annual rainfall of 850 mm, and a mean annual temperature of 12.6 °C. This territory includes an area with a low population density, reduced road traffic, as well as low levels of photochemical smog and fine particles. The morphological key characteristics used for the plant identification were taken from Flora d’Italia [
23]. The fruits were kept in cooled bags and then stored in a deep-freezer at −20 °C for subsequent analysis. Three samples were used and all the assays were carried out in triplicate. The results were given by the average values and the errors by the standard deviation (SD).
4.2. Plant Extraction and HPLC-DAD–ESI/MS Analysis of Phenolic Acids and Flavone/Ols
HPLC separation of the phenolic acids and flavone/ols extract was performed according to Guimarães and colleagues [
24]. Briefly, each sample of dried and ground fruit was extracted with methanol: water 80:20 (
v/
v) at room temperature, 150 rpm, for 1 h. The extract was filtered through Whatman No. 4 paper. The residue was then re-extracted twice with additional 30 mL portions of methanol: water 80:20 (
v/
v). The combined extracts were evaporated at 35 °C (rotary evaporator Büchi R-210 (Marshall Scientific, Hampton, VA, USA,) to remove methanol. For purification, the aqueous phase was deposited onto a C-18 SepPak
®-Vac 3 cc cartridge (Phenomenex, Torrance, CA, USA).
The extracts were analyzed using a Hewlett–Packard 1100 chromatograph (Agilent Technologies, San Diego, CA, USA) with a diode array detector (DAD) coupled to an HP Chem Station (rev. A.05.04) data-processing station. A Waters Spherisorb S3 ODS-2 C18, 3 µm (4.6 × 150 mm) column thermostat set at 35 °C was used. The solvents used were: (A) 0.1% formic acid in water; (B) acetonitrile. Double online detection was carried out in the DAD using 280 and 370 nm as the preferred wavelengths and in a mass spectrometer (MS) connected to the HPLC system via the DAD cell outlet.
MS detection was performed in an API 3200 Qtrap (Applied Biosystems, Darmstadt, Germany) equipped with an ESI source and a triple quadrupole ion trap mass analyzer that was controlled by the Analyst 5.1 software (Merck, Saint Louis, MO, USA). The MS detector was programmed for recording in two consecutive modes: enhanced MS (EMS) and enhanced product ion (EPI) analysis. EMS was employed to show the full spectra, so as to obtain an overview of all of the ions in each sample.
The phenolic compounds in the samples were characterized according to their UV and mass spectra and retention times compared to available standards. For the quantitative analysis of phenolic compounds, a five-level calibration curve was obtained by the injection of known concentrations (2.5–100 µg/mL) of different standard compounds: caffeic acid, chlorogenic acid, gallic acid, isorhamnetin 3-O-glucoside, isorhamnetin 3-O-rutinoside, kaempferol 3-O-glucoside, kaempferol 3-O-rutinoside, quercetin 3-O-glucoside and quercetin 3-O-rutinoside. The results were expressed in mg per 100 g of dry weight (dw).
4.3. Plant Extraction and HPLC-DAD–ESI/MS Analysis of Anthocyanins
The analysis of the anthocyanins extract was performed according to Guimarães and colleagues [
24]. Briefly, they were extracted with methanol containing 0.5% trifluoroacetic acid (TFA) and filtered through a Whatman No. 4 paper. The residue was then re-extracted twice with additional 30 mL portions of 0.5% TFA in methanol. The combined extracts were evaporated at 35 °C to remove the methanol, and redissolved in water. For purification, the extract solution was deposited onto a C-18 SepPak
®- Vac 3 cc cartridge (Phenomenex).
The extracts were analyzed using the HPLC system and separation was achieved on an AQUA®-(Phenomenex) reverse phase C18 column (5 µm, 150 × 4.6 mm i.d.) with the thermostat set at 35 °C. The solvents used were: (A) 0.1% TFA in water; and (B) 100% acetonitrile. Double detection was carried out by DAD, using 520 nm as the preferred wavelength, and MS, using the same equipment described above. The EMS and ESI methods were used for the acquisition of the full spectra and fragmentation patterns of the precursor ions, respectively.
The anthocyanins present in the samples were characterized according to their UV and mass spectra and retention times, and comparison with authentic standards. For quantitative analysis, a five-level calibration curve was obtained by the injection of known concentrations (50–0.25 µg/mL) of different standard compounds: cyaniding 3-O-glucoside and peonidin 3-O-glucoside. The results were expressed in µg per 100 g of dry weight (dw).
4.4. Cell Cultures
Human colorectal carcinoma cells (HCT116), human colorectal adenocarcinoma cells (SW480), human cervical cancer cells (HeLa), human bronchoalveolar adenocarcinoma cells (A549), human gingival fibroblasts, and rat intestinal epithelial cells (IEC-6) were provided by the American Type Culture Collection (ATCC, Manassas, VA, USA) and used according to Meschini et al. [
12].
4.5. Cell Treatments
The PsT extraction was performed by macerating the vegetable material in a water/alcohol solvent (60° of alcohol) for varying periods, from a few hours to several days. The drying process was performed using the conventional methods for evaporation under reduced pressure, spray drying, or lyophilization. The dry weight of PsT used for the cell culture treatments ranged from 86 mg to 0.017 mg of the total. Cells were treated with PsT hydroalcoholic solution (86 mg/mL), or with different solutions (50, 10, 5, 4.5, 4, 2, 1, 0.5, 0.1 mg/mL) obtained after the progressive dilution of PsT 86 mg/mL with a complex blend of amino acids, vitamins and minerals, called nutraceutical activator complex (NAC), for 24 h [
12].
The experiments were also performed treating cells with PsT diluted with other vehicles, such as phosphate saline buffer (PBS, Sigma-Aldrich, Saint Louis, MO, USA) or 0.9% physiological solution (PS), to demonstrate that the (PsT + NAC)® combined action was the most effective against tumor cells.
To perform a positive control of apoptosis induction, the cells were treated with Staurosporine (STS, 1 µM, Sigma-Aldrich, Saint Louis, MO, USA) for 24 h [
13].
4.6. MTT Assay
Cell viability was assessed by (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) MTT assay (Sigma Aldrich, Saint Louis, MO, USA).
After removing the cell medium, untreated and (PsT + NAC)
® treated cells were washed with PBS and incubated with 0.5 mg/mL MTT solution for 2 h at 37 °C. After removing the MTT solution, the samples were lysed by 100 μL DMSO, and analyzed by a microplate reader (Bio-Rad, Hercules, CA, USA) at 570 nm. Cell viability (%) was calculated as follows: (absorbance mean value of the treated sample/absorbance mean value of the control sample) × 100 [
25].
4.7. Detection of Mitochondrial Membrane Potential
Cationic fluorescent probe tetramethylrhodamine methyl ester (TMRM, Molecular Probes Inc., Eugene, OR, USA) was used to monitor the loss of mitochondrial membrane potential [
26]. Untreated and treated HCT 116 and SW480 cell suspensions were stained with TMRM solution (25 µg/mL) for 10 min at 37 °C and analyzed by flow cytometry.
4.8. Cloning Efficacy Assay
Untreated and treated HCT116 and SW480 cells were detached and plated (1 × 103) per 60 mm tissue culture dish and allowed to grow in culture medium for 15 days. After growth, cell colonies were fixed with 95% ethanol for 15 min, and stained with a methylene blue solution in 80% ethanol for 2 h. Only colonies composed of more than 50 cells were evaluated. The cloning formation rate (%) was calculated by dividing the number of colonies of treated cells, and the number of colonies of untreated cells.
4.9. Annexin V-FITC/PI Assay
Annexin V-fluorescein isothiocyanate (FITC)/Propidium iodide (PI) staining was used to investigate cell death induced by the combined treatment PsT® + NAC. After treatment for 24 h, cells were processed using an Annexin V-FITC/PI apoptosis detection kit (eBioscence, London, UK). They were detached, centrifuged and re-suspended in binding buffer 1X. Cell suspensions were then incubated with 5 µL of Annexin V-FITC solution for 15 min. After washing with binding buffer 1X, cells were incubated with 5 µL of PI and immediately analyzed by flow cytometer.
4.10. Cell Cycle Analysis
Untreated and treated HCT 116 and SW480 cell pellets were fixed in 70% ethanol in PBS at 4 °C for 1 h, washed twice and then re-suspended in PBS containing 100 µg/mL ribonuclease (RNAse, Sigma-Aldrich, St. Louis, MO, USA). Cellular DNA was labelled with 40 µg/mL PI in PBS and stored at 37 °C, for at least 30 min. After this, incubation cells were analyzed by flow cytometry.
4.11. Flow Cytometric Analyses
Flow cytometric analyses were carried out by a BD LSR II flow cytometer (Becton, Dickinson & Company, Franklin Lakes, NJ, USA) equipped with a 15 mW, 488 nm, air-cooled argon ion laser and a Kimmon HeCd 325 nm laser. At least 10,000 events were acquired in lin or log mode. The percentage of depolarized/hyperpolarized cells, quantitative analysis of apoptosis, and cell cycle distribution were performed using FACS Diva Software (Becton, Dickinson & Company).
4.12. Statistical Analyses
The distribution of each measurement was examined for the assumption of normality with the Shapiro–Wilk test. One-way Analysis of Variance (ANOVA) was applied to detect differences between the control and treatments. Bonferroni post hoc analysis was applied to reveal differences between all treated samples in each cell line. The alpha level was set at p < 0.05.