Isaria fumosorosea KCh J2 Entomopathogenic Strain as an Effective Biocatalyst for Steroid Compound Transformations
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Screening Procedure
3.3. Screening Procedure for DHEA
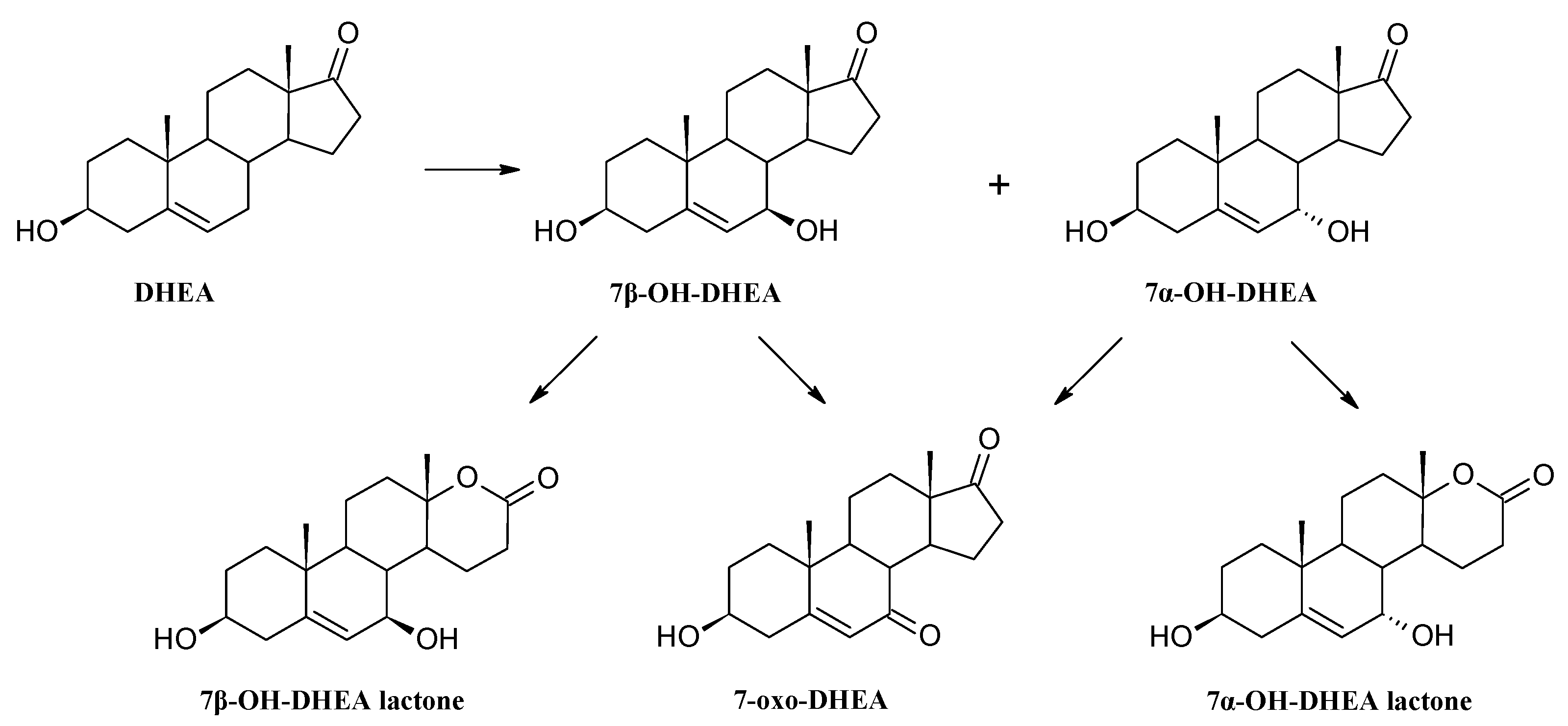
3.4. Establishing the DHEA Transformation Pathway
3.5. Transformation Procedure for Different DHEA Concentrations
3.6. Preparative Biotransformation
3.7. Analytical Methods
3.8. Spectral Data of Isolated Metabolites
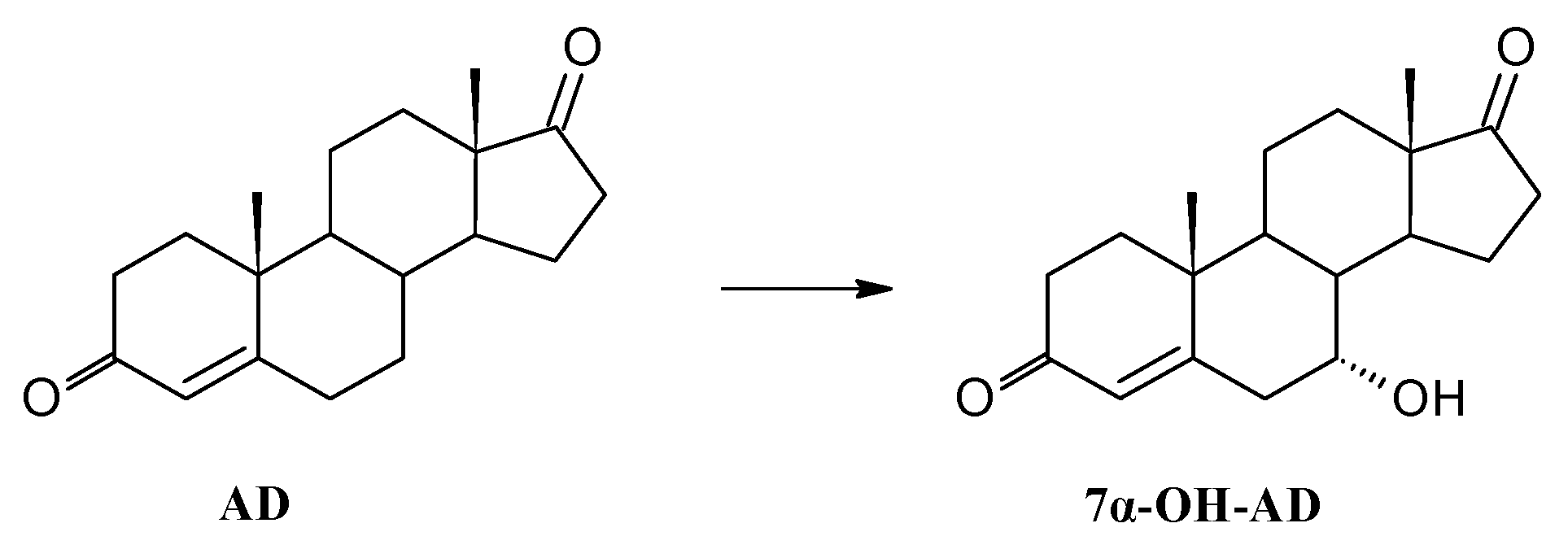
3.8.1. Transformation of Androstenedione (AD)
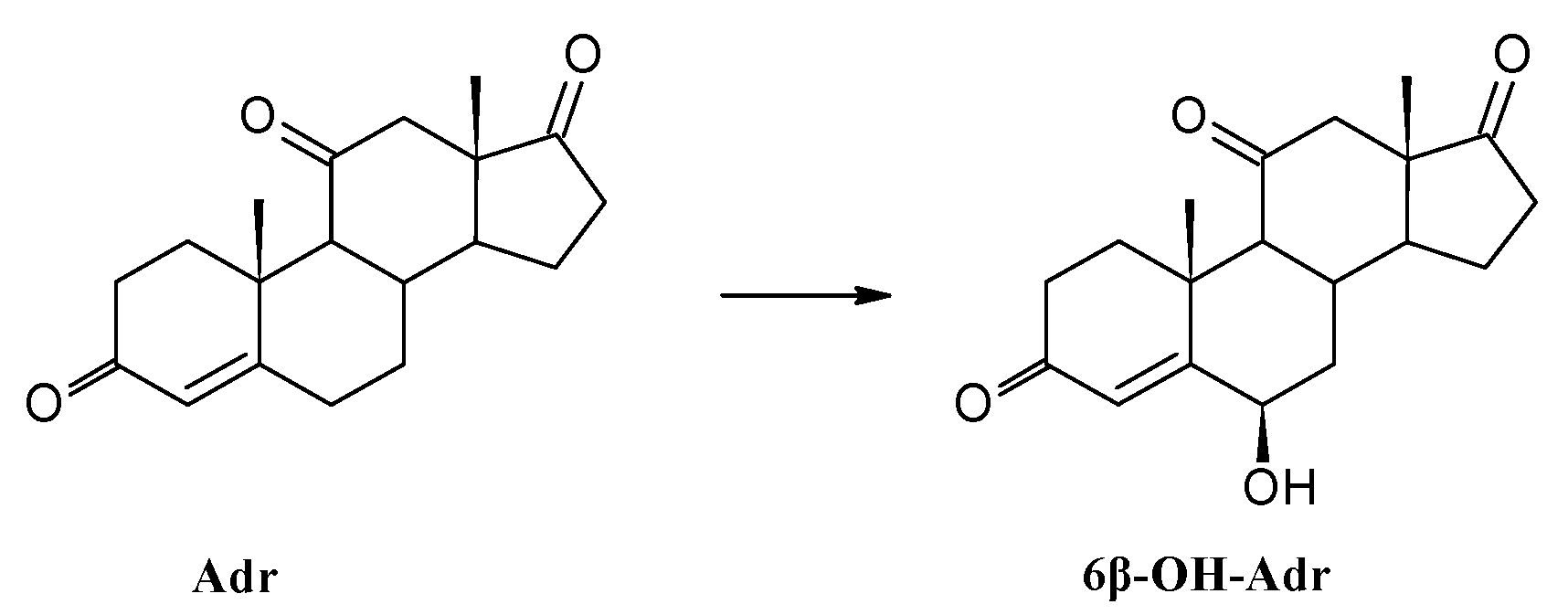
3.8.2. Transformation of Adrenosterone (Adr)
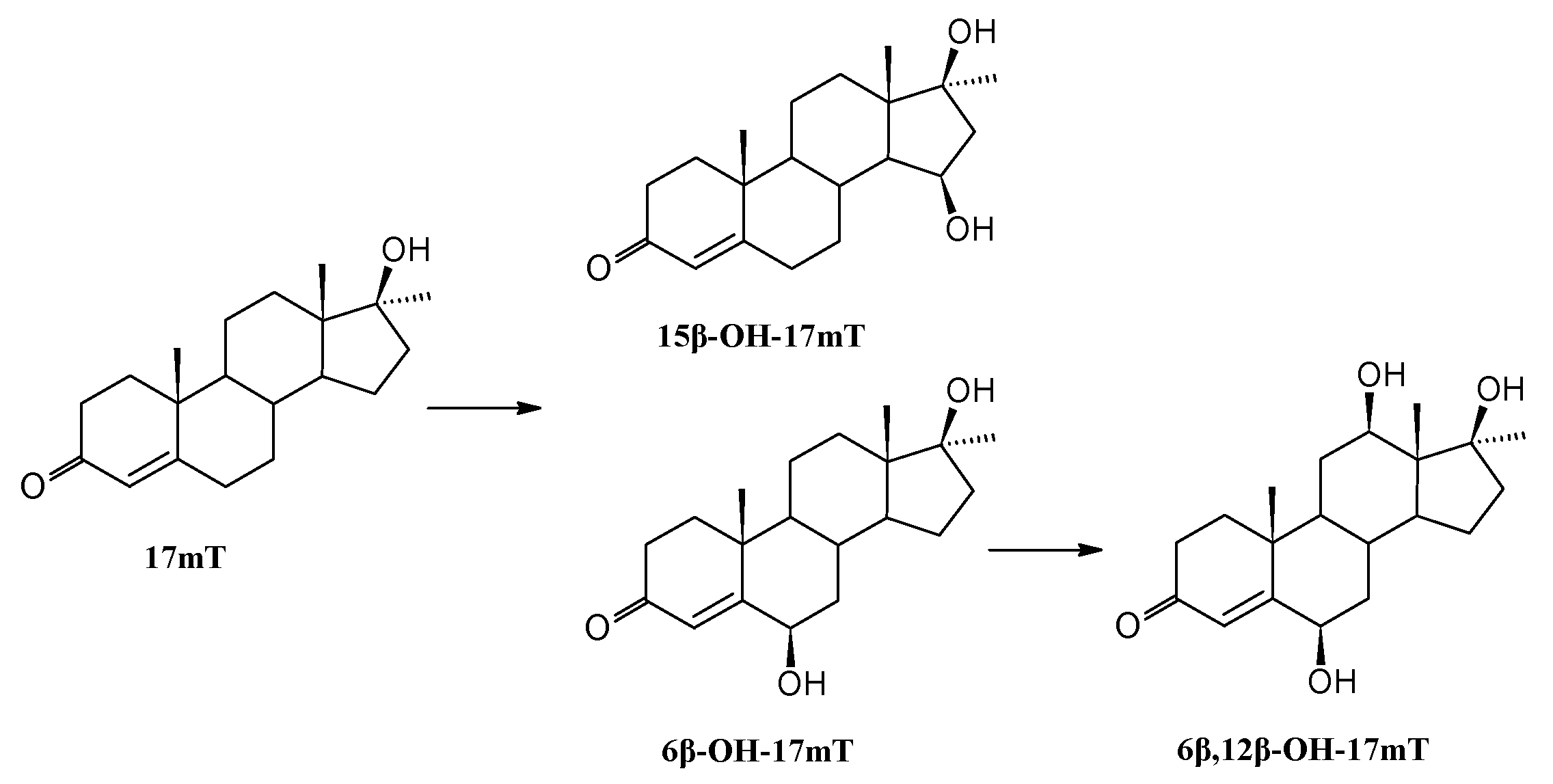
3.8.3. Transformation of 17α-Methyltestosterone (17mT)
3.8.4. Transformation of Progesterone (P)
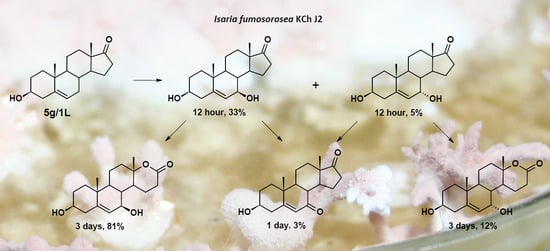
3.8.5. Transformation of dehydroepiandrosterone (DHEA)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crabb, T.A.; Saul, J.A.; Williams, R.O. Microbiological transformations. Part 4. Microbiological transformations of 5α-androstan-17-ones and of 17a-aza-d-homo-5α -androstan-17-ones with the fungus Cunninghamella elegans. J. Chem. Soc. Perk. Trans. 1 1981, 1041–1045. [Google Scholar] [CrossRef]
- Žnidaršič-Plazl, P.; Plazl, I. Development of a continuous steroid biotransformation process and product extraction within microchannel system. Catal. Today 2010, 157, 315–320. [Google Scholar] [CrossRef]
- Schmid, A.; Dordick, J.S.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Industrial biocatalysis today and tomorrow. Nature 2001, 409, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Kołek, T.; Milecka, N.; Świzdor, A.; Panek, A.; Białońska, A. Hydroxylation of DHEA, androstenediol and epiandrosterone by Mortierella isabellina AM212. Evidence indicating that both constitutive and inducible hydroxylases catalyze 7α- as well as 7β-hydroxylations of 5-ene substrates. Org. Biomol. Chem. 2011, 9, 5414–5422. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, H.N.; Khera, R.A. Biological transformations of steroidal compounds: A review. Steroids 2012, 77, 1267–1290. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, E.; Urbaniak, M.; Kancelista, A.; Dymarska, M.; Kostrzewa-Susłow, E.; Stępień, Ł.; Janeczko, T. Biotransformation of dehydroepiandrosterone (DHEA) by environmental strains of filamentous fungi. RSC Adv. 2017, 7, 31493–31501. [Google Scholar] [CrossRef]
- Janeczko, T.; Świzdor, A.; Dmochowska-Gładysz, J.; Białońska, A.; Ciunik, Z.; Kostrzewa-Susłow, E. Novel metabolites of dehydroepiandrosterone and progesterone obtained in Didymosphearia igniaria KCH 6670 culture. J. Mol. Catal. B Enzym. 2012, 82, 24–31. [Google Scholar] [CrossRef]
- Katz, M.; Gans, E.H. Topical corticosteroids, structure-activity and the glucocorticoid receptor: Discovery and development—A process of “Planned Serendipity”. J. Pharm. Sci. 2008, 97, 2936–2947. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, T.; Milecka, N.; Kostrzewa-Susłow, E. Industrial importance of microbial hydroxylation of steroids. Przem. Chem. 2012, 91, 767–771. [Google Scholar]
- Brazzini, B.; Pimpinelli, N. New and established topical corticosteroids in dermatology: Clinical pharmacology and therapeutic use. Am. J. Clin. Dermatol. 2002, 3, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Borges, K.B.; de Souza Borges, W.; Durán-Patrón, R.; Pupo, M.T.; Bonato, P.S.; Collado, I.G. Stereoselective biotransformations using fungi as biocatalysts. Tetrahedron-Asymmetry 2009, 20, 385–397. [Google Scholar] [CrossRef]
- Loria, R.M. Immune up-regulation and tumor apoptosis by androstene steroids. Steroids 2002, 67, 953–966. [Google Scholar] [CrossRef]
- Pelissier, M.-A.; Trap, C.; Malewiak, M.-I.; Morfin, R. Antioxidant effects of dehydroepiandrosterone and 7alpha-hydroxy-dehydroepiandrosterone in the rat colon, intestine and liver. Steroids 2004, 69, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Hampl, R.; Lapčík, O.; Hill, M.; Klak, J.; Kasal, A.; Nováček, A.; Šterzl, I.; Šterzl, J.; Stárka, L. 7-Hydroxydehydroepiandrosterone-a natural antiglucocorticoid and a candidate for steroid replacement therapy? Physiol. Res. 2000, 49, S107–S112. [Google Scholar] [PubMed]
- Duskova, M.; Simunkova, K.; Hill, M.; Starka, L. 7-Hydroxylated derivatives of dehydroepiandrosterone as possibly related to menstrual mood change in healthy women. Endocr. Regul. 2011, 45, 131–137. [Google Scholar] [CrossRef] [PubMed]
- El Kihel, L. Oxidative metabolism of dehydroepiandrosterone (DHEA) and biologically active oxygenated metabolites of DHEA and epiandrosterone (EpiA)-Recent reports. Steroids 2012, 77, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, L.M.; Balthazart, J. Steroids and neuroprotection: New advances. Front. Neuroendocrinol. 2009, 30, v–ix. [Google Scholar] [CrossRef] [PubMed]
- Huppert, F.A.; Van Niekerk, J.K. Dehydroepiandrosterone (DHEA) supplementation for cognitive function. Cochrane Database Syst. Rev. 2001, 2. [Google Scholar] [CrossRef]
- Bazin, M.-A.; El Kihel, L.; Boulouard, M.; Bouët, V.; Rault, S. The effects of DHEA, 3β-hydroxy-5α-androstane-6,17-dione, and 7-amino-DHEA analogues on short term and long term memory in the mouse. Steroids 2009, 74, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Hennebert, O.; Pelissier, M.A.; Le Mee, S.; Wülfert, E.; Morfin, R. Anti-inflammatory effects and changes in prostaglandin patterns induced by 7β-hydroxy-epiandrosterone in rats with colitis. J. Steroid Biochem. Mol. Biol. 2008, 110, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lobastova, T.G.; Gulevskaya, S.A.; Sukhodolskaya, G.V.; Turchin, K.F.; Donova, M.V. Screening of mycelial fungi for 7α- and 7β-hydroxylase activity towards dehydroepiandrosterone. Biocatal. Biotransform. 2007, 25, 434–442. [Google Scholar] [CrossRef]
- Milecka-Tronina, N.; Kołek, T.; Świzdor, A.; Panek, A. Hydroxylation of DHEA and its analogues by Absidia coerulea AM93. Can an inducible microbial hydroxylase catalyze 7α- and 7β-hydroxylation of 5-ene and 5α-dihydro C19-steroids? Bioorgan. Med. Chem. 2014, 22, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Kołek, T. Biotransformation XLVII: Transformations of 5-ene steroids in Fusarium culmorum culture. J. Steroid Biochem. Mol. Biol. 1999, 71, 83–90. [Google Scholar] [CrossRef]
- Koshimura, M.; Utsukihara, T.; Hara, A.; Mizobuchi, S.; Horiuchi, C.A.; Kuniyoshi, M. Hydroxylation of steroid compounds by Gelasinospora retispora. J. Mol. Catal. B Enzym. 2010, 67, 72–77. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, D.; Chen, Z.; Ruan, H.; Ge, W. Biotransformation of 3β-hydroxy-5-en-steroids by Mucor silvaticus. Biocatal. Biotransform. 2013, 31, 168–174. [Google Scholar] [CrossRef]
- Bensasson, C.M.; Hanson, J.R.; Hunter, A.C. The hydroxylation of Δ5-androstenes by Cephalosporium aphidicola. Phytochemistry 1998, 49, 2355–2358. [Google Scholar] [CrossRef]
- Yildirim, K. Microbial hydroxylation of some steroids by Aspergillus wentii MRC 200316. Collect. Czechoslov. Chem. Commun. 2010, 75, 1273–1281. [Google Scholar] [CrossRef]
- Janeczko, T.; Dmochowska-Gładysz, J.; Kostrzewa-Susłow, E.; Białońska, A.; Ciunik, Z. Biotransformations of steroid compounds by Chaetomium sp. KCH 6651. Steroids 2009, 74, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.J.; Zhou, B.; Lai, T.C.; Su, H.L.; Yang, H.Y. Study on Biotransformation Products of Androstenedione by Paecilomyces victoriae. Adv. Mater. Res. 2013, 807, 414–417. [Google Scholar] [CrossRef]
- Huszcza, E.; Dmochowska-Gladysz, J. Transformations of testosterone and related steroids in Absidia glauca culture. J. Basic Microbiol. 2003, 43, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zenk, J.L.; Helmer, T.R.; Kassen, L.J.; Kuskowski, M.A. The effect of 7-Keto Naturalean™ on weight loss: A randomized, double-blind, placebo-controlled trial. Curr. Ther. Res. Clin. Exp. 2002, 63, 263–272. [Google Scholar] [CrossRef]
- Ihler, G.; Chami-Stemmann, H. 7-oxo-DHEA and Raynaud’s phenomenon. Med. Hypotheses 2003, 60, 391–397. [Google Scholar] [CrossRef]
- Kalman, D.; Colker, C.; Swain, M.; Torina, G. A randomized, double-blind, placebo-controlled study of 3-acetyl-7-oxo-dehydroepiandrosterone in healthy overweight adults. Curr. Ther. Res. 2000, 61, 435–442. [Google Scholar] [CrossRef]
- Hampl, R.; Sulcová, J.; Bílek, R.; Hill, M. How short-term transdermal treatment of men with 7-oxo-dehydroepiandrosterone influence thyroid function. Physiol. Res. 2006, 55, 49–54. [Google Scholar] [PubMed]
- Yadav, M.R.; Barmade, M.A.; Tamboli, R.S.; Murumkar, P.R. Developing steroidal aromatase inhibitors-an effective armament to win the battle against breast cancer. Eur. J. Med. Chem. 2015, 105, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Cepa, M.M.D.S.; Tavares da Silva, E.J.; Correia-da-Silva, G.; Roleira, F.M.F.; Teixeira, N.A.A. Structure-activity relationships of new A,D-ring modified steroids as aromatase inhibitors: Design, synthesis, and biological activity evaluation. J. Med. Chem. 2005, 48, 6379–6385. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, Y.; Chen, X.; Feng, J.; Wu, Q.; Zhu, D. Biotransformations of steroids to testololactone by a multifunctional strain Penicillium simplicissimum WY134-2. Tetrahedron 2014, 70, 41–46. [Google Scholar] [CrossRef]
- Świzdor, A. Baeyer-Villiger oxidation of some C19 steroids by Penicillium lanosocoeruleum. Molecules 2013, 18, 13812–13822. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Evans, H.C.; Latgé, J.-P. Atlas of Entomopathogenic Fungi, 1st edition; Springer: Berlin/Heidelberg, Germany, 1988; pp. 5–16. ISBN 978-3-662-05892-3. [Google Scholar]
- Gibson, D.M.; Donzelli, B.G.G.; Krasnoff, S.B.; Keyhani, N.O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 2014, 31, 1287–1305. [Google Scholar] [CrossRef] [PubMed]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lücking, R.; et al. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef] [PubMed]
- Heitman, J. Microbial pathogens in the fungal kingdom. Fungal Biol. Rev. 2011, 25, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Cantone, F.A.; Vandenberg, J.D. Intraspecific diversity in Paecilomyces fumosoroseus. Mycol. Res. 1998, 102, 209–215. [Google Scholar] [CrossRef]
- Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Manoch, L.; Samson, R.A. On the relationships of Paecilomyces sect. Isarioidea species. Mycol. Res. 2005, 109, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hao, Y.; Gao, T.; Huang, Y.; Ren, S.; Keyhani, N.O. The Ifchit1 chitinase gene acts as a critical virulence factor in the insect pathogenic fungus Isaria fumosorosea. Appl. Microbiol. Biotechnol. 2016, 100, 5491–5503. [Google Scholar] [CrossRef] [PubMed]
- Avery, P.B.; Wekesa, V.W.; Hunter, W.B.; Hall, D.G.; Cindy, L.; Osborne, L.S.; Powell, C.A.; Rogers, M.E. Effects of the fungus Isaria fumosorosea (Hypocreales : Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (emiptera : Psyllidae). Biocontrol. Sci. Technol. 2011, 21, 1065–1078. [Google Scholar] [CrossRef]
- Hunter, A.W.B.; Avery, P.B.; Pick, D.; Powell, C.A. Broad spectrum potential of Isaria fumosorosea against insect pests of citrus. Sci. Notes 2011, 94, 1051–1054. [Google Scholar]
- Pick, D.A.; Avery, P.B.; Hunter, W.B.; Powell, C.A.; Arthurs, S.P. Effect of Isaria fumosorosea (Hypocreales: Cordycipitaceae) and Lysiphlebus testaceipes, (Hymenoptera: Braconidae) on the brown citrus aphid: Preliminary assessment of a compatibility study. Fla. Entomol. 2012, 95, 764–766. [Google Scholar] [CrossRef]
- Wang, C.; Gao, T.; Huang, Y.; Huang, Z. Effect of Ifchit1 gene of Isaria fumosorosea on mortality, oviposition and oxidase activities of Bemisia tabaci. Biocontrol. Sci. Technol. 2017, 27, 485–495. [Google Scholar] [CrossRef]
- Ali, S.; Huang, Z.; Ren, S. Media composition influences on growth, enzyme activity, and virulence of the entomopathogen hyphomycete Isaria fumosoroseus. Entomol. Exp. Appl. 2009, 131, 30–38. [Google Scholar] [CrossRef]
- Ali, S.; Huang, Z.; Ren, S. Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biocontrol agent against diamondback moth. J. Pest Sci. 2010, 83, 361–370. [Google Scholar] [CrossRef]
- Ortiz-Urquiza, A.; Keyhani, N. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, N.; Ortiz-Urquiza, A.; Huarte-Bonnet, C.; Zhang, S.; Keyhani, N.O. Targeting of insect epicuticular lipids by the entomopathogenic fungus Beauveria bassiana: Hydrocarbon oxidation within the context of a host-pathogen interaction. Front. Microbiol. 2013, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Gunatilaka, A.A.L. Microbial metabolism of 1-aminoanthracene by Beauveria bassiana. Bioorgan. Med. Chem. 2008, 16, 5085–5089. [Google Scholar] [CrossRef] [PubMed]
- Olivo, H.F.; Peeples, T.L.; Rı́os, M.-Y.; Velázquez, F.; Kim, J.-W.; Narang, S. Microbial C-hydroxylation and β-4-O-methylglucosidation of methyl-benzamide 7-azanorbornane ethers with Beauveria bassiana. J. Mol. Catal. B Enzym. 2003, 21, 97–105. [Google Scholar] [CrossRef]
- Tronina, T.; Bartmańska, A.; Milczarek, M.; Wietrzyk, J.; Popłoński, J.; Rój, E.; Huszcza, E. Antioxidant and antiproliferative activity of glycosides obtained by biotransformation of xanthohumol. Bioorgan. Med. Chem. Lett. 2013, 23, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Bartmańska, A.; Huszcza, E.; Tronina, T. Transformation of isoxanthohumol by fungi. J. Mol. Catal. B Enzym. 2009, 61, 221–224. [Google Scholar] [CrossRef]
- Bartmańska, A.; Tronina, T.; Huszcza, E. Transformation of 8-prenylnaringenin by Absidia coerulea and Beauveria bassiana. Bioorgan. Med. Chem. Lett. 2012, 22, 6451–6453. [Google Scholar] [CrossRef] [PubMed]
- Sordon, S.; Popłoński, J.; Tronina, T.; Huszcza, E. Microbial glycosylation of daidzein, genistein and biochanin A: Two new glucosides of biochanin A. Molecules 2017, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Tronina, T.; Strugała, P.; Popłoński, J.; Włoch, A.; Sordon, S.; Bartmańska, A.; Huszcza, E. The influence of glycosylation of natural and synthetic prenylated flavonoids on binding to human serum albumin and inhibition of cyclooxygenases COX-1 and COX-2. Molecules 2017, 22, 1230. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wei, Q.; Chen, H.; Chen, S.; Xu, W.; Qiu, G.; Liang, S.; Hu, X. Microbial transformation of androst-4-ene-3,17-dione by Beauveria bassiana. Steroids 2006, 71, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Nicolau, F.; Peeples, T.L. Optimization of the 11α-hydroxylation of steroid DHEA by solvent-adapted Beauveria bassiana. Biocatal. Biotransform. 2017, 35, 103–109. [Google Scholar] [CrossRef]
- Gonzalez, R.; Nicolau, F.; Peeples, T. N-alkane solvent-enhanced biotransformation of steroid DHEA by Beauveria bassiana as biocatalyst. J. Adv. Biol. Biotechnol. 2015, 2, 30–37. [Google Scholar] [CrossRef]
- Gao, Q.; Qiao, Y.; Shen, Y.; Wang, M.; Wang, X.; Liu, Y. Screening for strains with 11α-hydroxylase activity for 17α-hydroxy progesterone biotransformation. Steroids 2017, 124, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Huszcza, E.; Dmochowska-Gładysz, J.; Bartmańska, A. Transformations of steroids by Beauveria bassiana. Z. Naturforsch. C 2005, 60, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Świzdor, A.; Kołek, T.; Panek, A.; Białońska, A. Microbial Baeyer-Villiger oxidation of steroidal ketones using Beauveria bassiana: Presence of an 11α-hydroxyl group essential to generation of d-homo lactones. Biochim. BBA-Mol. Cell Biol. Lipids 2011, 1811, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Świzdor, A.; Panek, A.; Milecka-Tronina, N. Microbial Baeyer-Villiger oxidation of 5α-steroids using Beauveria bassiana. A stereochemical requirement for the 11α-hydroxylation and the lactonization pathway. Steroids 2014, 82, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lobastova, T.G.; Khomutov, S.M.; Donova, M.V. Formation of hydroxylated steroid lactones from dehydroepiandrosterone by Spicaria fumoso-rosea F-881. Appl. Biochem. Microbiol. 2015, 51, 180–187. [Google Scholar] [CrossRef]
- Urech, J.; Vischer, E.; Wettstein, A. Substratspezifische hydroxylierungen von steroiden mittels pilz-stämmen der gattung Gibberella. Mikrobiologische Reaktionen, 9. Mitteilung. Helv. Chim. Acta 1960, 43, 1077–1086. [Google Scholar] [CrossRef]
- Nassiri-Koopaei, N.; Mogharabi, M.; Amini, M.; Shafiee, A.; Faramarzi, M.A. Fungal transformation of methyltestosterone by the soil ascomycete Acremonium strictum to some hydroxy derivatives of 17-methylsteroid. Chem. Nat. Compd. 2013, 49, 665–670. [Google Scholar] [CrossRef]
- Faramarzi, M.A.; Aghelnejad, M.; Tabatabaei Yazdi, M.; Amini, M.; Hajarolasvadi, N. Metabolism of androst-4-en-3,17-dione by the filamentous fungus Neurospora crassa. Steroids 2008, 73, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, M.A.; Badiee, M.; Yazdi, M.T.; Amini, M.; Torshabi, M. Formation of hydroxysteroid derivatives from androst-4-en-3,17-dione by the filamentous fungus Mucor racemosus. J. Mol. Catal. B Enzym. 2008, 50, 7–12. [Google Scholar] [CrossRef]
- Lehman, L.R.; Stewart, J.D. Filamentous fungi: Potentially useful catalysts for the biohydroxylations of non-activated carbon centers. Curr. Org. Chem. 2001, 5, 439–470. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Khan, N.T.; Musharraf, S.G.; Anjum, S.; Atta-ur-Rahman. Biotransformation of adrenosterone by filamentous fungus, Cunninghamella elegans. Steroids 2007, 72, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Brannon, D.R.; Parrish, F.W.; Wiley, B.J.; Long Jr, L. Microbial transformation of a series of andogens with Aspergillus tamarii. J. Org. Chem. 1967, 32, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Musharraf, S.G.; Atta-Ur-Rahman; Choudhary, M.I.; Sultan, S. Microbial transformation of (+)-adrenosterone. Nat. Prod. Lett. 2002, 16, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Robinzon, B.; Prough, R.A. A novel NADP+-dependent dehydrogenase activity for 7α/β- and 11β-hydroxysteroids in human liver nuclei: A third 11β-hydroxysteroid dehydrogenase. Arch. Biochem. Biophys. 2009, 486, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Pompon, D.; Urban, P.; Morfin, R. Inter-conversion of 7α- and 7β-hydroxy-dehydroepiandrosterone by the human 11β-hydroxysteroid dehydrogenase type 1. J. Steroid Biochem. Mol. Biol. 2006, 99, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Marwah, A.; Marwah, P.; Lardy, H. Ergosteroids: VI. Metabolism of dehydroepiandrosterone by rat liver in vitro : A liquid chromatographic-mass spectrometric study. J. Chromatogr. B 2002, 767, 285–299. [Google Scholar] [CrossRef]
- Hennebert, O.; Montes, M.; Favre-Reguillon, A.; Chermette, H.; Ferroud, C.; Morfin, R. Epimerase activity of the human 11β-hydroxysteroid dehydrogenase type 1 on 7-hydroxylated C19-steroids. J. Steroid Biochem. Mol. Biol. 2009, 114, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Chalbot, S. Human liver S9 fractions: Metabolism of dehydroepiandrosterone, epiandrosterone, and related 7-hydroxylated derivatives. Drug Metab. Dispos. 2005, 33, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Dymarska, M.; Grzeszczuk, J.; Urbaniak, M.; Janeczko, T.; Pląskowska, E.; Stępień, Ł.; Kostrzewa-Susłow, E. Glycosylation of 6-methylflavone by the strain Isaria fumosorosea KCH J2. PLoS ONE 2017, 12, e.0184885. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the compounds are available from the authors. |
| Atom Number | Products | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7α-OH-AD | 6β-OH-Adr | 6β-OH-17mT | 6β-OH-17mT a | 15β-OH-17mT | 6β,12β-OH-17mT b | 7α-OH-DHEA | 7β-OH-DHEA | 7α-OH-DHEA-Lactone | 7β-OH-DHEA-Lactone | 7-oxo-DHEA | |
| 1 | 35.40 | 36.10 | 38.36 | 38.50 | 34.11 | 36.58 | 37.07 | 37.02 | 36.84 | 36.72 | 36.60 |
| 2 | 33.89 | 34.11 | 34.39 | 35.08 | 32.87 | 33.89 | 31.39 | 31.60 | 31.32 | 31.53 | 31.25 |
| 3 | 198.83 | 200.67 | 200.53 | 198.91 | 199.71 | 199.22 | 71.25 | 71.30 | 71.20 | 71.11 | 70.44 |
| 4 | 127.03 | 127.17 | 126.55 | 126.46 | 124.07 | 125.21 | 42.06 | 41.77 | 42.02 | 41.47 | 41.99 |
| 5 | 167.25 | 166.61 | 168.38 | 169.45 | 171.20 | 168.84 | 146.64 | 143.78 | 146.29 | 144.00 | 166.27 |
| 6 | 41.01 | 72.13 | 73.25 | 73.40 | 32.78 | 71.07 | 123.67 | 125.65 | 123.43 | 125.15 | 126.06 |
| 7 | 67.01 | 38.10 | 39.05 | 40.11 | 32.22 | 37.52 | 64.37 | 72.91 | 63.44 | 72.21 | 201.20 |
| 8 | 39.37 | 30.65 | 30.70 | 31.82 | 35.94 | 29.34 | 37.32 | 40.54 | 40.24 | 43.42 | 44.47 |
| 9 | 45.31 | 63.13 | 53.77 | 55.32 | 54.32 | 51.68 | 42.72 | 48.37 | 41.76 | 48.13 | 50.22 |
| 10 | 38.55 | 37.68 | 38.22 | 39.14 | 38.95 | 38.27 | 37.63 | 36.78 | 37.54 | 36.52 | 38.54 |
| 11 | 20.15 | 207.41 | 20.78 | 21.85 | 20.70 | 29.54 | 20.19 | 20.51 | 21.70 | 22.07 | 20.72 |
| 12 | 30.98 | 50.50 | 31.54 | 32.79 | 31.47 | 71.83 | 31.18 | 31.36 | 38.53 | 39.15 | 30.85 |
| 13 | 47.31 | 50.44 | 45.60 | 46.58 | 44.89 | 48.41 | 47.23 | 47.89 | 83.50 | 83.46 | 48.00 |
| 14 | 45.64 | 49.71 | 50.29 | 51.57 | 54.77 | 49.35 | 45.05 | 51.34 | 40.15 | 47.20 | 45.88 |
| 15 | 21.25 | 21.69 | 23.30 | 24.37 | 69.16 | 22.67 | 22.02 | 24.31 | 19.86 | 21.55 | 24.31 |
| 16 | 35.71 | 36.03 | 37.29 | 39.89 | 51.97 | 38.02 | 35.91 | 36.10 | 28.86 | 29.26 | 35.77 |
| 17 | 220.41 | 217.24 | 81.73 | 80.90 | 81.40 | 80.42 | 221.30 | 221.31 | 171.83 | 171.79 | 220.52 |
| 18 | 13.49 | 14.91 | 14.11 | 14.71 | 16.67 | 8.75 | 13.39 | 13.70 | 20.08 | 20.38 | 13.89 |
| 19 | 17.01 | 19.32 | 19.68 | 19.94 | 17.47 | 18.86 | 18.38 | 19.29 | 18.33 | 19.07 | 17.57 |
| 20 | 25.91 | 26.64 | 25.41 | 25.83 | |||||||
| Substrate | Compounds Found in the Reaction Mixture (%) | Biotransformation Time (Days) | ||
|---|---|---|---|---|
| 1 | 3 | 7 | ||
| Androstenedione | AD | - | - | - |
| 7α-OH-AD | 76 | 71 | 64 | |
| Adrenosterone | Adr | 38 | 28 | 11 |
| 6β-OH-Adr | 57 | 67 | 84 | |
| 17α-Methyltestosterone | 17mT | - | - | - |
| 6β-OH-17mT | 76 | 49 | 20 | |
| 15β-OH-17mT | 11 | 9 | 8 | |
| 6β,12β-OH-17mT | 4 | 67 | 84 | |
| Concentration of Substrate (g/L) | Compounds Found in the Reaction Mixture (%) | Biotransformation Time (h) | ||||
|---|---|---|---|---|---|---|
| 3 | 12 | 24 | 72 | 168 | ||
| 0.1 | DHEA | 7 | - | - | - | - |
| 7α-OH-DHEA | 15 | 2 | - | - | - | |
| 7β-OH-DHEA | 75 | 34 | 18 | 8 | - | |
| 7-oxo-DHEA | 2 | 9 | 6 | - | - | |
| 7α-OH-DHEA lactone | - | 13 | 20 | 22 | 22 | |
| 7β-OH-DHEA lactone | - | 41 | 52 | 60 | 58 | |
| 0.5 | DHEA | 74 | 2 | - | - | - |
| 7α-OH-DHEA | 5 | 1 | - | - | - | |
| 7β-OH-DHEA | 21 | 16 | - | - | - | |
| 7-oxo-DHEA | - | 1 | - | - | - | |
| 7α-OH-DHEA lactone | - | 15 | 18 | 17 | 8 | |
| 7β-OH-DHEA lactone | - | 62 | 76 | 72 | 74 | |
| 1.0 | DHEA | 75 | 1 | - | - | - |
| 7α-OH-DHEA | 5 | 2 | - | - | - | |
| 7β-OH-DHEA | 20 | 36 | - | - | - | |
| 7-oxo-DHEA | - | 2 | - | - | - | |
| 7α-OH-DHEA lactone | - | 12 | 19 | 20 | 15 | |
| 7β-OH-DHEA lactone | - | 45 | 76 | 70 | 66 | |
| 2.0 | DHEA | 89 | 8 | - | - | - |
| 7α-OH-DHEA | 2 | 7 | - | - | - | |
| 7β-OH-DHEA | 9 | 58 | 2 | - | - | |
| 7-oxo-DHEA | - | 4 | - | - | - | |
| 7α-OH-DHEA lactone | - | 1 | 16 | 14 | 12 | |
| 7β-OH-DHEA lactone | - | 20 | 78 | 78 | 79 | |
| 5.0 | DHEA | 96 | 56 | 26 | - | - |
| 7α-OH-DHEA | 1 | 5 | 6 | - | - | |
| 7β-OH-DHEA | 3 | 33 | 37 | - | - | |
| 7-oxo-DHEA | - | 1 | 3 | - | - | |
| 7α-OH-DHEA lactone | - | - | 2 | 12 | 11 | |
| 7β-OH-DHEA lactone | - | 4 | 23 | 81 | 75 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, E.; Dymarska, M.; Kostrzewa-Susłow, E.; Janeczko, T. Isaria fumosorosea KCh J2 Entomopathogenic Strain as an Effective Biocatalyst for Steroid Compound Transformations. Molecules 2017, 22, 1511. https://doi.org/10.3390/molecules22091511
Kozłowska E, Dymarska M, Kostrzewa-Susłow E, Janeczko T. Isaria fumosorosea KCh J2 Entomopathogenic Strain as an Effective Biocatalyst for Steroid Compound Transformations. Molecules. 2017; 22(9):1511. https://doi.org/10.3390/molecules22091511
Chicago/Turabian StyleKozłowska, Ewa, Monika Dymarska, Edyta Kostrzewa-Susłow, and Tomasz Janeczko. 2017. "Isaria fumosorosea KCh J2 Entomopathogenic Strain as an Effective Biocatalyst for Steroid Compound Transformations" Molecules 22, no. 9: 1511. https://doi.org/10.3390/molecules22091511
APA StyleKozłowska, E., Dymarska, M., Kostrzewa-Susłow, E., & Janeczko, T. (2017). Isaria fumosorosea KCh J2 Entomopathogenic Strain as an Effective Biocatalyst for Steroid Compound Transformations. Molecules, 22(9), 1511. https://doi.org/10.3390/molecules22091511