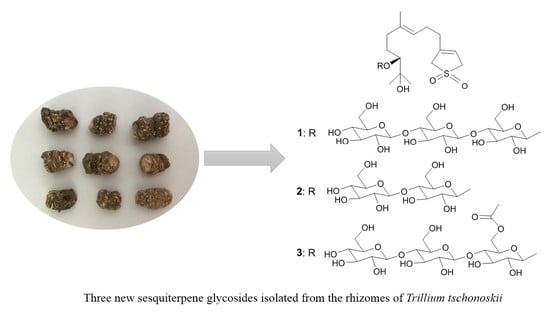
Three New Sesquiterpene Glycosides from the Rhizomes of Trillium tschonoskii
Abstract
:1. Introduction
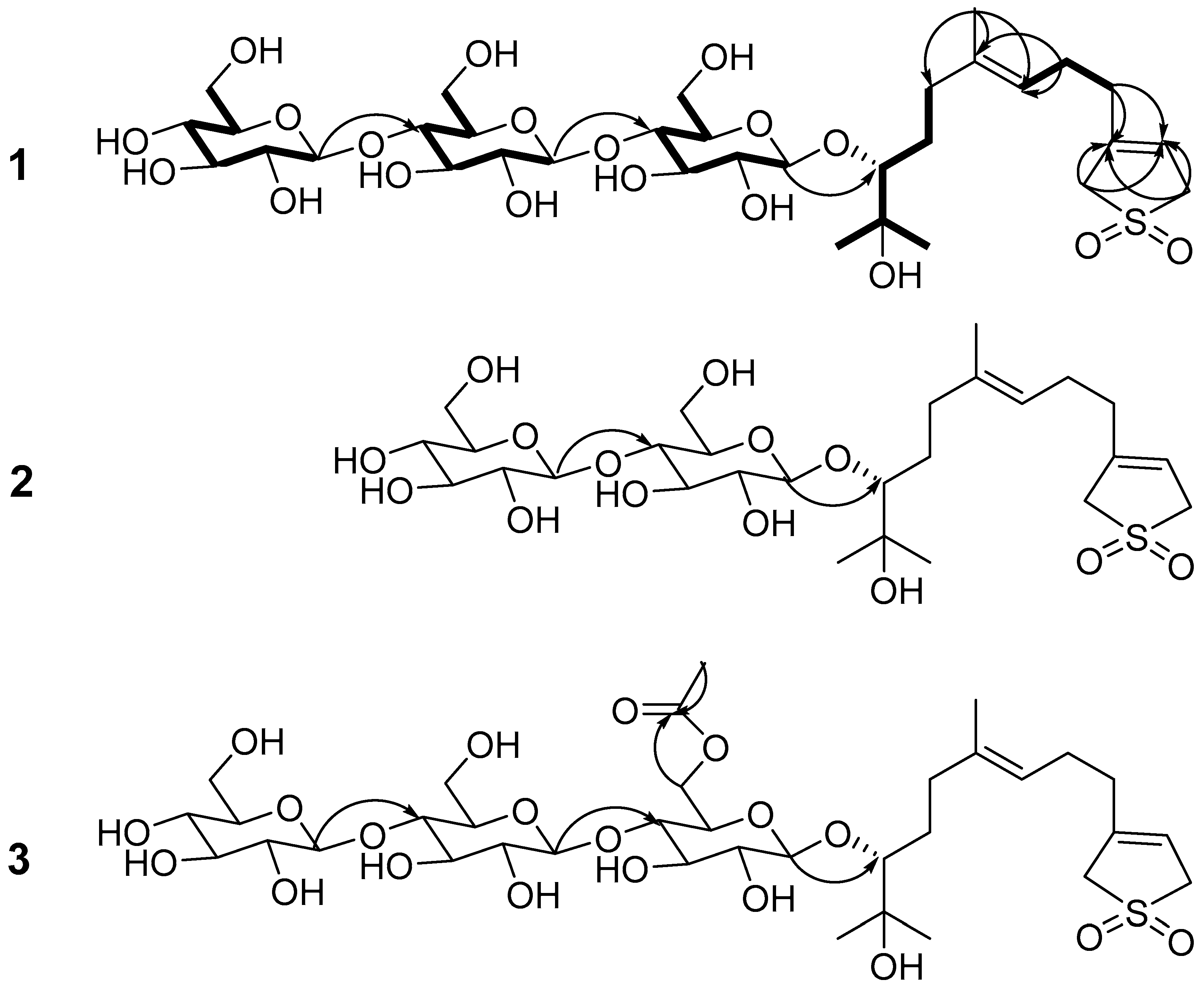
2. Results and Discussion
3. Experimental
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis and GC-MS Analysis
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Wu, Z.Y.; Yu, D.J.; Cui, H.B. Flora Republicae Popularis Sinicae; Academia Sinicae: Beijing, China, 1978. [Google Scholar]
- Xie, W. The Compilation of Chinese Herbal Medicine; People’s Medical Publishing House: Beijing, China, 2000. [Google Scholar]
- Wang, J.; Zou, K.; Zhang, Y.; Liu, C.; Wu, J.; Zhou, Y.; Dan, F.; Zhang, Y. An 18-norspirostanol saponin with inhibitory action against COX-2 production from the underground part of Trillium tschonoskii. Chem. Pharm. Bull. 2007, 55, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhai, Z.; Li, N.; Jin, H.; Chen, J.; Yuan, S.; Wang, L.; Zhang, J.; Li, Y.; Yun, J.; et al. Steroidal saponin of Trillium tschonoskii. Reverses multidrug resistance of hepatocellular carcinoma. Phytomedicine 2013, 20, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Xiao, D.; Han, J.; Yue, Z.; Sun, Y.; Fan, L.; Zhang, F.; Meng, J.; Zhang, R.; et al. Trillium tschonoskii steroidal saponins suppress the growth of colorectal Cancer cells In Vitro and In Vivo. J. Ethnopharmacol. 2015, 168, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.W.; Peng, C.; Zhou, Q.M.; Yang, H.; Guo, L.; Xiong, L. Spirostanols from the roots and rhizomes of Trillium tschonoskii. Phytochem. Lett. 2015, 14, 134–137. [Google Scholar] [CrossRef]
- Yu, L.L.; Zou, K.; Wang, J.Z.; Zhu, L.B.; Zhou, Y.; Yang, J. Study on the anti-inflammatory, analgesic and thrombosis effects of extract of Trillium tschonoskii Maxim. Shizhen J. Tradit. Chin. Med. Res. 2008, 19, 1178–1180. [Google Scholar]
- Wei, J.C.; Man, S.L.; Gao, W.Y.; Chai, X.; Zhao, W.S.; Wang, Y.; Jing, S.S. Steroidal saponins from the rhizomes of Trillium tschonoskii Maxim. Biochem. Syst. Ecol. 2012, 14, 112–116. [Google Scholar] [CrossRef]
- Hayes, P.Y.; Lehmann, R.; Penman, K.; Kitching, W.; De Voss, J.J. Steroidal saponins from the roots of Trillium erectum (Beth root). Phytochemistry 2009, 70, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Shafiqur, R.; Ismail, M.; Shah, M.R.; Adhikari, A.; Anis, I.; Ahmad, M.S.; Khurram, M. Govanoside A, a new steroidal saponin from rhizomes of Trillium govanianum. Steroids 2015, 104, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, A.; Mimaki, Y. Steroidal glycosides from the underground parts of Trillium erectum and their cytotoxic activity. Phytochemistry 2008, 69, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Yanai, Y.; Ikeda, T.; Okawa, M.; Nohara, T. Steroids from the underground parts of Trillium kamtschaticum. Chem. Pharma. Bull. 2003, 51, 1328–1331. [Google Scholar] [CrossRef]
- Ono, M.; Takamura, C.; Sugita, F.; Masuoka, C.; Yoshimitsu, H.; Ikeda, T.; Nohara, T. Two new steroid glycosides and a new sesquiterpenoid glycoside from the underground parts of Trillium kamtschaticum. Chem. Pharm. Bull. 2007, 55, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Song, X.; Wang, X.; Mei, Q.; Li, Z.; Cui, J.; Tang, Z.; Yue, Z. Two new compounds from the roots and rhizomes of Trillium tschonoskii. Phytochem. Lett. 2014, 10, 113–117. [Google Scholar] [CrossRef]
- Abraham, W.R.; Arfmanna, H.A.; Giersch, W. Microbial hydroxylation of precursors of sinensal. Z. Für Naturforsch. C 1992, 47, 851–858. [Google Scholar]
- Seo, S.; Tomita, Y.; Tori, K.; Yoshimura, Y. Determination of the absolute configuration of a secondary hydroxy group in a chiral secondary alcohol using glycosidation shifts in carbon-13 nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 1978, 100, 3331–3339. [Google Scholar] [CrossRef]
- Miyase, T.; Ueno, A.; Takizawa, N.; Kobayashi, H.; Oguchi, H. Studies on the glycosides of Epimedium grandiflorum MORR. var. thunbergianum (MIQ.) NAKAI. I. Chem. Pharm. Bull. 1987, 35, 3713–3719. [Google Scholar] [CrossRef]
- Zhang, B.J.; Teng, X.F.; Bao, M.F.; Zhong, X.H.; Ni, L.; Cai, X.H. Cytotoxic indole alkaloids from Tabernaemontana officinalis. Phytochemistry 2015, 120, 46–52. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–3 are available from the authors. |
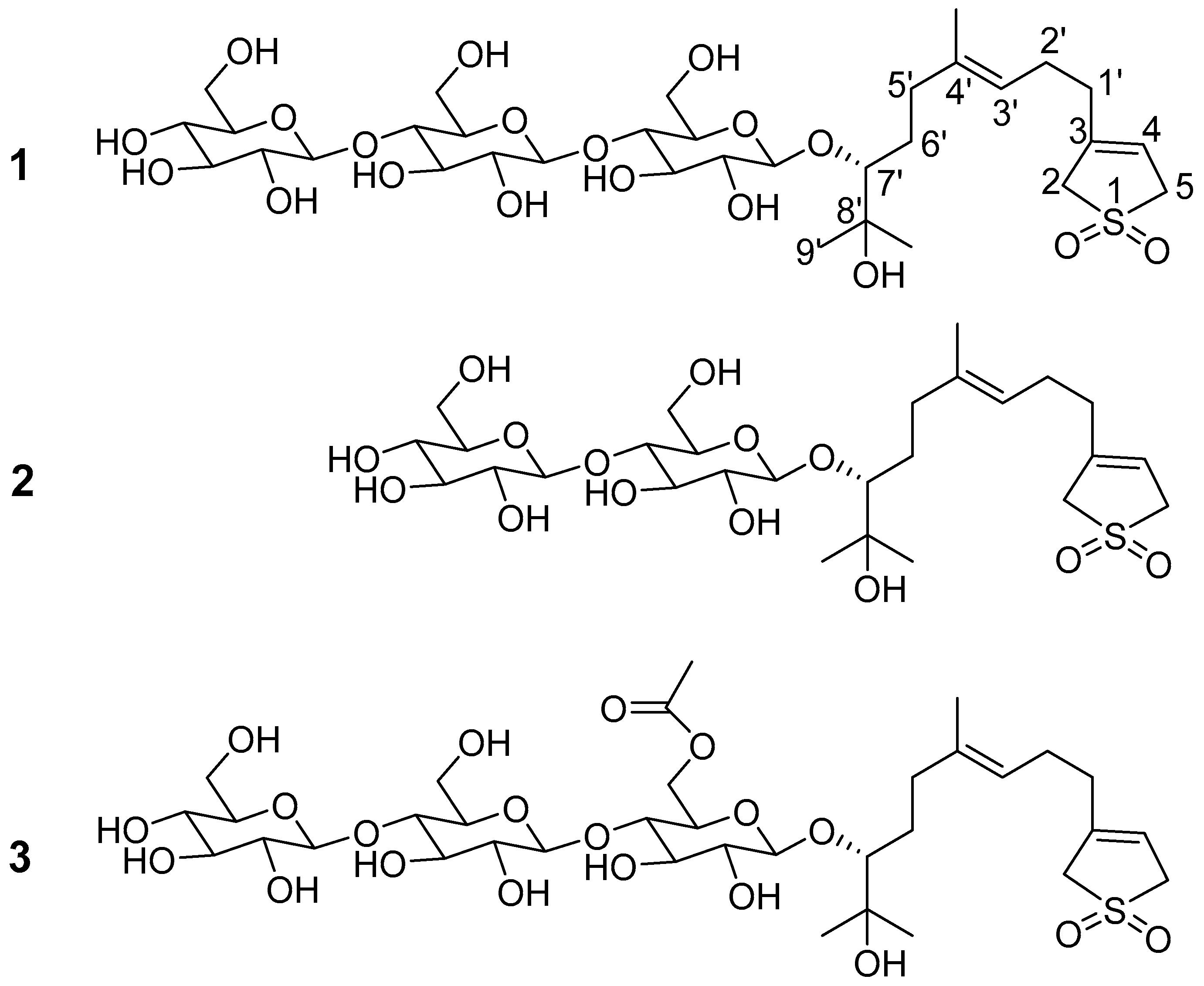
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 2 | 58.0 | 3.85 (2H, m) | 58.0 | 3.85 (2H, m) | 58.0 | 3.85 (2H, m) |
| 3 | 138.7 | 138.7 | 138.7 | |||
| 4 | 117.8 | 5.58 (1H, m) | 117.8 | 5.58 (1H, m) | 117.8 | 5.58 (1H, m) |
| 5 | 57.4 | 3.89 (2H, m) | 57.4 | 3.89 (2H, m) | 57.4 | 3.89 (2H, m) |
| 1′ | 33.1 | 2.07 (2H, m) | 33.1 | 2.07 (2H, m) | 33.1 | 2.07 (2H, m) |
| 2′ | 25.6 | 2.09 (2H, m) | 25.6 | 2.09 (2H, m) | 25.6 | 2.09 (2H, m) |
| 3′ | 123.6 | 5.34 (1H, br t, J = 7.2Hz) | 123.6 | 5.34 (1H, br t, J = 7.2Hz) | 123.6 | 5.34 (1H, br t, J = 7.2Hz) |
| 4′ | 136.8 | 136.8 | 136.8 | |||
| 5′ | 36.2 | 2.74 (1H, m) | 36.1 | 2.74 (1H, m) | 36.1 | 2.74 (1H, m) |
| 2.50 (1H, m) | 2.50 (1H, m) | 2.50 (1H, m) | ||||
| 6′ | 30.8 | 1.84 (1H, m) | 30.8 | 1.84 (1H, m) | 30.8 | 1.84 (1H, m) |
| 1.76 (1H, m) | 1.76 (1H, m) | 1.76 (1H, m) | ||||
| 7′ | 90.0 | 3.75 (1H, dd, J = 1.5, 9.5Hz) | 90.0 | 3.75 (1H, dd, J = 1.5, 9.5Hz) | 90.4 | 3.75 (1H, dd, J = 1.5, 9.5Hz) |
| 8′ | 71.9 | 71.9 | 71.9 | |||
| 9′ | 25.3 | 1.37 (3H, s) | 25.3 | 1.37 (3H, s) | 25.5 | 1.37 (3H, s) |
| 8′-CH3 | 26.8 | 1.32 (3H, s) | 26.8 | 1.32 (3H, s) | 26.7 | 1.32 (3H, s) |
| 4′-CH3 | 16.1 | 1.61 (3H, s) | 16.1 | 1.61 (3H, s) | 16.1 | 1.61 (3H, s) |
| Glc-1′ | 105.7 | 4.89 (1H, d, J = 7.8 Hz) | 105.7 | 4.91 (1H, d, J = 7.8Hz) | 105.6 | 4.86 (1H, d, J = 7.8 Hz) |
| Glc-2′ | 74.3 | 4.05 (1H, o) | 74.8 | 4.05 (1H, o) | 71.9 | 4.23 (1H, o) |
| Glc-3′ | 76.5 | 4.22 (1H, o) | 78.5 | 4.00 (1H, o) | 76.5 | 4.22 (1H, o) |
| Glc-4′ | 80.9 | 4.28 (1H, o) | 81.3 | 4.31 (1H, o) | 81.3 | 4.23 (1H, o) |
| Glc-5′ | 76.4 | 3.95 (1H, o) | 76.8 | 4.28 (1H, o) | 76.8 | 4.00 (1H, o) |
| Glc-6′ | 61.9 | 4.47 (1H, o) | 62.0 | 4.50 (2H, o) | 62.1 | 4.58 (1H, o) |
| 4.47 (1H, o) | 4.44 (1H, o) | |||||
| Glc-1′′ | 105.0 | 5.12 (1H, d, J = 7.8 Hz) | 105.0 | 5.18 (1H, d, J = 7.8Hz) | 105.0 | 5.00 (1H, d, J = 7.8 Hz) |
| Glc-2′′ | 74.7 | 4.05 (1H, o) | 75.0 | 4.05 (1H, o) | 74.2 | 4.03 (1H, o) |
| Glc-3′′ | 76.7 | 4.22 (1H, o) | 78.3 | 4.17 (1H, o) | 78.5 | 4.01 (1H, o) |
| Glc-4′′ | 80.9 | 4.28 (1H, o) | 71.6 | 4.17 (1H, o) | 81.5 | 4.00 (1H, o) |
| Glc-5′′ | 78.5 | 3.96 (1H, o) | 76.6 | 3.95 (1H, o) | 73.2 | 4.13 (1H, o) |
| Glc-6′′ | 61.8 | 4.49 (1H, o) | 62.5 | 4.52 (1H, dd, J = 2.4, 11.4 Hz) | 64.3 | 5.13 (1H, dd, J = 2.4, 11.4 Hz) |
| 4.49 (1H, o) | 4.29 (1H, o) | 4.77 (1H, o) | ||||
| Glc-1′′′ | 104.5 | 5.15 (1H, d, J = 7.8 Hz) | 104.7 | 5.13 (1H, d, J = 7.8 Hz) | ||
| Glc-2′′′ | 75.0 | 4.05 (1H, o) | 74.8 | 4.05 (1H, o) | ||
| Glc-3′′′ | 76.6 | 4.17 (1H, o) | 76.6 | 3.95 (1H, o) | ||
| Glc-4′′′ | 71.5 | 4.17 (1H, o) | 71.6 | 4.15 (1H, o) | ||
| Glc-5′′′ | 78.2 | 3.95 (1H, o) | 78.2 | 4.17 (1H, o) | ||
| Glc-6′′′ | 62.5 | 4.52 (1H, dd, J = 2.4, 11.4 Hz) | 62.5 | 4.52 (1H, dd, J = 2.4, 11.4 Hz) | ||
| 4.29 (1H, o) | 4.28 (1H, o) | |||||
| CH3CO | 20.8 | 2.08 (3H, s) | ||||
| CH3CO | 170.8 | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Yang, Y.-J.; Sun, X.-G.; Zhang, J.; Zhao, Y.; Wang, B.; Ding, Q.-Z.; Guo, B.-L.; Ma, B.-P. Three New Sesquiterpene Glycosides from the Rhizomes of Trillium tschonoskii. Molecules 2017, 22, 1283. https://doi.org/10.3390/molecules22081283
Yang J, Yang Y-J, Sun X-G, Zhang J, Zhao Y, Wang B, Ding Q-Z, Guo B-L, Ma B-P. Three New Sesquiterpene Glycosides from the Rhizomes of Trillium tschonoskii. Molecules. 2017; 22(8):1283. https://doi.org/10.3390/molecules22081283
Chicago/Turabian StyleYang, Jie, Yin-Jun Yang, Xin-Guang Sun, Jie Zhang, Yang Zhao, Bei Wang, Qian-Zhi Ding, Bao-Lin Guo, and Bai-Ping Ma. 2017. "Three New Sesquiterpene Glycosides from the Rhizomes of Trillium tschonoskii" Molecules 22, no. 8: 1283. https://doi.org/10.3390/molecules22081283
APA StyleYang, J., Yang, Y.-J., Sun, X.-G., Zhang, J., Zhao, Y., Wang, B., Ding, Q.-Z., Guo, B.-L., & Ma, B.-P. (2017). Three New Sesquiterpene Glycosides from the Rhizomes of Trillium tschonoskii. Molecules, 22(8), 1283. https://doi.org/10.3390/molecules22081283