Radiolabeled Dendrimers for Nuclear Medicine Applications
Abstract
:1. Introduction
2. Radiolabeled Dendrimers
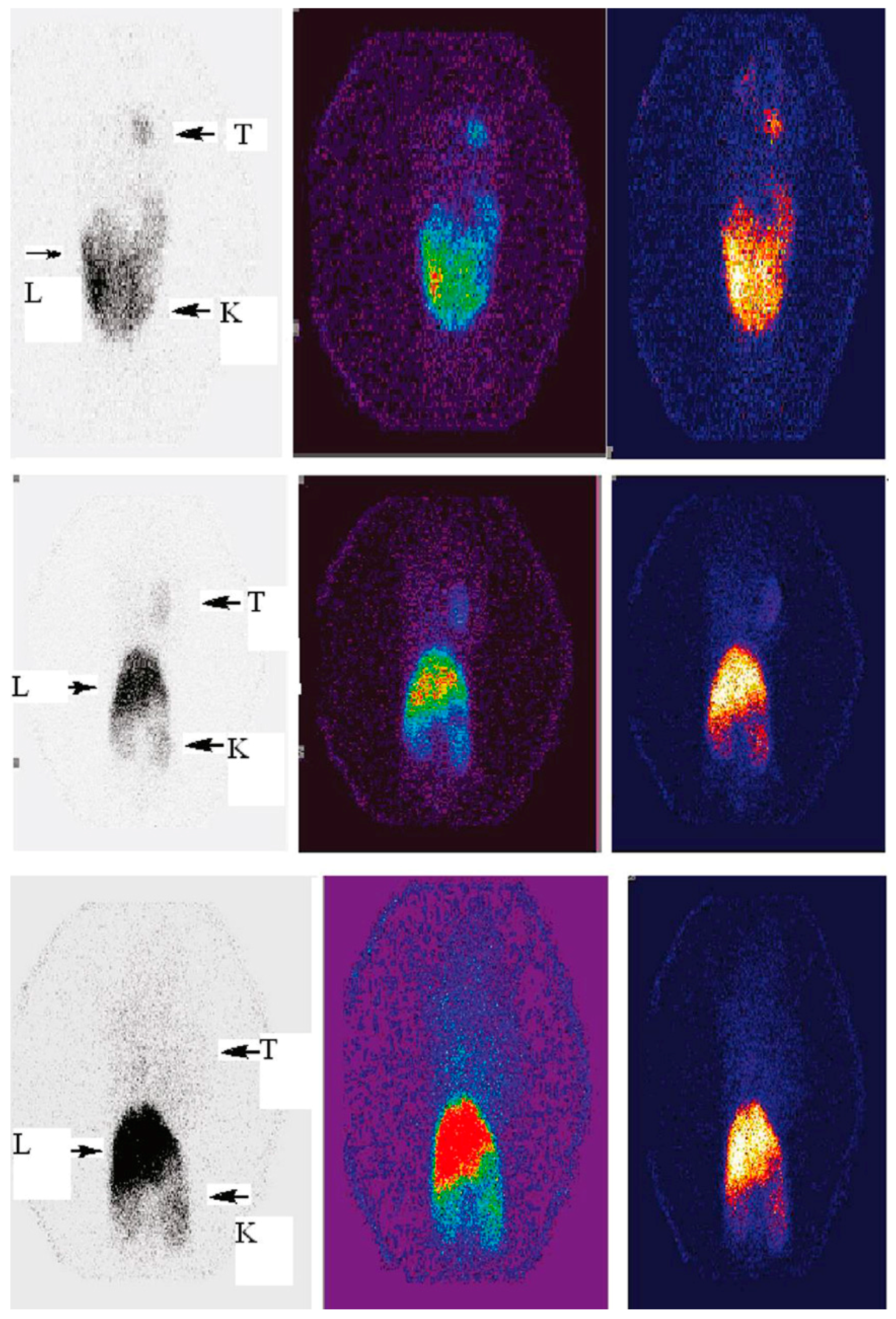
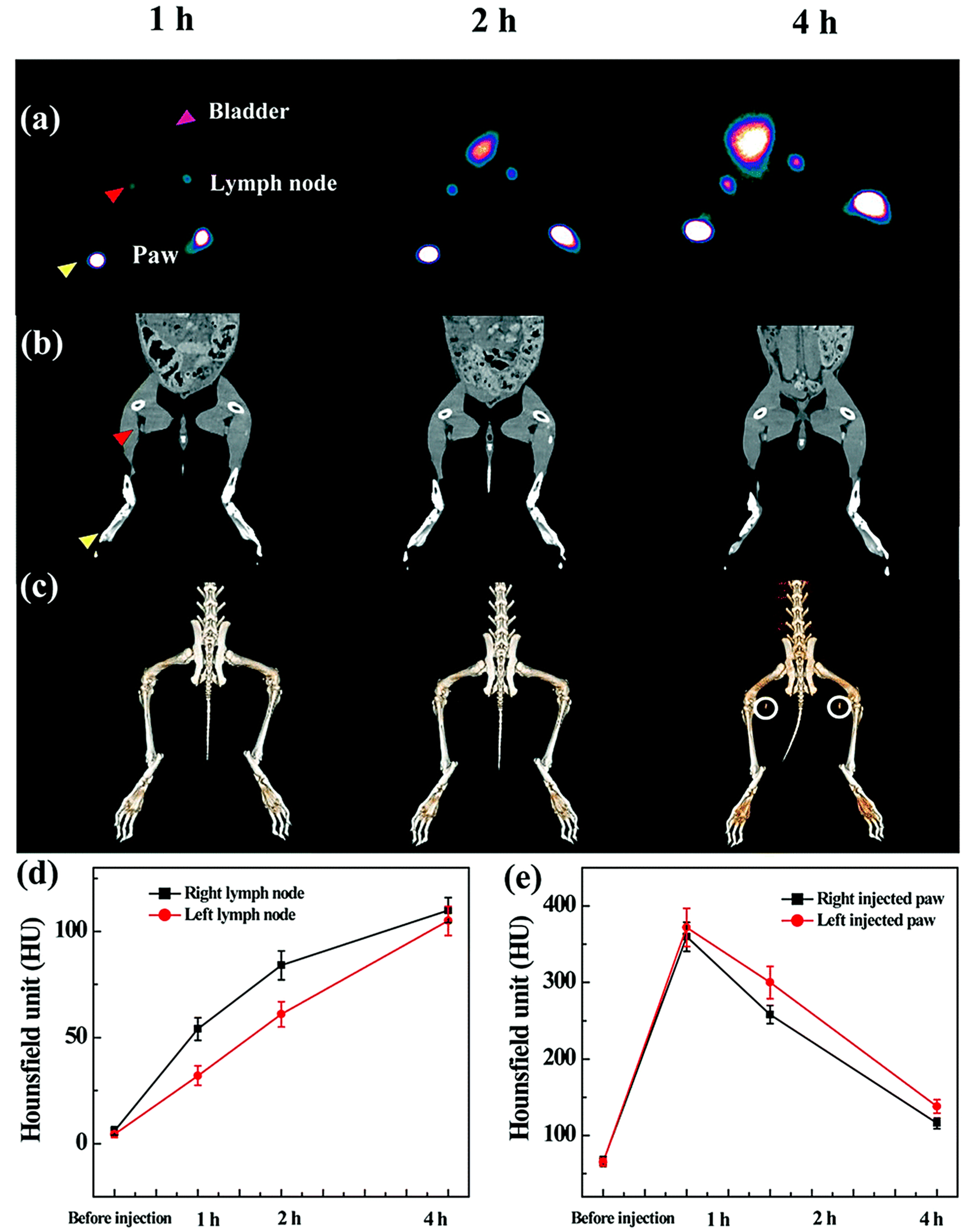
3. Radiolabeled Dendrimers for SPECT Imaging
4. Radiolabeled Dendrimers for PET Imaging
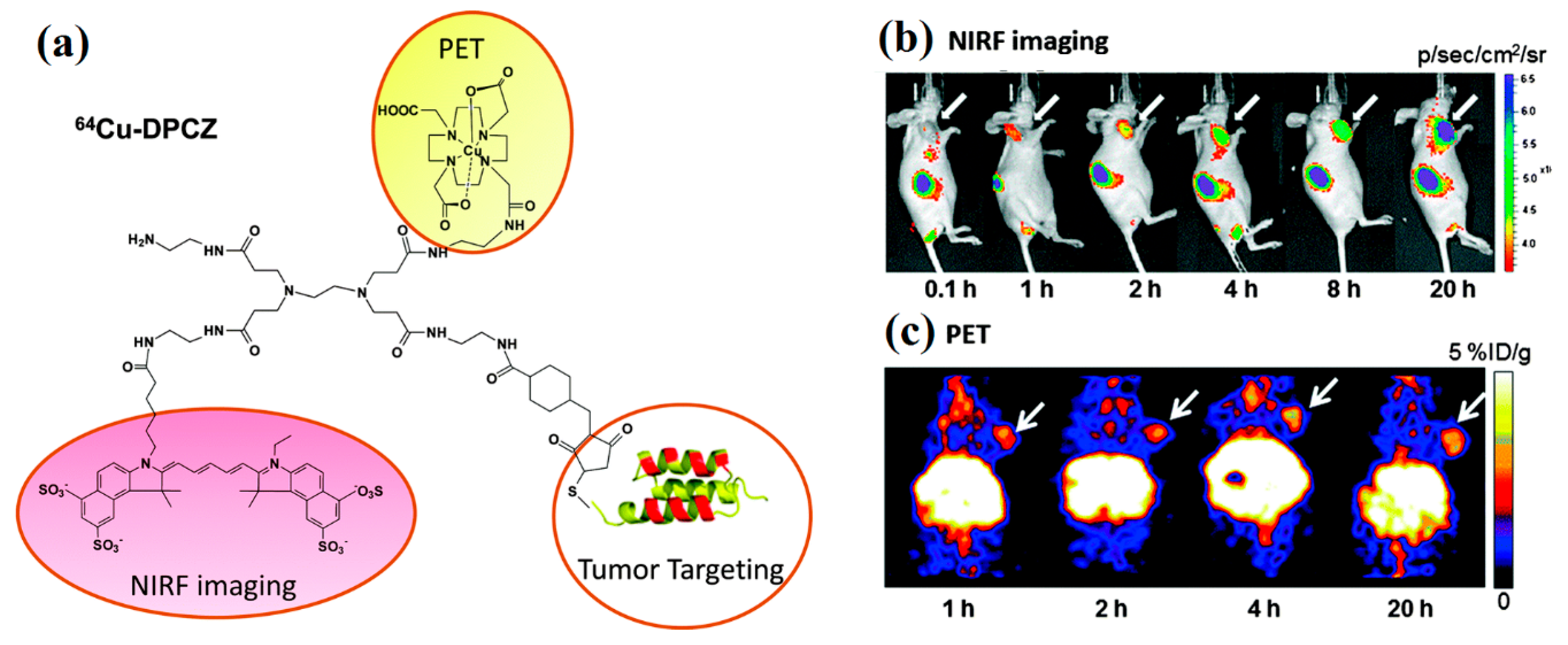
4.1. Cancer Imaging
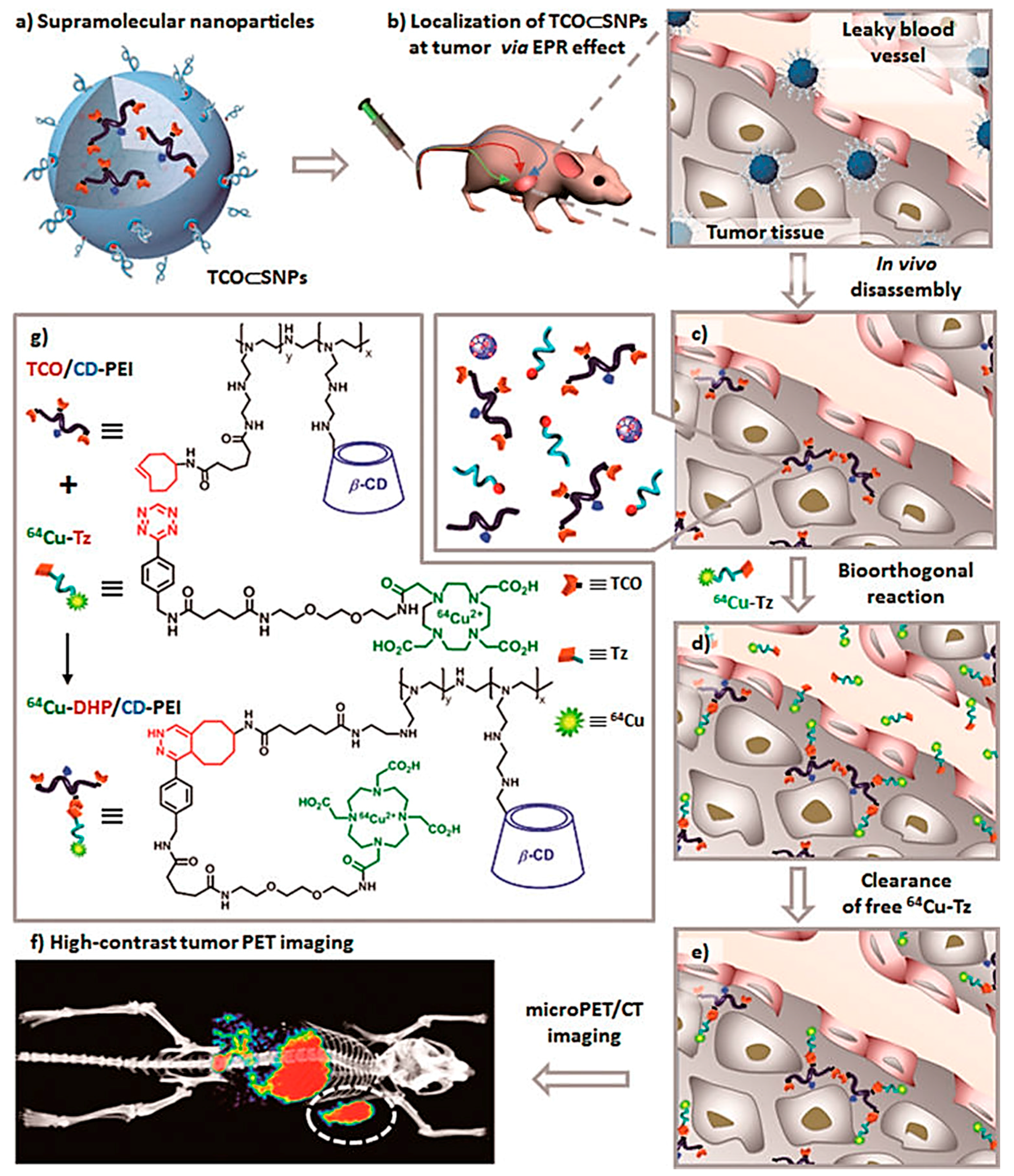
4.2. Other Applications
5. Radiolabeled Dendrimers for Radionuclide Therapy
6. Conclusions and Outlooks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stabin, M.; Brill, A.B. Physics Applications in Nuclear Medicine: 2007. J. Nucl. Med. 2008, 49, 20N–25N. [Google Scholar] [PubMed]
- Phelps, M.E.; Coleman, R.E. Nuclear Medicine in the New Millennium. J. Nucl. Med. 2000, 41, 1–4. [Google Scholar] [PubMed]
- Schöder, H.; Erdi, Y.E.; Larson, S.M.; Yeung, H.W.D. PET/CT: A new imaging technology in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1419–1437. [Google Scholar]
- Signore, A.; Glaudemans, A.W.J.M. The molecular imaging approach to image infections and inflammation by nuclear medicine techniques. Ann. Nucl. Med. 2011, 25, 681–700. [Google Scholar] [CrossRef] [PubMed]
- Oyen, W.J.G.; Bodei, L.; Giammarile, F.; Maecke, H.R.; Tennvall, J.; Luster, M.; Brans, B. Targeted therapy in nuclear medicine—Current status and future prospects. Ann. Oncol. 2007, 18, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Ametamey, S.M.; Honer, M.; Schubiger, P.A. Molecular Imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Dobrucki, L.W.; Sinusas, A.J. PET and SPECT in cardiovascular molecular imaging. Nat. Rev. Cardiol. 2010, 7, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.M.; Abrunhosa, A.J.; Ramos, P.; Pauwels, E.K.J. Molecular imaging with SPECT as a tool for drug development. Adv. Drug Deliv. Rev. 2011, 63, 547–554. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, M.R.; Sgouros, G.; Finn, R.D.; Humm, J.L.; Jurcic, J.G.; Larson, S.M.; Scheinberg, D.A. Radioimmunotherapy with alpha-emitting nuclides. Eur. J. Nucl. Med. 1998, 25, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- McQuade, P.; Rowland, D.J.; Lewis, J.S.; Welch, M.J. Positron-emitting isotopes produced on biomedical cyclotrons. Curr. Med. Chem. 2005, 12, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Ruth, T.J. The Production of Radionuclides for Radiotracers in Nuclear Medicine. Rev. Accel Sci. Technol. 2009, 2, 17–33. [Google Scholar] [CrossRef]
- Gnanasegaran, G.; Ballinger, J.R. Molecular imaging agents for SPECT (and SPECT/CT). Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 26–35. [Google Scholar] [CrossRef]
- Ercan, M.T.; Caglar, M. Therapeutic radiopharmaceuticals. Curr. Pharm. Des. 2000, 6, 1085–1121. [Google Scholar] [PubMed]
- Banerjee, S.; Ambikalmajan Pillai, M.R.; Ramamoorthy, N. Evolution of TC-99m in diagnostic radiopharmaceuticals. Semin. Nucl. Med. 2001, 31, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Pacak, K.; Eisenhofer, G.; Goldstein, D.S. Functional Imaging of Endocrine Tumors: Role of Positron Emission Tomography. Endocr. Rev. 2004, 25, 568–580. [Google Scholar] [CrossRef]
- Thobois, S.; Jahanshahi, M.; Pinto, S.; Frackowiak, R.; Limousin-Dowsey, P. PET and SPECT functional imaging studies in Parkinsonian syndromes: From the lesion to its consequences. Neuroimage 2004, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Simonetti, G. Fusion imaging in nuclear medicine—Applications of dual-modality systems in oncology. Cancer Biother. Radiopharm. 2004, 19, 1–10. [Google Scholar] [CrossRef]
- Even-Sapir, E.; Keidar, Z.; Bar-Shalom, R. Hybrid Imaging (SPECT/CT and PET/CT)—Improving the Diagnostic Accuracy of Functional/Metabolic and Anatomic Imaging. Semin. Nucl. Med. 2009, 39, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Delso, G.; Fürst, S.; Jakoby, B.; Ladebeck, R.; Ganter, C.; Nekolla, S.G.; Schwaiger, M.; Ziegler, S.I. Performance Measurements of the Siemens mMR Integrated Whole-Body PET/MR Scanner. J. Nucl. Med. 2011, 52, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Mariani, G.; Bruselli, L.; Kuwert, T.; Kim, E.E.; Flotats, A.; Israel, O.; Dondi, M.; Watanabe, N. A review on the clinical uses of SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1959–1985. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wehrl, H.F.; Sauter, A.W.; Divine, M.R.; Pichler, B.J. Combined PET/MR: A technology becomes mature. J. Nucl. Med. 2015, 56, 165–168. [Google Scholar] [CrossRef]
- Hall, L.T.; Struck, A.F.; Perlman, S.B. Clinical Molecular Imaging with PET Agents Other than 18F-FDG. Curr. Pharm. Biotechnol. 2010, 11, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Riehemann, K.; Schneider, S.W.; Luger, T.A.; Godin, B.; Ferrari, M.; Fuchs, H. Nanomedicine—Challenge and Perspectives. Angew. Chem. Int. Ed. Engl. 2009, 48, 872–897. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.L.; Hansen, A.E.; Gabizon, A.; Andresen, T.L. Liposome imaging agents in personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1417–1435. [Google Scholar] [CrossRef]
- Guo, J.; Hong, H.; Chen, G.; Shi, S.; Zheng, Q.; Zhang, Y.; Theuer, C.P.; Barnhart, T.E.; Cai, W.; Gong, S. Image-guided and tumor-targeted drug delivery with radiolabeled unimolecular micelles. Biomaterials 2013, 34, 8323–8332. [Google Scholar] [CrossRef] [PubMed]
- Avcıbaşı, U.; Avcıbaşı, N.; Akalın, H.A.; Ediz, M.; Demiroğlu, H.; Gümüşer, F.G.; Özçalışkan, E.; Türkcan, C.; Uygun, D.A.; Akgöl, S. Synthesis and biodistribution of novel magnetic-poly(HEMA–APH) nanopolymer radiolabeled with iodine-131 and investigation its fate in vivo for cancer therapy. J. Nanopart. Res. 2013, 15, 2021. [Google Scholar] [CrossRef]
- Bouziotis, P.; Psimadas, D.; Tsotakos, T.; Stamopoulos, D.; Tsoukalas, C. Radiolabeled iron oxide nanoparticles as dual-modality SPECT/MRI and PET/MRI agents. Curr. Top. Med. Chem. 2012, 12, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Shi, X. Dendrimer-based molecular imaging contrast agents. Prog. Polym. Sci. 2015, 44, 1–27. [Google Scholar] [CrossRef]
- Ghobril, C.; Lamanna, G.; Kueny-Stotz, M.; Garofalo, A.; Billotey, C.; Felder-Flesch, D. Dendrimers in nuclear medical imaging. New J. Chem. 2012, 36, 310–323. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Grinstaff, M.W. Biomedical applications of dendrimers: A tutorial. Chem. Soc. Rev. 2011, 40, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About Dendrimers: Structure, Physical Properties, and Applications. Chem. Rev. 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, P.; Kumar, A.; Kumar, S.; Chaudhary, R.K.; Gulyás, B. Nanoparticles in practice for molecular-imaging applications: An overview. Acta Biomater. 2016, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Hong, H.; Cai, W. Image-Guided Drug Delivery with Single-Photon Emission Computed Tomography: A Review of Literature. Curr. Drug Targets 2015, 16, 592–609. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Tang, J.; Sheng, Y.; Hu, H.; Shen, Y. Macromolecular MRI contrast agents: Structures, properties and applications. Prog. Polym. Sci. 2013, 38, 462–502. [Google Scholar] [CrossRef]
- Kesharwani, P.; Iyer, A.K. Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discov. Today 2015, 20, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Wen, S.; Chen, Q.; Zheng, L.; Shen, M.; Zhao, J.; Zhang, G.; Shi, X. Targeted Tumor Computed Tomography Imaging Using Low-Generation Dendrimer-Stabilized Gold Nanoparticles. Chemistry 2013, 19, 6409–6416. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Thomas, T.P.; Peters, J.; Kotlyar, A.; Myc, A.; Baker, J.J.R. Tumor angiogenic vasculature targeting with PAMAM dendrimer-RGD conjugates. Chem. Commun. (Camb.) 2005, 5739–5741. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, S.; Sun, H.; Baker, J.R. Improved biocompatibility of surface functionalized dendrimer-entrapped gold nanoparticles. Soft Matter 2007, 3, 71–74. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, L.; Li, Y.; Xu, T. Design of biocompatible dendrimers for cancer diagnosis and therapy: Current status and future perspectives. Chem. Soc. Rev. 2011, 40, 2673–2703. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Mahmood, U. Molecular Imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Michalski, M.H.; Chen, X. Molecular imaging in cancer treatment. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 358–377. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. Molecular Imaging in Cancer. Science 2006, 312, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.L.; Willowson, K.P. An Evidence-Based Review of Quantitative SPECT Imaging and Potential Clinical Applications. J. Nucl. Med. 2013, 54, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Volkert, W.A.; Goeckeler, W.F.; Ehrhardt, G.J.; Ketring, A.R. Therapeutic Radionuclides: Production and Decay Property Considerations. J. Nucl. Med. 1991, 32, 174–185. [Google Scholar] [PubMed]
- Zalutsky, M.R.; Pozzi, O.R. Radioimmunotherapy with alpha-particle emitting radionuclides. Q. J. Nucl. Med. Mol. Imaging 2004, 48, 289–296. [Google Scholar] [PubMed]
- Kwekkeboom, D.J.; de Herder, W.W.; van Eijck, C.H.J.; Kam, B.L.; van Essen, M.; Teunissen, J.J.M.; Krenning, E.P. Peptide Receptor Radionuclide Therapy in Patients With Gastroenteropancreatic Neuroendocrine Tumors. Semin. Nucl. Med. 2010, 40, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Psimadas, D.; Bouziotis, P.; Georgoulias, P.; Valotassiou, V.; Tsotakos, T.; Loudos, G. Radiolabeling approaches of nanoparticles with 99mTc. Contrast Med. Mol. Imaging 2013, 8, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Stockhofe, K.; Postema, J.M.; Schieferstein, H.; Ross, T.L. Radiolabeling of Nanoparticles and Polymers for PET Imaging. Pharmaceuticals 2014, 7, 392–418. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosula, S.; Goldsmith, S.J.; Hamacher, K.A.; Kostakoglu, L.; Konishi, S.; Milowski, M.I.; Nanus, D.M.; Bander, N.H. Prediction of Myelotoxicity Based on Bone Marrow Radiation-Absorbed Dose: Radioimmunotherapy Studies Using 90Y- and 177Lu-Labeled J591 Antibodies Specific for Prostate-Specific Membrane Antigen. J. Nucl. Med. 2005, 46, 850–858. [Google Scholar] [PubMed]
- Sudipta, C.; Shuang, L. 99mTc and 111In-Labeling of Small Biomolecules: Bifunctional Chelators and Related Coordination Chemistry. Curr. Top. Med. Chem. 2010, 10, 1113–1134. [Google Scholar]
- Sarko, D.; Eisenhut, M.; Haberkorn, U.; Mier, W. Bifunctional Chelators in the Design and Application of Radiopharmaceuticals for Oncological Diseases. Curr. Med. Chem. 2012, 19, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Nwe, K.; Bernardo, M.; Regino, C.A.S.; Williams, M.; Brechbiel, M.W. Comparison of MRI properties between derivatized DTPA and DOTA gadolinium-dendrimer conjugates. Bioorg. Med. Chem. 2010, 18, 5925–5931. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, H.; Liu, H.; Wen, S.; Peng, C.; Shen, M.; Zhang, G.; Shi, X. Multifunctional Dendrimer-Entrapped Gold Nanoparticles Modified with RGD Peptide for Targeted Computed Tomography/Magnetic Resonance Dual-Modal Imaging of Tumors. Anal. Chem. 2015, 87, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Wu, C.; Kim, M.-K.; Paik, C.H.; Carrasquillo, J.A.; Brechbiel, M.W. Evaluation of the in vivo Biodistribution of Indium-111 and Yttrium-88 Labeled Dendrimer-1B4M-DTPA and Its Conjugation with Anti-Tac Monoclonal Antibody. Bioconj. Chem. 1999, 10, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Niu, G.; Wang, F.; Chen, X. 68Ga-labeled NOTA-RGD-BBN peptide for dual integrin and GRPR-targeted tumor imaging. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Bass, L.A.; Wang, M.; Welch, M.J.; Anderson, C.J. In vivo Transchelation of Copper-64 from TETA-Octreotide to Superoxide Dismutase in Rat Liver. Bioconj. Chem. 2000, 11, 527–532. [Google Scholar] [CrossRef]
- Sundin, J.; Tolmachev, V.; Koziorowski, J.; Carlsson, J.; Lundqvist, H.; Welt, S.; Larson, S.; Sundin, A. High yield direct 76Br-bromination of monoclonal antibodies using chloramine-T. Nucl. Med. Biol. 1999, 26, 923–929. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Keliher, E.J.; Thurber, G.M.; Nahrendorf, M.; Weissleder, R. 18F labeled nanoparticles for in vivo PET-CT imaging. Bioconj. Chem. 2009, 20, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Welch, M.J. Nanoparticles Labeled with Positron Emitting Nuclides: Advantages, Methods, and Applications. Bioconj. Chem. 2012, 23, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Xu, X.; Zhu, H.; Huang, L.; Zhang, X.; Qi, Y.; Shen, Y.-M. Radiosynthesis and micro-SPECT imaging of 99mTc-dendrimer poly(amido)-amine folic acid conjugate. Bioorg. Med. Chem. Lett. 2010, 20, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Xu, X.; Zhang, X.; Zhu, H.; Huang, L.; Qi, Y.; Shen, Y.-M. Synthesis, Biodistribution, and Microsingle Photon Emission Computed Tomography (SPECT) Imaging Study of Technetium-99m Labeled PEGylated Dendrimer Poly(amidoamine) (PAMAM)-Folic Acid Conjugates. J. Med. Chem. 2010, 53, 3262–3272. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Wang, X.; Guo, X.; Zhang, X.; Qi, Y.; Shen, Y.-M. Radiosynthesis, biodistribution and micro-SPECT imaging study of dendrimer–avidin conjugate. Bioorg. Med. Chem. 2011, 19, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Guo, Z.; Gao, M.; Shi, C.; Xu, D.; You, L.; Wu, X.; Su, X.; Zhuang, R.; Pan, W.; et al. Synthesis and preliminary evaluation of a 99mTc-labeled folate-PAMAM dendrimer for FR imaging. Chem. Biol. Drug Des. 2017, 89, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Lusic, H.; Grinstaff, M.W. X-ray-Computed Tomography Contrast Agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.I.; Bae, Y.; Bard, A.J. Strong Blue Photoluminescence and ECL from OH-Terminated PAMAM Dendrimers in the Absence of Gold Nanoparticles. J. Am. Chem. Soc. 2004, 126, 8358–8359. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Li, K.; Cai, H.; Chen, Q.; Shen, M.; Huang, Y.; Peng, C.; Hou, W.; Zhu, M.; Zhang, G.; et al. Multifunctional dendrimer-entrapped gold nanoparticles for dual mode CT/MR imaging applications. Biomaterials 2013, 34, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Criscione, J.M.; Dobrucki, L.W.; Zhuang, Z.W.; Papademetris, X.; Simons, M.; Sinusas, A.J.; Fahmy, T.M. Development and Application of a Multimodal Contrast Agent for SPECT/CT Hybrid Imaging. Bioconj. Chem. 2011, 22, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, Z.; Xu, X.; Luo, Y.; Peng, C.; Shen, M.; Shi, X. 99mTc-Labeled Multifunctional Low-Generation Dendrimer-Entrapped Gold Nanoparticles for Targeted SPECT/CT Dual-Mode Imaging of Tumors. ACS Appl. Mater. Interfaces 2016, 8, 19883–19891. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimochi, M.; Hayama, K.; Toyama, M.; Sasagawa, I.; Tsubokawa, N. Dual-modality imaging with 99mTc and fluorescent indocyanine green using surface-modified silica nanoparticles for biopsy of the sentinel lymph node: An animal study. EJNMMI Res. 2013, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhao, L.; Zhao, Q.; Li, D.; Liu, C.; Yu, Z.; Shen, M.; Majoral, J.-P.; Mignani, S.; Zhao, J.; et al. A promising dual mode SPECT/CT imaging platform based on 99mTc-labeled multifunctional dendrimer-entrapped gold nanoparticles. J. Mater. Chem. B 2017, 5, 3810–3815. [Google Scholar] [CrossRef]
- Mirzaii, M.; Seyyedi, S.; Sadeghi, M.; Gholamzadeh, Z. Cadmium electrodeposition on copper substrate for cyclotron production of 111In radionuclide. J. Radioanal. Nucl. Chem. 2010, 284, 333–339. [Google Scholar] [CrossRef]
- Merkel, O.M.; Mintzer, M.A.; Sitterberg, J.; Bakowsky, U.; Simanek, E.E.; Kissel, T. Triazine Dendrimers as Nonviral Gene Delivery Systems: Effects of Molecular Structure on Biological Activity. Bioconj. Chem. 2009, 20, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Merkel, O.M.; Kissel, T.; Simanek, E.E. Polycationic triazine-based dendrimers: Effect of peripheral groups on transfection efficiency. New J. Chem. 2009, 33, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Merkel, O.M.; Zheng, M.; Mintzer, M.A.; Pavan, G.M.; Librizzi, D.; Maly, M.; Höffken, H.; Danani, A.; Simanek, E.E.; Kissel, T. Molecular modeling and in vivo imaging can identify successful flexible triazine dendrimer-based siRNA delivery systems. J. Control. Release 2011, 153, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.; Cai, Z.; Reilly, R.M. Trastuzumab Labeled to High Specific Activity with 111In by Conjugation to G4 PAMAM Dendrimers Derivatized with Multiple DTPA Chelators Exhibits Increased Cytotoxic Potency on HER2-Positive Breast Cancer Cells. Pharm. Res. 2013, 30, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Niki, Y.; Ogawa, M.; Magata, Y. Prolonged local retention of subcutaneously injected polymers monitored by noninvasive SPECT imaging. Int. J. Pharm. 2014, 476, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Iwamiya, Y.; Kurosaki, T.; Ogawa, M.; Magata, Y.; Sasaki, H.; Ohshima, T.; Maeda, M.; Mukai, T. Radiolabeled γ-polyglutamic acid complex as a nano-platform for sentinel lymph node imaging. J. Control. Release 2014, 194, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Niki, Y.; Ogawa, M.; Makiura, R.; Magata, Y.; Kojima, C. Optimization of dendrimer structure for sentinel lymph node imaging: Effects of generation and terminal group. Nanomedicine 2015, 11, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Rose, K.; Hughes, G.J.; Magistretti, P.J. [mono[125I]iodo-Tyr10,MetO17]-vasoactive intestinal polypeptide. Preparation, characterization, and use for radioimmunoassay and receptor binding. J. Biol. Chem. 1986, 261, 5320–5327. [Google Scholar] [PubMed]
- Lv, J.; Cao, X.F.; Zhu, B. 125I Radioactive Seeds Implantation Therapy for Hepatocellular Carcinoma. Gastroenterol. Res. 2009, 2, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Luo, J.; Jain, T.; Riggs, J.W.; Tseng, H.P.; Henderson, P.T.; Cherry, S.R.; Rowland, D.; Lam, K.S. Biodistribution and pharmacokinetics of a telodendrimer micellar paclitaxel nanoformulation in a mouse xenograft model of ovarian cancer. Int. J. Nanomed. 2012, 7, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lo, S.-T.; Lim, J.; da Costa, V.C.P.; Ramezani, S.; Öz, O.K.; Pavan, G.M.; Annunziata, O.; Sun, X.; Simanek, E.E. Design, Synthesis and Biological Assessment of a Triazine Dendrimer with Approximately 16 Paclitaxel Groups and 8 PEG Groups. Mol. Pharm. 2013, 10, 4452–4461. [Google Scholar] [CrossRef] [PubMed]
- Ledingham, K.W.D.; McKenna, P.; McCanny, T.; Shimizu, S.; Yang, J.M.; Robson, L.; Zweit, J.; Gillies, J.M.; Bailey, J.; Chimon, G.N.; et al. High power laser production of short-lived isotopes for positron emission tomography. J. Phys. D Appl. Phys. 2004, 37, 2341. [Google Scholar] [CrossRef]
- Sharma, R.; Xu, Y.; Kim, S.W.; Schueller, M.J.; Alexoff, D.; Smith, S.D.; Wang, W.; Schlyer, D. Carbon-11 radiolabeling of iron-oxide nanoparticles for dual-modality PET/MR imaging. Nanoscale 2013, 5, 7476–7483. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Campaña, C.; Gómez-Vallejo, V.; Puigivila, M.; Martín, A.; Calvo-Fernández, T.; Moya, S.E.; Ziolo, R.F.; Reese, T.; Llop, J. Biodistribution of Different Sized Nanoparticles Assessed by Positron Emission Tomography: A General Strategy for Direct Activation of Metal Oxide Particles. ACS Nano 2013, 7, 3498–3505. [Google Scholar] [CrossRef] [PubMed]
- Trembleau, L.; Simpson, M.; Cheyne, R.W.; Escofet, I.; Appleyard, M.V.C.A.L.; Murray, K.; Sharp, S.; Thompson, A.M.; Smith, T.A.D. Development of 18F-fluorinatable dendrons and their application to cancer cell targeting. New J. Chem. 2011, 35, 2496–2502. [Google Scholar] [CrossRef]
- Bruskin, A.; Sivaev, I.; Persson, M.; Lundqvist, H.; Carlsson, J.; Sjöberg, S.; Tolmachev, V. Radiobromination of monoclonal antibody using potassium [76Br] (4 isothiocyanatobenzyl-ammonio)-bromo-decahydro-closo-dodecaborate (Bromo-DABI). Nucl. Med. Biol. 2004, 31, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Winberg, K.J.; Persson, M.; Malmström, P.-U.; Sjöberg, S.; Tolmachev, V. Radiobromination of anti-HER2/neu/ErbB-2 monoclonal antibody using the p-isothiocyanatobenzene derivative of the [76Br]undecahydro-bromo-7,8-dicarba-nido-undecaborate(1-) ion. Nucl. Med. Biol. 2004, 31, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.; Rossin, R.; Shokeen, M.; Hagooly, A.; Ananth, A.; Capoccia, B.; Guillaudeu, S.; Abendschein, D.; Anderson, C.J.; Welch, M.J.; et al. Biodegradable dendritic positron-emitting nanoprobes for the noninvasive imaging of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Blower, P.J.; Lewis, J.S.; Zweit, J. Copper radionuclides and radiopharmaceuticals in nuclear medicine. Nucl. Med. Biol. 1996, 23, 957–980. [Google Scholar] [CrossRef]
- Fani, M.; André, J.P.; Maecke, H.R. 68Ga-PET: A powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Med. Mol. Imaging 2008, 3, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, Z.; Ren, G.; Xu, Y.; Cheng, Z. A novel Affibody bioconjugate for dual-modality imaging of ovarian cancer. Chem. Commun. (Camb.) 2014, 50, 12832–12835. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, T.-Y.; Luo, Y.; Liu, Q.; Xiao, W.; Guo, W.; Lac, D.; Zhang, H.; Feng, C.; Wachsmann-Hogiu, S.; et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun. 2014, 5, 4712. [Google Scholar] [CrossRef] [PubMed]
- Van Duijnhoven, S.M.J.; Rossin, R.; van den Bosch, S.M.; Wheatcroft, M.P.; Hudson, P.J.; Robillard, M.S. Diabody Pretargeting with Click Chemistry in vivo. J. Nucl. Med. 2015, 56, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Kraeber-Bodéré, F.; Salaun, P.-Y.; Oudoux, A.; Goldenberg, D.M.; Chatal, J.-F.; Barbet, J. Pretargeted radioimmunotherapy in rapidly progressing, metastatic, medullary thyroid cancer. Cancer 2010, 116, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Choi, J.-S.; Garcia, M.A.; Xing, Y.; Chen, K.-J.; Chen, Y.-M.; Jiang, Z.K.; Ro, T.; Wu, L.; Stout, D.B.; et al. Pretargeted Positron Emission Tomography Imaging That Employs Supramolecular Nanoparticles with in vivo Bioorthogonal Chemistry. ACS Nano 2016, 10, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Ratib, O.; Nkoulou, R.; Schwaiger, M. Cardiovascular clinical applications of PET/MRI. Clin. Transl. Imaging 2013, 1, 65–71. [Google Scholar] [CrossRef]
- Glaudemans, A.W.J.M.; de Vries, E.F.J.; Galli, F.; Dierckx, R.A.J.O.; Slart, R.H.J.A.; Signore, A. The use of (18)F-FDG-PET/CT for Diagnosis and Treatment Monitoring of Inflammatory and Infectious Diseases. Clin. Dev. Immunol. 2013, 2013, 623036. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, P.; Porkka, K.; Hoffman, J.A.; Ruoslahti, E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med. 2002, 8, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Fogal, V.; Zhang, L.; Krajewski, S.; Ruoslahti, E. Mitochondrial/Cell-Surface Protein p32/gC1qR as a Molecular Target in Tumor Cells and Tumor Stroma. Cancer Res. 2008, 68, 7210–7218. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, J.; Kotamraju, V.R.; Seo, J.W.; Agemy, L.; Fogal, V.; Mahakian, L.M.; Peters, D.; Roth, L.; Gagnon, M.K.J.; Ferrara, K.W.; et al. Specific penetration and accumulation of a homing peptide within atherosclerotic plaques of apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. USA 2011, 108, 7154–7159. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Gröger, D.; Bergmann, R.; Pietzsch, J.; Steinbach, J.; Graham, B.; Spiccia, L.; Berthon, F.; Czarny, B.; Devel, L.; et al. Synthesis and Biodistribution Studies of 3H- and 64Cu-Labeled Dendritic Polyglycerol and Dendritic Polyglycerol Sulfate. Bioconj. Chem. 2015, 26, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Eriksson, L. Physics of pure and non-pure positron emitters for PET: A review and a discussion. EJNMMI Phys. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Siwu, E.R.O.; Minami, K.; Hasegawa, K.; Nozaki, S.; Kanayama, Y.; Koyama, K.; Chen, W.C.; Paulson, J.C.; Watanabe, Y.; et al. Noninvasive Imaging of Dendrimer-Type N-Glycan Clusters: In Vivo Dynamics Dependence on Oligosaccharide Structure. Angew. Chem. Int. Ed. Engl. 2010, 49, 8195–8200. [Google Scholar] [CrossRef] [PubMed]
- Ghai, A.; Singh, B.; Panwar Hazari, P.; Schultz, M.K.; Parmar, A.; Kumar, P.; Sharma, S.; Dhawan, D.; Kumar Mishra, A. Radiolabeling optimization and characterization of 68Ga labeled DOTA–polyamido-amine dendrimer conjugate—Animal biodistribution and PET imaging results. Appl. Radiat. Isot. 2015, 105, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, S.; Wang, F.; Liu, S.; Chen, X. Noninvasive imaging of tumor integrin expression using 18F-labeled RGD dimer peptide with PEG4 linkers. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1296–1307. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Xiong, Z.; Cheng, Z.; Fisher, D.R.; Liu, S.; Gambhir, S.S.; Chen, X. microPET Imaging of Glioma Integrin αvβ3 Expression Using 64Cu-Labeled Tetrameric RGD Peptide. J. Nucl. Med. 2005, 46, 1707–1718. [Google Scholar] [PubMed]
- Wu, Z.; Li, Z.; Chen, K.; Cai, W.; He, L.; Chin, F.T.; Li, F.; Chen, X. microPET of tumor integrin αvβ3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4). J. Nucl. Med. 2007, 48, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Dijkgraaf, I.; Yim, C.-B.; Franssen, G.M.; Schuit, R.C.; Luurtsema, G.; Liu, S.; Oyen, W.J.G.; Boerman, O.C. PET imaging of αvβ3 integrin expression in tumours with 68Ga-labelled mono-, di- and tetrameric RGD peptides. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-B.; Cai, W.; Cao, Q.; Chen, K.; Wu, Z.; He, L.; Chen, X. 64Cu-Labeled Tetrameric and Octameric RGD Peptides for Small-Animal PET of Tumor αvβ3 Integrin Expression. J. Nucl. Med. 2007, 48, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Wängler, C.; Maschauer, S.; Prante, O.; Schäfer, M.; Schirrmacher, R.; Bartenstein, P.; Eisenhut, M.; Wängler, B. Multimerization of cRGD Peptides by Click Chemistry: Synthetic Strategies, Chemical Limitations, and Influence on Biological Properties. ChemBioChem 2010, 11, 2168–2181. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Wängler, B.; Wängler, C. Optimized Solid Phase-Assisted Synthesis of Dendrons Applicable as Scaffolds for Radiolabeled Bioactive Multivalent Compounds Intended for Molecular Imaging. Molecules 2014, 19, 6952–6974. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.; Michler, C.; Wängler, B.; Bartenstein, P.; Fischer, G.; Schirrmacher, R.; Wängler, C. PESIN Multimerization Improves Receptor Avidities and in Vivo Tumor Targeting Properties to GRPR-Overexpressing Tumors. Bioconj. Chem. 2014, 25, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Pool, S.E.; Krenning, E.P.; Koning, G.A.; van Eijck, C.H.J.; Teunissen, J.J.M.; Kam, B.; Valkema, R.; Kwekkeboom, D.J.; de Jong, M. Preclinical and Clinical Studies of Peptide Receptor Radionuclide Therapy. Semin. Nucl. Med. 2010, 40, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Phillips, W.T.; Bao, A.; Brenner, A.J.; Goins, B.A. Image-guided interventional therapy for cancer with radiotherapeutic nanoparticles. Adv. Drug Deliv. Rev. 2014, 76, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.T.; Holland, N.B. Multifunctional nanoparticles for use in theranostic applications. Drug Deliv. Transl. Res. 2015, 5, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, J.; Cheng, Y.; Xiong, Z.; Tang, Y.; Guo, L.; Shi, X.; Zhao, J. Chlorotoxin-Conjugated Multifunctional Dendrimers Labeled with Radionuclide 131I for Single Photon Emission Computed Tomography Imaging and Radiotherapy of Gliomas. ACS Appl. Mater. Interfaces 2015, 7, 19798–19808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhao, L.; Cheng, Y.; Xiong, Z.; Tang, Y.; Shen, M.; Zhao, J.; Shi, X. Radionuclide 131I-labeled multifunctional dendrimers for targeted SPECT imaging and radiotherapy of tumors. Nanoscale 2015, 7, 18169–18178. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhu, J.; Zhao, L.; Xiong, Z.; Tang, Y.; Liu, C.; Guo, L.; Qiao, W.; Shi, X.; Zhao, J. 131I-labeled multifunctional dendrimers modified with BmK CT for targeted SPECT imaging and radiotherapy of gliomas. Nanomedicine 2016, 11, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Biersack, H.-J.; Palmedo, H.; Andris, A.; Rogenhofer, S.; Knapp, F.F.; Guhlke, S.; Ezziddin, S.; Bucerius, J.; von Mallek, D. Palliation and Survival After Repeated 188Re-HEDP Therapy of Hormone-Refractory Bone Metastases of Prostate Cancer: A Retrospective Analysis. J. Nucl. Med. 2011, 52, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiong, Q.; Yang, X.; Huang, Z.; He, L. Preparation and Imaging Research on 188Re-DTPA-Deoxyglucose in MCF-7 Tumor-Bearing Mice. Cancer Biother. Radiopharm. 2007, 22, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Guhlke, S.; Beets, A.L.; Oetjen, K.; Mirzadeh, S.; Biersack, H.-J.; Knapp, F.F. Simple New Method for Effective Concentration of 188Re Solutions from Alumina-Based 188W–188Re Generator. J. Nucl. Med. 2000, 41, 1271–1278. [Google Scholar] [PubMed]
- Cui, W.; Zhang, Y.; Xu, X.; Shen, Y.-M. Synthesis and 188Re Radiolabelling of Dendrimer Polyamide Amine (PAMAM) Folic Acid Conjugate. Med. Chem. 2012, 8, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Kunikowska, J.; Królicki, L.; Hubalewska-Dydejczyk, A.; Mikołajczak, R.; Sowa-Staszczak, A.; Pawlak, D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: Which is a better therapy option? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Schoffelen, R.; van der Graaf, W.T.A.; Franssen, G.; Sharkey, R.M.; Goldenberg, D.M.; McBride, W.J.; Rossi, E.A.; Eek, A.; Oyen, W.J.G.; Boerman, O.C. Pretargeted 177Lu Radioimmunotherapy of Carcinoembryonic Antigen–Expressing Human Colonic Tumors in Mice. J. Nucl. Med. 2010, 51, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Laznickova, A.; Biricova, V.; Laznicek, M.; Hermann, P. Mono(pyridine-N-oxide) DOTA analog and its G1/G4-PAMAM dendrimer conjugates labeled with 177Lu: Radiolabeling and biodistribution studies. Appl. Radiat. Isot. 2014, 84, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, L.; Tassano, M.; Cabrera, M.; Zamboni, C.B.; Fernández, M.; Anjos, R.M.; Cabral, P. Development of 177Lu-DOTA-Dendrimer and Determination of Its Effect on Metal and Ion Levels in Tumor Tissue. Cancer Biother. Radiopharm. 2015, 30, 405–409. [Google Scholar] [CrossRef] [PubMed]
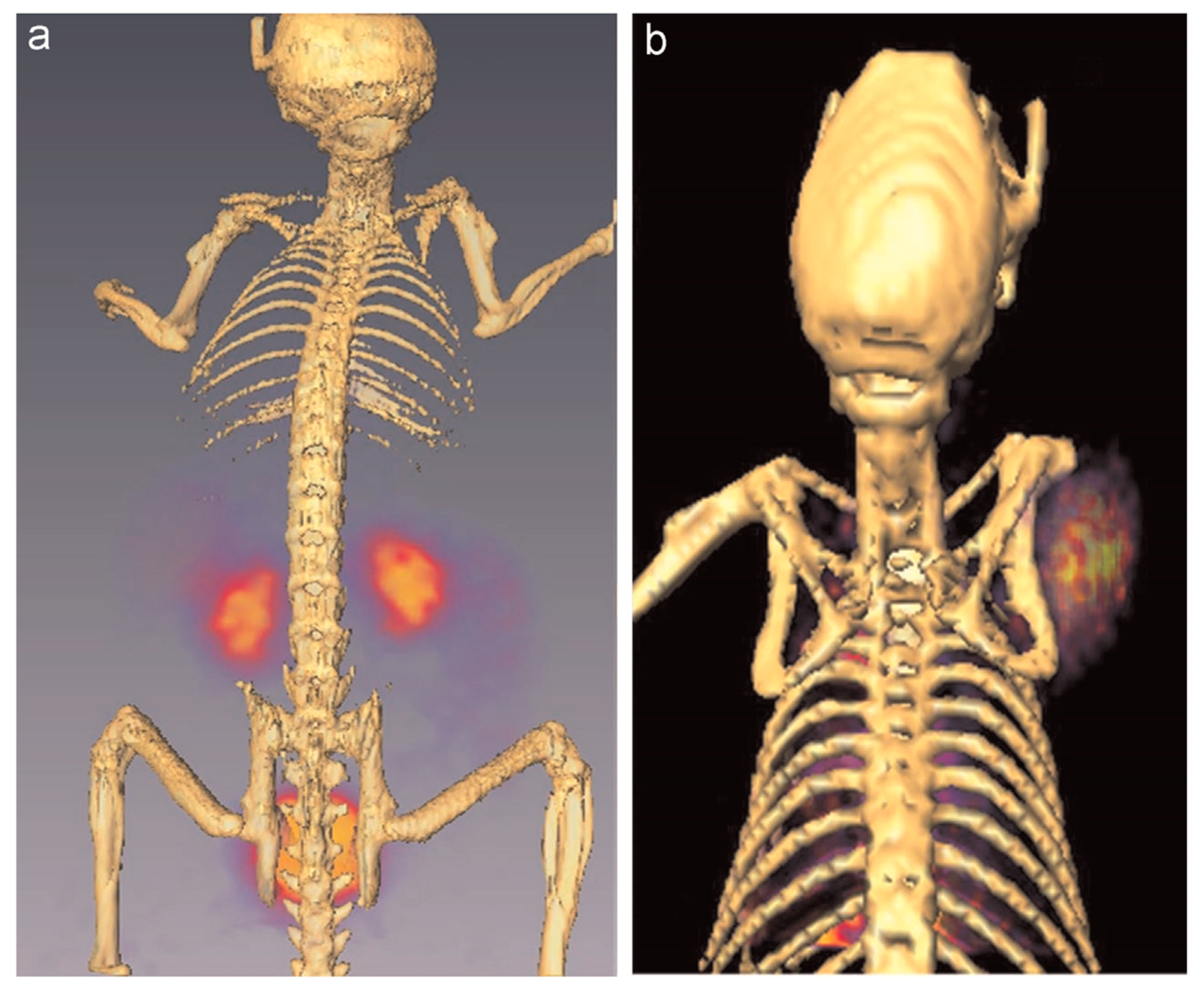
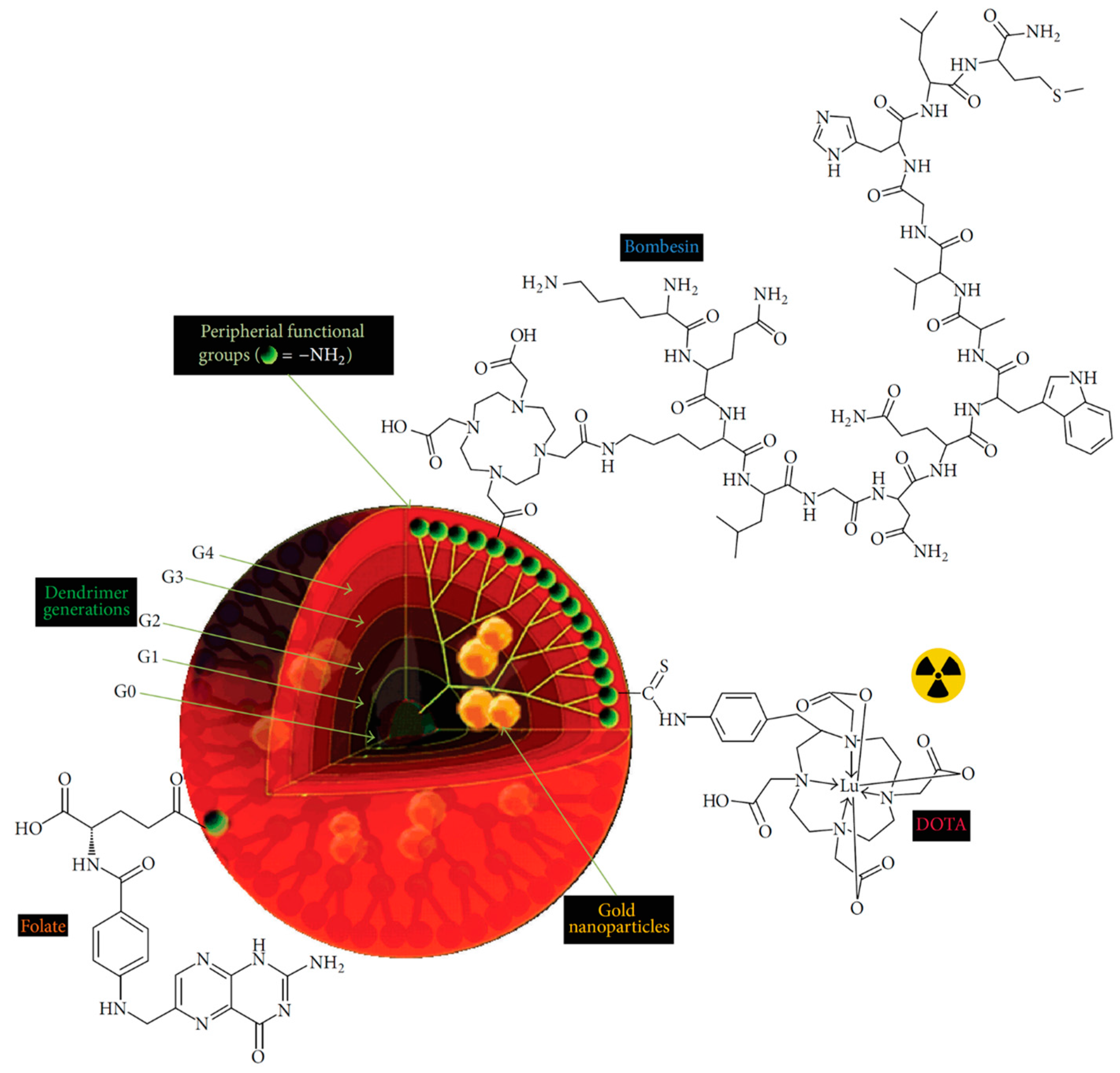
- Mendoza-Nava, H.; Ferro-Flores, G.; de María Ramírez, F.; Ocampo-García, B.; Santos-Cuevas, C.; Aranda-Lara, L.; Azorín-Vega, E.; Morales-Avila, E.; Isaac-Olivé, E. 177Lu-Dendrimer Conjugated to Folate and Bombesin with Gold Nanoparticles in the Dendritic Cavity: A Potential Theranostic Radiopharmaceutical. J. Nanomater. 2016, 2016, 1039258. [Google Scholar]
- Meyer, J.-P.; Houghton, J.L.; Kozlowski, P.; Abdel-Atti, D.; Reiner, T.; Pillarsetty, N.V.K.; Scholz, W.W.; Zeglis, B.M.; Lewis, J.S. 18F-Based Pretargeted PET Imaging Based on Bioorthogonal Diels–Alder Click Chemistry. Bioconj. Chem. 2016, 27, 298–301. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Zhu, M.; Li, Y.; Xing, Y.; Zhao, J. Radiolabeled Dendrimers for Nuclear Medicine Applications. Molecules 2017, 22, 1350. https://doi.org/10.3390/molecules22091350
Zhao L, Zhu M, Li Y, Xing Y, Zhao J. Radiolabeled Dendrimers for Nuclear Medicine Applications. Molecules. 2017; 22(9):1350. https://doi.org/10.3390/molecules22091350
Chicago/Turabian StyleZhao, Lingzhou, Meilin Zhu, Yujie Li, Yan Xing, and Jinhua Zhao. 2017. "Radiolabeled Dendrimers for Nuclear Medicine Applications" Molecules 22, no. 9: 1350. https://doi.org/10.3390/molecules22091350
APA StyleZhao, L., Zhu, M., Li, Y., Xing, Y., & Zhao, J. (2017). Radiolabeled Dendrimers for Nuclear Medicine Applications. Molecules, 22(9), 1350. https://doi.org/10.3390/molecules22091350






