Lactose Binding Induces Opposing Dynamics Changes in Human Galectins Revealed by NMR-Based Hydrogen–Deuterium Exchange
Abstract
1. Introduction
2. Results
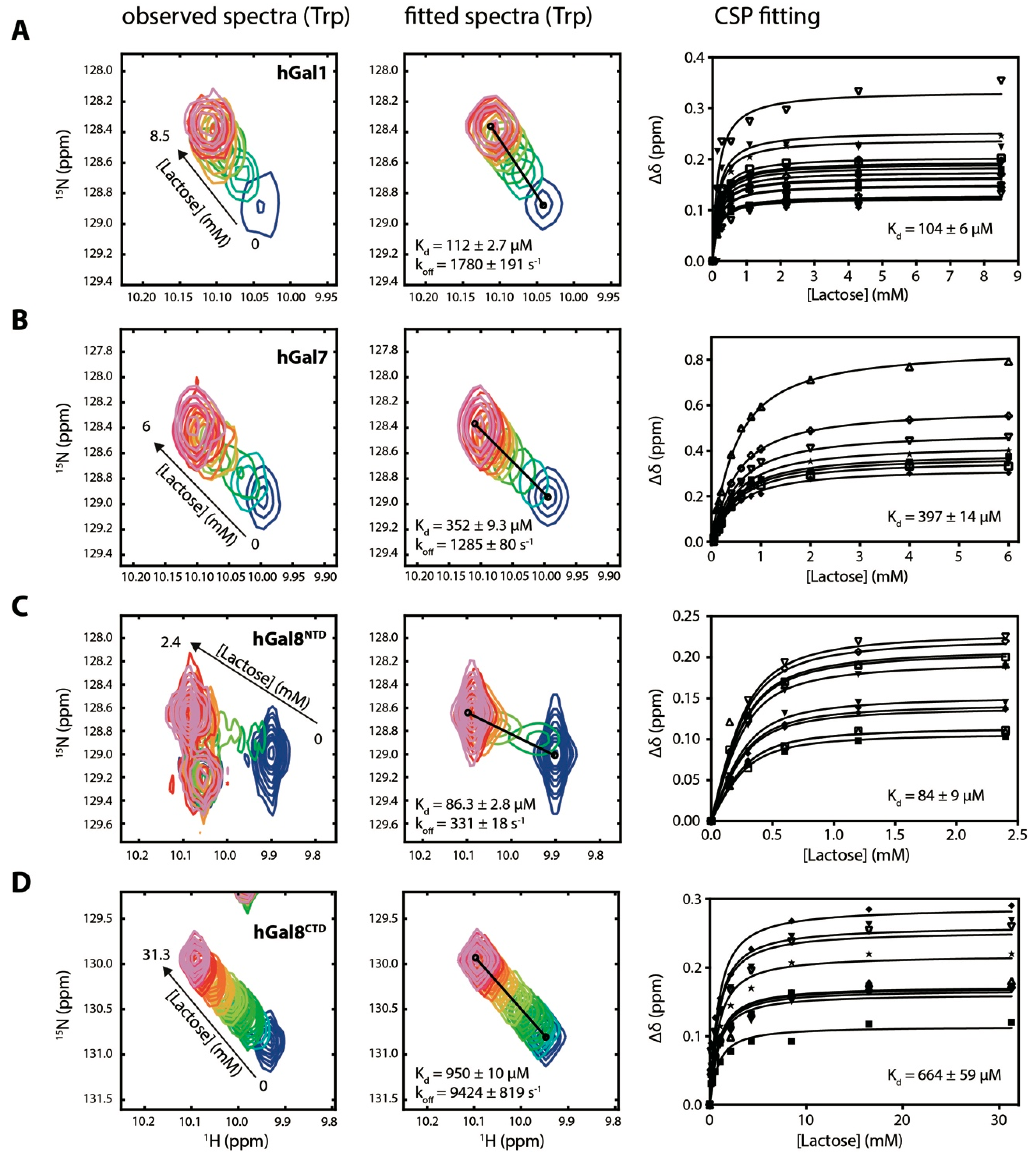
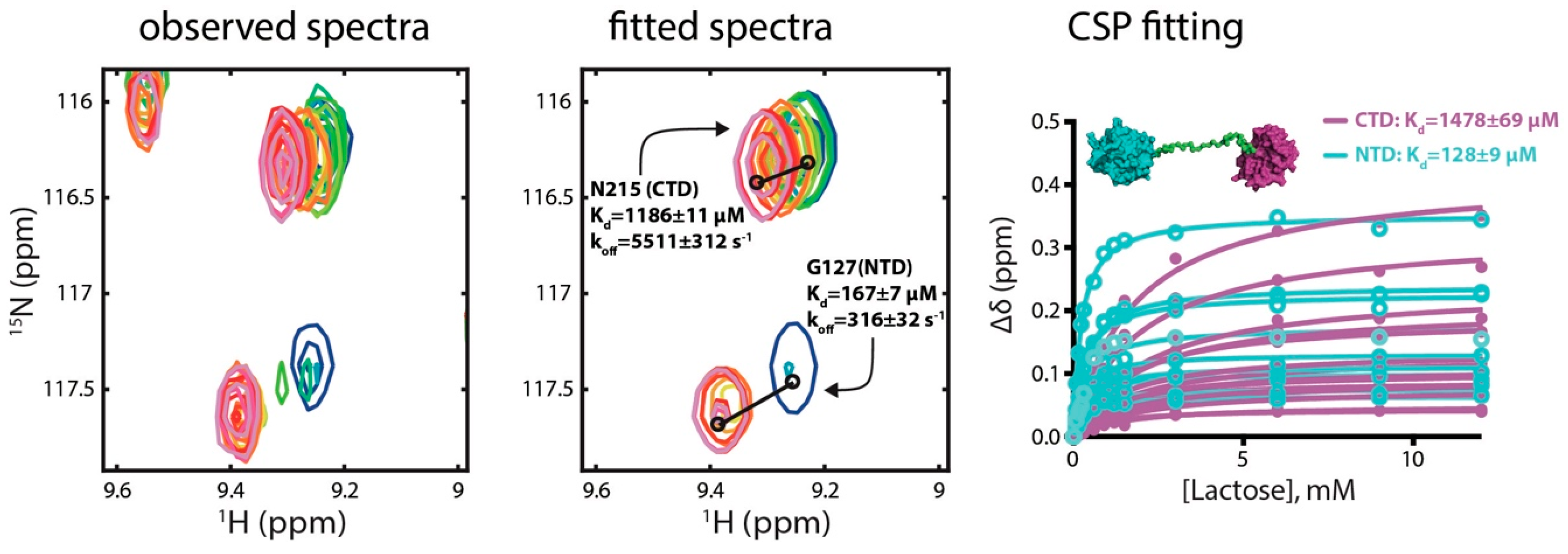
2.1. Determination of Lactose Binding Affinity to Galectins
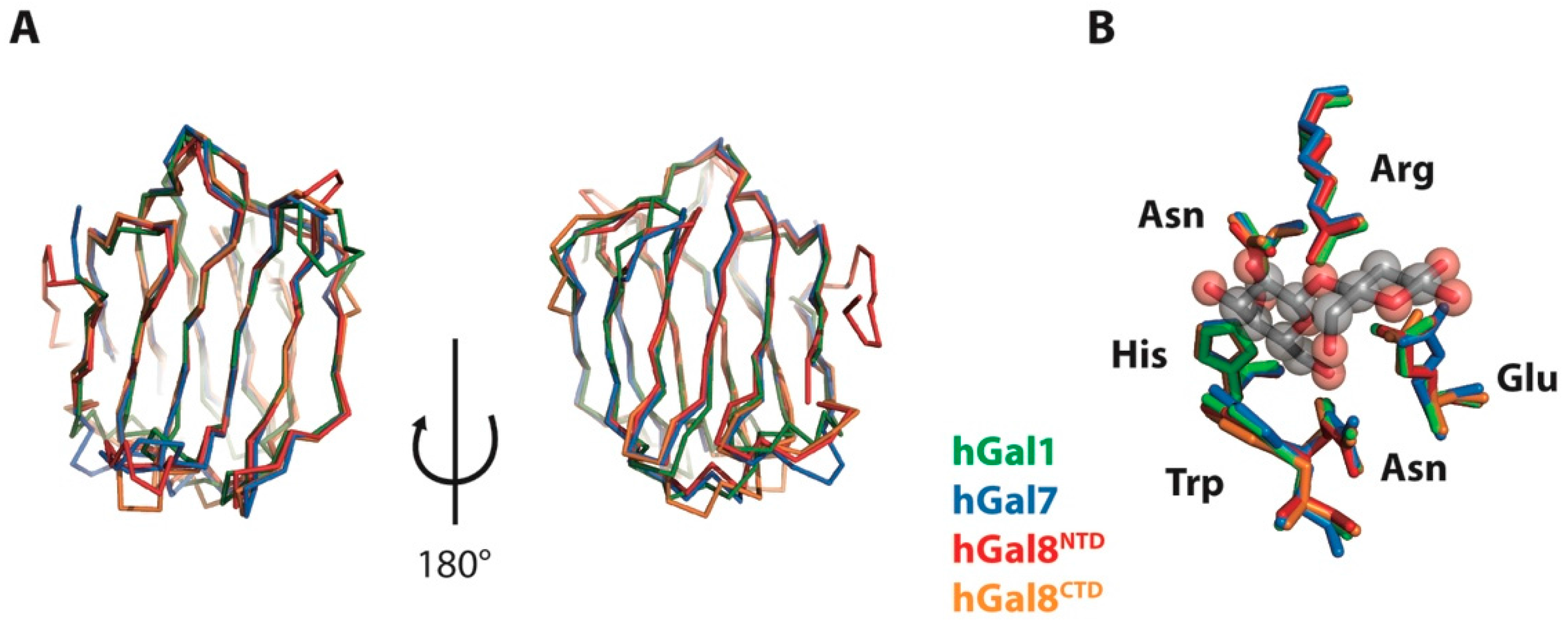
2.2. Structural Perturbations of Galectins upon Lactose Binding
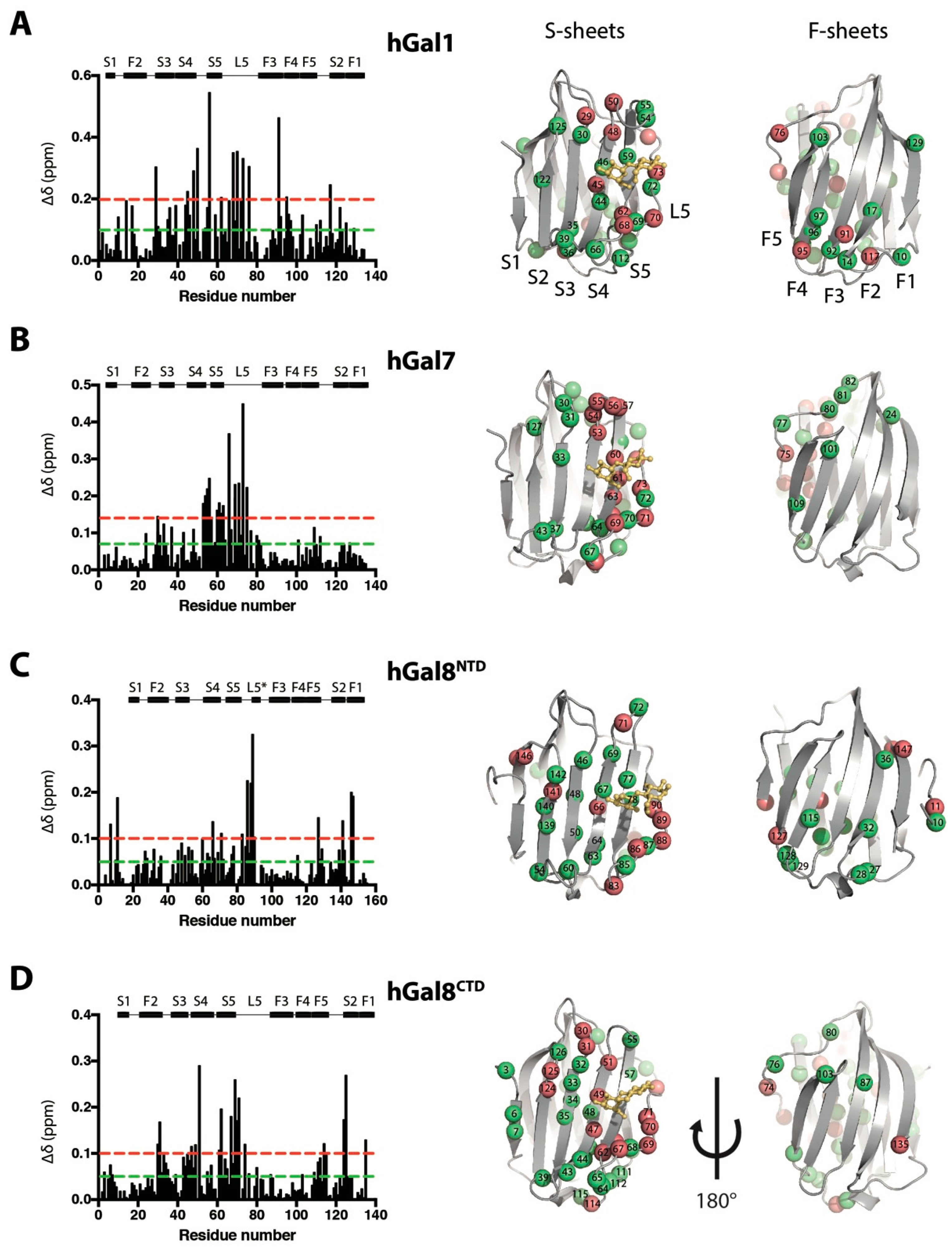
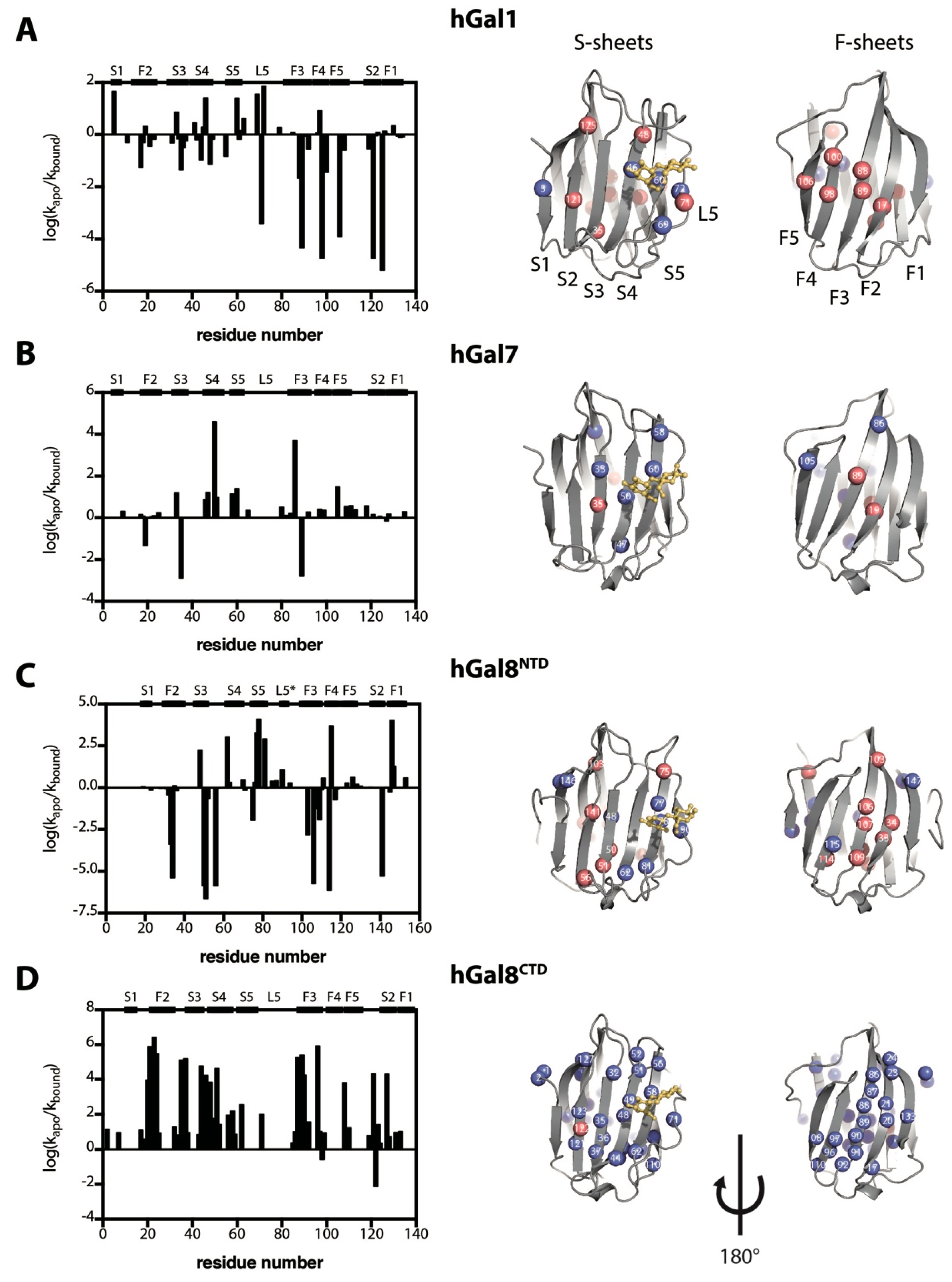
2.3. Impacts of Lactose Binding on the Dynamics of Galectins Monitored by NMR–HDX
3. Discussion
3.1. Comparing Kd Values with Literature Values
3.2. Structural and Dynamics Impact on Galectins upon Lactose Binding
4. Materials and Methods
4.1. Preparation of Recombinant Galectins
4.2. Intrinsic Fluorescence Spectroscopy
4.3. Bio-Layer Interferometry
4.4. NMR Titration Experiments
4.5. Isothermal Titration Calorimetry
4.6. NMR Hydrogen–Deuterium Exchange
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cummings, R.D.; Liu, F. Galectins. In Essentials of Glycobiology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009; pp. 1–12. ISBN 9780879697709. [Google Scholar]
- Méndez-Huergo, S.P.; Blidner, A.G.; Rabinovich, G.A. Galectins: Emerging regulatory checkpoints linking tumor immunity and angiogenesis. Curr. Opin. Immunol. 2017, 45, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Cerliani, J.P.; Blidner, A.G.; Toscano, M.A.; Croci, D.O.; Rabinovich, G.A. Translating the “Sugar Code” into Immune and Vascular Signaling Programs. Trends Biochem. Sci. 2017, 42, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Vespa, G.N.; Lewis, L.A.; Kozak, K.R.; Moran, M.; Nguyen, J.T.; Baum, L.G.; Miceli, M.C. Galectin-1 specifically modulates TCR signals to enhance TCR apoptosis but inhibit IL-2 production and proliferation. J. Immunol. 1999, 162, 799–806. [Google Scholar] [PubMed]
- Santucci, L.; Fiorucci, S.; Cammilleri, F.; Servillo, G.; Federici, B.; Morelli, A. Galectin-1 exerts immunomodulatory and protective effects on concanavalin A-induced hepatitis in mice. Hepatology 2000, 31, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Garin, M.I.; Chu, C.C.; Golshayan, D.; Cernuda-Morollon, E.; Wait, R.; Garín, M.I.; Chu, N.C.; Golshayan, D.; Cernuda-Morollón, E.; Wait, R.; et al. Galectin-1: A key effector of regulation mediated by CD4+CD25+ T cells. Blood 2007, 109, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Stillman, B.N.; Hsu, D.K.; Pang, M.; Brewer, C.F.; Johnson, P.; Liu, F.-T.; Baum, L.G. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 2006, 176, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Said, N.; Amin, S.; Wu, H.K.; Bruce, A.; Garate, M.; Hsu, D.K.; Kuwabara, I.; Liu, F.-T.; Panjwani, N. Galectins-3 and -7, but not galectin-1, play a role in re-epithelialization of wounds. J. Biol. Chem. 2002, 277, 42299–42305. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wu, H.K.; Bruce, A.; Wollenberg, K.; Panjwani, N. Detection of differentially expressed genes in healing mouse corneas, using cDNA microarrays. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2897–2904. [Google Scholar]
- Moisan, S.; Demers, M.; Mercier, J.; Magnaldo, T.; Potworowski, E.F.; St-Pierre, Y. Upregulation of galectin-7 in murine lymphoma cells is associated with progression toward an aggressive phenotype. Leukemia 2003, 17, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Kopitz, J.; Andre, S.; von Reitzenstein, C.; Versluis, K.; Kaltner, H.; André, S.; von Reitzenstein, C.; Versluis, K.; Kaltner, H.; Pieters, R.J.; et al. Homodimeric galectin-7 (p53-induced gene 1) is a negative growth regulator for human neuroblastoma cells. Oncogene 2003, 22, 6277–6288. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Kuwabara, I.; Liu, F.-T. Suppression of tumor growth by galectin-7 gene transfer. Cancer Res. 2004, 64, 5672–5676. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.L.M.; Wandel, M.P.; von Muhlinen, N.; Foeglein, Á.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Brumell, J.H. Microbiology: A sweet way of sensing danger. Nature 2012, 482, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-W.; Beom Hong, S.; Hoe Kim, J.; Hoon Kwon, D.; Kyu Song, H. Structural basis for recognition of autophagic receptor NDP52 by the sugar receptor galectin-8. Nat. Commun. 2013, 4, 1613. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, C.; Ouellet, M.; Giguère, D.; Ohtake, R.; Roy, R.; Sato, S.; Tremblay, M.J. Galectin-1-specific inhibitors as a new class of compounds to treat HIV-1 infection. Antimicrob. Agents Chemother. 2012, 56, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Cumpstey, I.; Carlsson, S.; Leffler, H.; Nilsson, U.J. Synthesis of a phenyl thio-β-d-galactopyranoside library from 1,5-difluoro-2,4-dinitrobenzene: Discovery of efficient and selective monosaccharide inhibitors of galectin-7. Org. Biomol. Chem. 2005, 3, 1922. [Google Scholar] [CrossRef] [PubMed]
- Fort, S.; Kim, H.S.; Hindsgaul, O. Screening for galectin-3 inhibitors from synthetic lacto-N-biose libraries using microscale affinity chromatography coupled to mass spectrometry. J. Org. Chem. 2006, 71, 7146–7154. [Google Scholar] [CrossRef] [PubMed]
- Van Scherpenzeel, M.; Moret, E.E.; Ballell, L.; Liskamp, R.M.J.; Nilsson, U.J.; Leffler, H.; Pieters, R.J. Synthesis and evaluation of new thiodigalactoside-based chemical probes to label galectin-3. ChemBioChem 2009, 10, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Salameh, B.A.; Cumpstey, I.; Sundin, A.; Leffler, H.; Nilsson, U.J. 1H-1,2,3-Triazol-1-yl thiodigalactoside derivatives as high affinity galectin-3 inhibitors. Bioorg. Med. Chem. 2010, 18, 5367–5378. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. Galecto Biotech. Nat. Biotechnol. 2013, 31, 481. [Google Scholar] [CrossRef] [PubMed]
- Saraboji, K.; Håkansson, M.; Genheden, S.; Diehl, C.; Qvist, J.; Weininger, U.; Nilsson, U.J.; Leffler, H.; Ryde, U.; Akke, M.; et al. The carbohydrate-binding site in galectin-3 is preorganized to recognize a sugarlike framework of oxygens: Ultra-high-resolution structures and water dynamics. Biochemistry 2012, 51, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.J.; Lin, H.Y.; Tu, Z.; Huang, B.S.; Wu, S.C.; Lin, C.H. Structural basis underlying the binding preference of human galectins-1, -3 and -7 for Galβ1-3/4GlcNAc. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.G.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta Gen. Subj. 2002, 1572, 232–254. [Google Scholar] [CrossRef]
- Sörme, P.; Kahl-knutson, B.; Wellmar, U.; Nilsson, U.J.; Leffler, H. Fluorescence polarization to study galectin-ligand interactions. Methods Enzymol. 2003, 362, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, E.; Miller, M.C.; Nesmelova, I.V.; López-Merino, L.; Berbís, M.A.; Nesmelov, Y.; Tkachev, Y.V.; Lagartera, L.; Daragan, V.A.; André, S.; et al. Lactose binding to human galectin-7 (p53-induced gene 1) induces long-range effects through the protein resulting in increased dimer stability and evidence for positive cooperativity. Glycobiology 2013, 23, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Nesmelova, I.V.; Ermakova, E.; Daragan, V.A.; Pang, M.; Menéndez, M.; Lagartera, L.; Solís, D.; Baum, L.G.; Mayo, K.H. Lactose binding to galectin-1 modulates structural dynamics, increases conformational entropy, and occurs with apparent negative cooperativity. J. Mol. Biol. 2010, 397, 1209–1930. [Google Scholar] [CrossRef] [PubMed]
- Nesmelova, I.V.; Pang, M.; Baum, L.G.; Mayo, K.H. 1H, 13C and 15N backbone and side-chain chemical shift assignments for the 29 kDa human galectin-1 protein dimer. Biomol. NMR Assign. 2008, 2, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Nesmelova, I.V.; Berbís, M.Á.; Miller, M.C.; Cañada, F.J.; André, S.; Jiménez-Barbero, J.; Gabius, H.J.; Mayo, K.H. 1H, 13C, and 15N backbone and side-chain chemical shift assignments for the 31 kDa human galectin-7 (p53-induced gene 1) homodimer, a pro-apoptotic lectin. Biomol. NMR Assign. 2012, 6, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.G.; Chien, C.-T.H.; Lin, C.-H.; Hsu, S.-T.D. NMR assignments of the C-terminal domain of human galectin-8. Biomol. NMR Assign. 2015. [Google Scholar] [CrossRef] [PubMed]
- Waudby, C.A.; Ramos, A.; Cabrita, L.D.; Christodoulou, J. Two-dimensional NMR lineshape analysis. Sci. Rep. 2016, 6, 24826. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-H.H.; Chien, C.-T.H.; Wu, H.-Y.; Huang, K.-F.; Wang, I.; Ho, M.-R.; Tu, I.-F.; Lee, I.-M.; Li, W.; Shih, Y.-L.; et al. A multivalent marine lectin from Crenomytilus grayanus possesses anti-cancer activity through recognizing globotriose Gb3. J. Am. Chem. Soc. 2016, 138, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
- López-Lucendo, M.F.; Solís, D.; André, S.; Hirabayashi, J.; Kasai, K.I.; Kaltner, H.; Gabius, H.J.; Romero, A. Growth-regulatory human galectin-1: Crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J. Mol. Biol. 2004, 343, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Leonidas, D.D.; Vatzaki, E.H.; Vorum, H.; Celis, J.E.; Madsen, P.; Acharya, K.R. Structural basis for the recognition of carbohydrates by human galectin-7. Biochemistry 1998, 37, 13930–13940. [Google Scholar] [CrossRef] [PubMed]
- Andersson, F.I.; Werrell, E.F.; McMorran, L.; Crone, W.J.K.; Das, C.; Hsu, S.T.D.; Jackson, S.E. The effect of Parkinson’s-disease-associated mutations on the deubiquitinating enzyme UCH-L1. J. Mol. Biol. 2011, 407, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.C.; Wetzel, S.; Zhang, H.; Crone, E.W.; Lee, Y.T.; Jackson, S.E.; Hsu, S.T.D. The knotted protein UCH-L1 exhibits partially unfolded forms under native conditions that share common structural features with its kinetic folding intermediates. J. Mol. Biol. 2016, 428, 2507–2520. [Google Scholar] [CrossRef] [PubMed]
- Ideo, H.; Seko, A.; Ishizuka, I.; Yamashita, K. The N-terminal carbohydrate recognition domain of galectin-8 recognizes specific glycosphingolipids with high affinity. Glycobiology 2003, 13, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.M.G.; Hoang, L.; Lin, Y.; Englander, S.W. Hydrogen exchange methods to study protein folding. Methods 2004, 34, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-T.D.; Peter, C.; van Gunsteren, W.F.; Bonvin, A.M.J.J. Entropy calculation of HIV-1 Env gp120, its receptor CD4, and their complex: An analysis of configurational entropy changes upon complexation. Biophys. J. 2005, 88, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Guardia, C.M.A.; Gauto, D.F.; Di Lella, S.; Rabinovich, G.A.; Martí, M.A.; Estrin, D.A. An integrated computational analysis of the structure, dynamics, and ligand binding interactions of the human galectin network. J. Chem. Inf. Model. 2011, 51, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-J.; Lin, H.-Y.; Tu, Z.; Lin, T.-C.; Wu, S.-C.; Tseng, Y.-Y.; Liu, F.-T.; Hsu, S.-T.D.; Lin, C.-H. Dual thio-digalactoside-binding modes of human galectins as the structural basis for the design of potent and selective inhibitors. Sci. Rep. 2016, 6, 29457. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Humana Press: New York, NY, USA, 2005; pp. 571–607. ISBN 978-1-58829-343-5. [Google Scholar]
- Wang, L.-W.; Liu, Y.-N.; Lyu, P.-C.; Jackson, S.E.; Hsu, S.-T.D. Comparative analysis of the folding dynamics and kinetics of an engineered knotted protein and its variants derived from HP0242 of Helicobacter pylori. J. Phys. Condens. Matter 2015, 27, 354106. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.; Chen, S.Y.; Hsu, S.T.D. Unraveling the folding mechanism of the smallest knotted protein, MJ0366. J. Phys. Chem. B 2015, 119, 4359–4370. [Google Scholar] [CrossRef] [PubMed]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Schanda, P.; Kupĉe, E.; Brutscher, B. SOFAST-HMQC experiments for recording two-dimensional heteronuclear correlation spectra of proteins within a few seconds. J. Biomol. NMR 2005, 33, 199–211. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The DNA plasmids corresponding to hGal1, hGal7 and hGal8 are available from the authors. |
| NMR (CSP) | NMR (TITAN) | Intrinsic Fluorescence | BLI | ITC | |
|---|---|---|---|---|---|
| hGal1 | 104 ± 6 | 112 ± 1 | 98 ± 8 | 110 ± 38 | 209 ± 13 |
| hGal7 | 397 ± 14 | 352 ± 9 | 331 ± 6 | 347 ± 180 | 400 ± 2 |
| hGal8NTD | 84 ± 9 | 86 ± 3 | 88 ± 2 | 89 ± 26 | 93 ± 1 |
| hGal8CTD | 664 ± 59 | 950 ± 10 | 772 ± 56 | 873 ± 526 | 893 ± 25 |
| hGal8full(NTD) | 128 ± 9 | 167 ± 7 | — | — | — |
| hGal8full(CTD) | 1478 ± 69 | 1186 ± 11 | — | — | — |
| koff (s−1) | |
|---|---|
| hGal1 | 1780 ± 191 |
| hGal7 | 1285 ± 80 |
| hGal8NTD | 331 ± 18 |
| hGal8CTD | 9424 ± 819 |
| hGal8full(NTD) | 316 ± 32 |
| hGal8full(CTD) | 5511 ± 312 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, C.-T.H.; Ho, M.-R.; Lin, C.-H.; Hsu, S.-T.D. Lactose Binding Induces Opposing Dynamics Changes in Human Galectins Revealed by NMR-Based Hydrogen–Deuterium Exchange. Molecules 2017, 22, 1357. https://doi.org/10.3390/molecules22081357
Chien C-TH, Ho M-R, Lin C-H, Hsu S-TD. Lactose Binding Induces Opposing Dynamics Changes in Human Galectins Revealed by NMR-Based Hydrogen–Deuterium Exchange. Molecules. 2017; 22(8):1357. https://doi.org/10.3390/molecules22081357
Chicago/Turabian StyleChien, Chih-Ta Henry, Meng-Ru Ho, Chung-Hung Lin, and Shang-Te Danny Hsu. 2017. "Lactose Binding Induces Opposing Dynamics Changes in Human Galectins Revealed by NMR-Based Hydrogen–Deuterium Exchange" Molecules 22, no. 8: 1357. https://doi.org/10.3390/molecules22081357
APA StyleChien, C.-T. H., Ho, M.-R., Lin, C.-H., & Hsu, S.-T. D. (2017). Lactose Binding Induces Opposing Dynamics Changes in Human Galectins Revealed by NMR-Based Hydrogen–Deuterium Exchange. Molecules, 22(8), 1357. https://doi.org/10.3390/molecules22081357






