Base-Mediated One-Pot Synthesis of Aliphatic Diazirines for Photoaffinity Labeling
Abstract
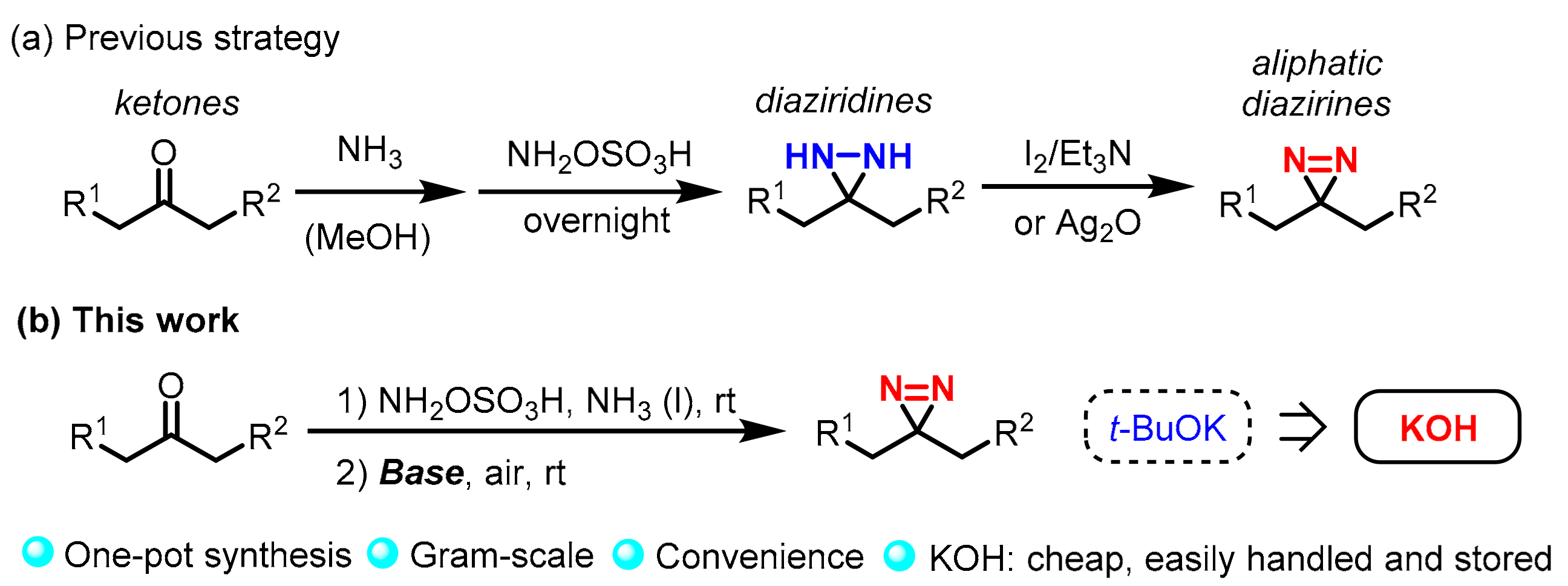
:1. Introduction
2. Results and Discussion
2.1. Reaction Optimization
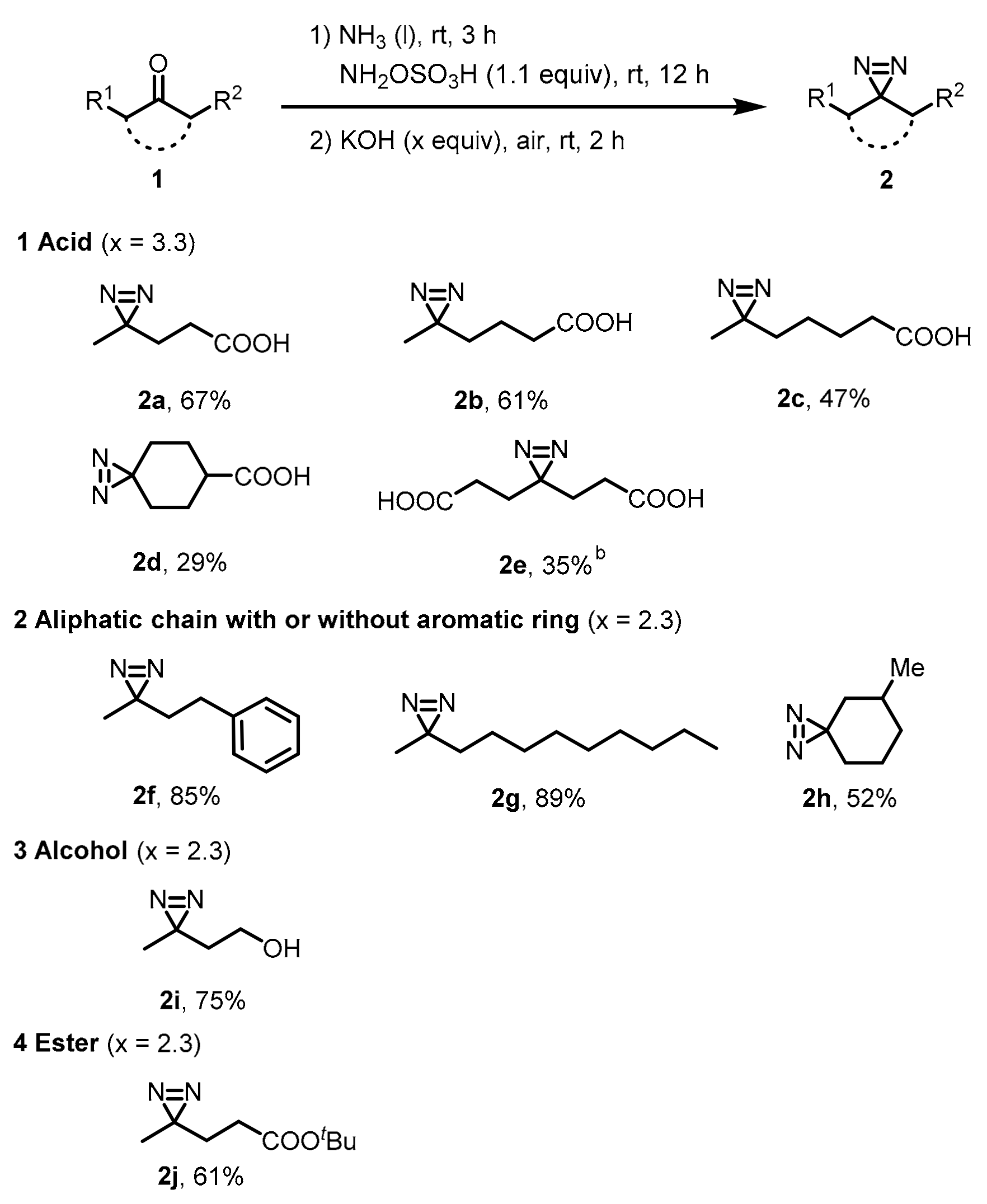
2.2. Scope Investigation
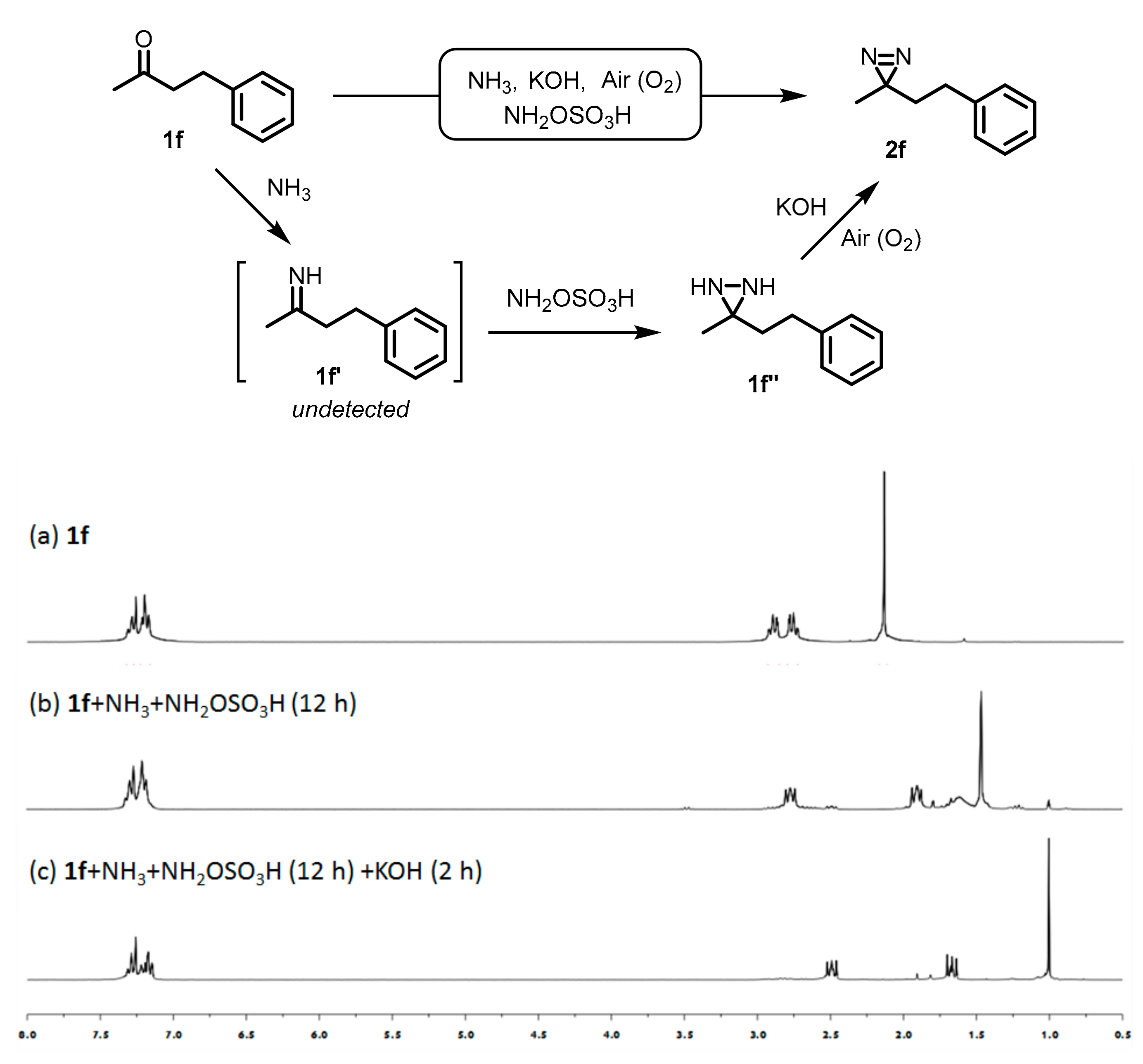
2.3. Kinetic Study of One-Pot Synthesis of Aliphatic Diaizirine
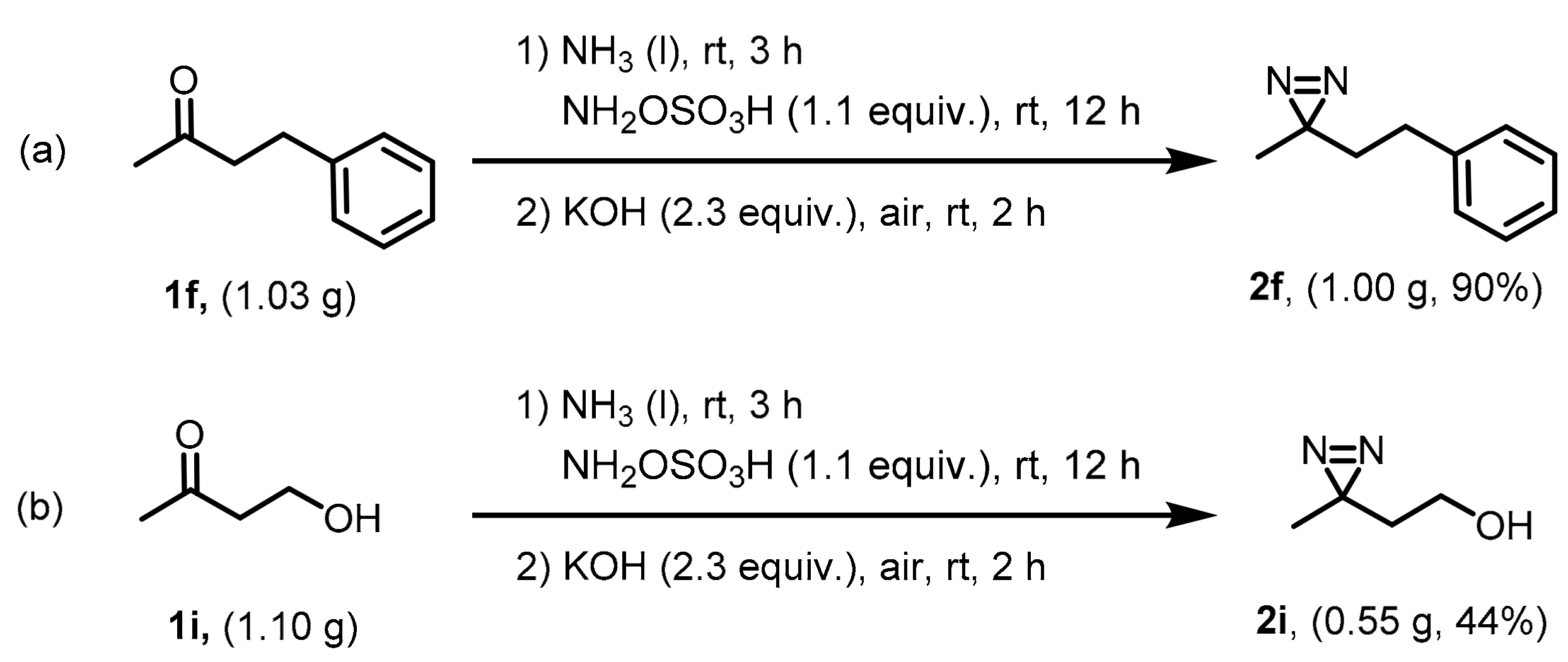
2.4. Gram-Scale Synthesis
3. Materials and Methods
3.1. General Procedures
3.2. Typical Procedures for One-Pot Synthesis of Aliphatic Diazirines from Corresponding Ketones
3.2.1. Acidic Substrates (1a–e)
3.2.2. Neutral Substrates (1f–j)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brunner, J. New photolabeling and cross-linking methods. Ann. Rev. Biochem. 1993, 62, 483–514. [Google Scholar] [CrossRef] [PubMed]
- Ruoho, A.E.; Kiefer, H.; Roeder, P.E.; Singer, S.J. The Mechanism of Photoaffinity Labeling. Proc. Natl. Acad. Sci. USA 1973, 70, 2567–2571. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, Y. Development and leading-edge application of innovative photoaffinity labeling. Chem. Pharm. Bull. 2015, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Casida, J.E. Molecular recognition of neonicotinoid insecticides: The determinants of life and death. Acc. Chem. Res. 2009, 42, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hatanaka, Y. Recent progress in diazirine-based photoaffinity labeling. Eur. J. Org. Chem. 2008, 2008, 2513–2523. [Google Scholar] [CrossRef]
- Fleet, G.W.J.; Porter, R.R.; Knowles, J.R. Affinity labeling of antibodies with aryl nitrene as reactive group. Nature 1969, 224, 511–512. [Google Scholar] [CrossRef]
- Ishida, A.; Wang, L.; Tachrim, Z.P.; Suzuki, T.; Sakihama, Y.; Hashidoko, Y.; Hashimoto, M. Comprehensive synthesis of photoreactive phenylthiourea derivatives for the photoaffinity labeling. Chemistryselect 2017, 2, 160–164. [Google Scholar] [CrossRef]
- Murai, Y.; Yoshida, T.; Wang, L.; Masuda, K.; Hashidoko, Y.; Monde, K.; Hatanaka, Y.; Hashimoto, M. Efficient synthesis of photoreactive 2-propoxyaniline derivatives as artificial sweeteners. Synlett 2016, 27, 946–950. [Google Scholar] [CrossRef]
- Wang, L.; Yoshida, T.; Muto, Y.; Murai, Y.; Tachrim, Z.P.; Ishida, A.; Nakagawa, S.; Sakihama, Y.; Hashidoko, Y.; Masuda, K.; et al. Synthesis of diazirine based photoreactive saccharin derivatives for the photoaffinity labeling of gustatory receptors. Eur. J. Org. Chem. 2015, 3129–3134. [Google Scholar] [CrossRef]
- Wang, L.; Hashidoko, Y.; Hashimoto, M. Co-solvent promoted O-benzylation with silver(I) oxide: Synthesis of 1′-benzylated sucrose derivatives, mechanistic studies and scope investigation. J. Org. Chem. 2016, 81, 4464–4474. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Murai, Y.; Yoshida, T.; Ishida, A.; Masuda, K.; Sakihama, Y.; Hashidoko, Y.; Hatanaka, Y.; Hashimoto, M. Alternative one-pot synthesis of (trifluoromethyl)phenyldiazirines from tosyloxime derivatives: Application for new synthesis of optically pure diazirinylphenylalanines for photoaffinity labeling. Org. Lett. 2015, 17, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Das, J. Aliphatic diazirines as photoaffinity probes for proteins: Recent developments. Chem. Rev. 2011, 111, 4405–4417. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click chemistry for drug development and diverse chemical–biology applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, T.; Pitscheider, M.; Sieber, S.A. Natural products and their biological targets: Proteomic and metabolomic labeling strategies. Angew. Chem. Int. Ed. 2010, 49, 2680–2698. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, Y.; Ojida, A.; Hamachi, I. Protein organic chemistry and applications for labeling and engineering in live-cell systems. Angew. Chem. Int. Ed. 2013, 52, 4088–4106. [Google Scholar] [CrossRef] [PubMed]
- Wratil, P.R.; Horstkorte, R.; Reutter, W. Metabolic glycoengineering with N-acyl side chain modified mannosamines. Angew. Chem. Int. Ed. 2016, 55, 9482–9512. [Google Scholar] [CrossRef] [PubMed]
- Church, R.F.R.; Kende, A.S.; Weiss, M.J. Diazirines. I. Some observations on the scope of the ammonia-hydroxylamine-O-sulfonic acid diaziridine synthesis. The preparation of certain steroid diaziridines and diazirines. J. Am. Chem. Soc. 1965, 87, 2665–2671. [Google Scholar] [CrossRef]
- Church, R.F.R.; Weiss, M.J. Diazirines. II. Synthesis and properties of small functionalized diazirine molecules. Observations on the reaction of a diaziridine with the iodine-iodide ion system. J. Org. Chem. 1970, 35, 2465–2471. [Google Scholar] [CrossRef]
- Wang, L.; Ishida, A.; Hashidoko, Y.; Hashimoto, M. Dehydrogenation of NH-NH bond triggered by potassium t-butoxide in liquid NH3. Angew. Chem. Int. Ed. 2017, 56, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Baars, H.; Beyer, A.; Kohlhepp, S.V.; Bolm, C. Transition-metal-free synthesis of benzimidazoles mediated by KOH/DMSO. Org. Lett. 2014, 16, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhuang, R.; Bao, L.; Tang, G.; Zhao, Y. KOH-mediated transition metal-free synthesis of imines from alcohols and amines. Green Chem. 2012, 14, 2384–2387. [Google Scholar] [CrossRef]
- Beyer, A.; Reucher, C.M.M.; Bolm, C. Potassium hydroxide/dimethyl sulfoxide promoted intramolecular cyclization for the synthesis of benzimidazol-2-ones. Org. Lett. 2011, 13, 2876–2879. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Kumar, A.V.; Swapna, K.; Rao, K.R. Copper oxide nanoparticle-catalyzed coupling of diaryl diselenide with aryl halides under ligand-free conditions. Org. Lett. 2009, 11, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.W.; Ikawa, T.; Tundel, R.E.; Buchwald, S.W. The selective reaction of aryl halides with KOH: Synthesis of phenols, aromatic ethers, and benzofurans. J. Am. Chem. Soc. 2006, 128, 10694–10695. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T.; Moriyama, K.; Kawakami, S.; Tsubaki, K. Powdered KOH in DMSO: An efficient base for asymmetric cyclization via memory of chirality at ambient temperature. J. Am. Chem. Soc. 2008, 130, 4153–4157. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Correia, B.E.; Niphakis, M.J.; Cravatt, B.F. Mapping the protein interaction landscape for fully functionalized small-molecule probes in human cells. J. Am. Chem. Soc. 2014, 136, 10777–10782. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, L.; Tan, X-L.; Qi, Y-K.; Feng, S.; Deng, H.; Yan, Y.; Zheng, J-S.; Liu, L.; Tian, C-L. Chemical synthesis of diubiquitin-based photoaffinity probes for selectively profiling ubiquitin-binding proteins. Angew. Chem. Int. Ed. 2017, 56, 2744–2748. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, E.; Shanmugasundararaj, S.; Wilkemeyer, M.F.; Dou, X.; Chen, S.; Charness, M.E.; Miller, K.W. An alcohol binding site on the neural cell adhesion molecule L1. Proc. Natl. Acad. Sci. USA. 2008, 105, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Shigdel, U.K.; Zhang, J.; He, C. Diazirine-based DNA photo-cross-linking probes for the study of protein–DNA interactions. Angew. Chem. Int. Ed. 2008, 47, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Morieux, P.; Salomé, C.; Park, K.D.; Stables, J.P.; Kohn, H. The structure-activity relationship of the 3-Oxy site in the anticonvulsant (R)-N-Benzyl 2-acetamido-3-methoxypropionamide. J. Med. Chem. 2010, 53, 5716–5726. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
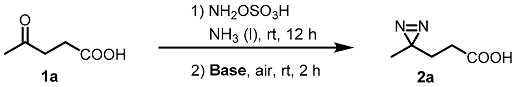
| Entry | Solvent | Base | Temp | Yield (%) b |
|---|---|---|---|---|
| 1 | NH3 | LiNH2 | RT | 36 |
| 2 | NH3 | NaNH2 | RT | 44 |
| 3 | NH3 | NaH | RT | 47 |
| 4 | NH3 | MgH2 | RT | 20 |
| 5 | NH3 | CaH2 | RT | 17 |
| 6 | NH3 | EtONa | RT | 45 |
| 7 | NH3 | MeONa | RT | 45 |
| 8 | NH3 | MeOK | RT | 63 |
| 9 | NH3 | NaOH | RT | 28 |
| 10 | NH3 | KOH | RT | 67 |
| 11 | NH3 | KOH | 0 °C | 11 |
| 12 | NH3/MeOH c | KOH | RT | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Tachrim, Z.P.; Kurokawa, N.; Ohashi, F.; Sakihama, Y.; Hashidoko, Y.; Hashimoto, M. Base-Mediated One-Pot Synthesis of Aliphatic Diazirines for Photoaffinity Labeling. Molecules 2017, 22, 1389. https://doi.org/10.3390/molecules22081389
Wang L, Tachrim ZP, Kurokawa N, Ohashi F, Sakihama Y, Hashidoko Y, Hashimoto M. Base-Mediated One-Pot Synthesis of Aliphatic Diazirines for Photoaffinity Labeling. Molecules. 2017; 22(8):1389. https://doi.org/10.3390/molecules22081389
Chicago/Turabian StyleWang, Lei, Zetryana Puteri Tachrim, Natsumi Kurokawa, Fumina Ohashi, Yasuko Sakihama, Yasuyuki Hashidoko, and Makoto Hashimoto. 2017. "Base-Mediated One-Pot Synthesis of Aliphatic Diazirines for Photoaffinity Labeling" Molecules 22, no. 8: 1389. https://doi.org/10.3390/molecules22081389
APA StyleWang, L., Tachrim, Z. P., Kurokawa, N., Ohashi, F., Sakihama, Y., Hashidoko, Y., & Hashimoto, M. (2017). Base-Mediated One-Pot Synthesis of Aliphatic Diazirines for Photoaffinity Labeling. Molecules, 22(8), 1389. https://doi.org/10.3390/molecules22081389





