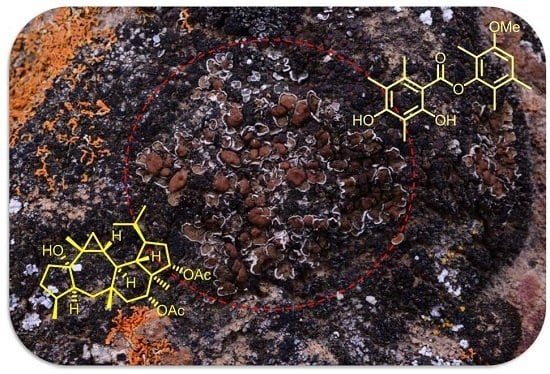
Gypmacrophin A, a Rare Pentacyclic Sesterterpenoid, Together with Three Depsides, Functioned as New Chemical Evidence for Gypsoplaca macrophylla (Zahlbr.) Timdal Identification
Abstract
1. Introduction
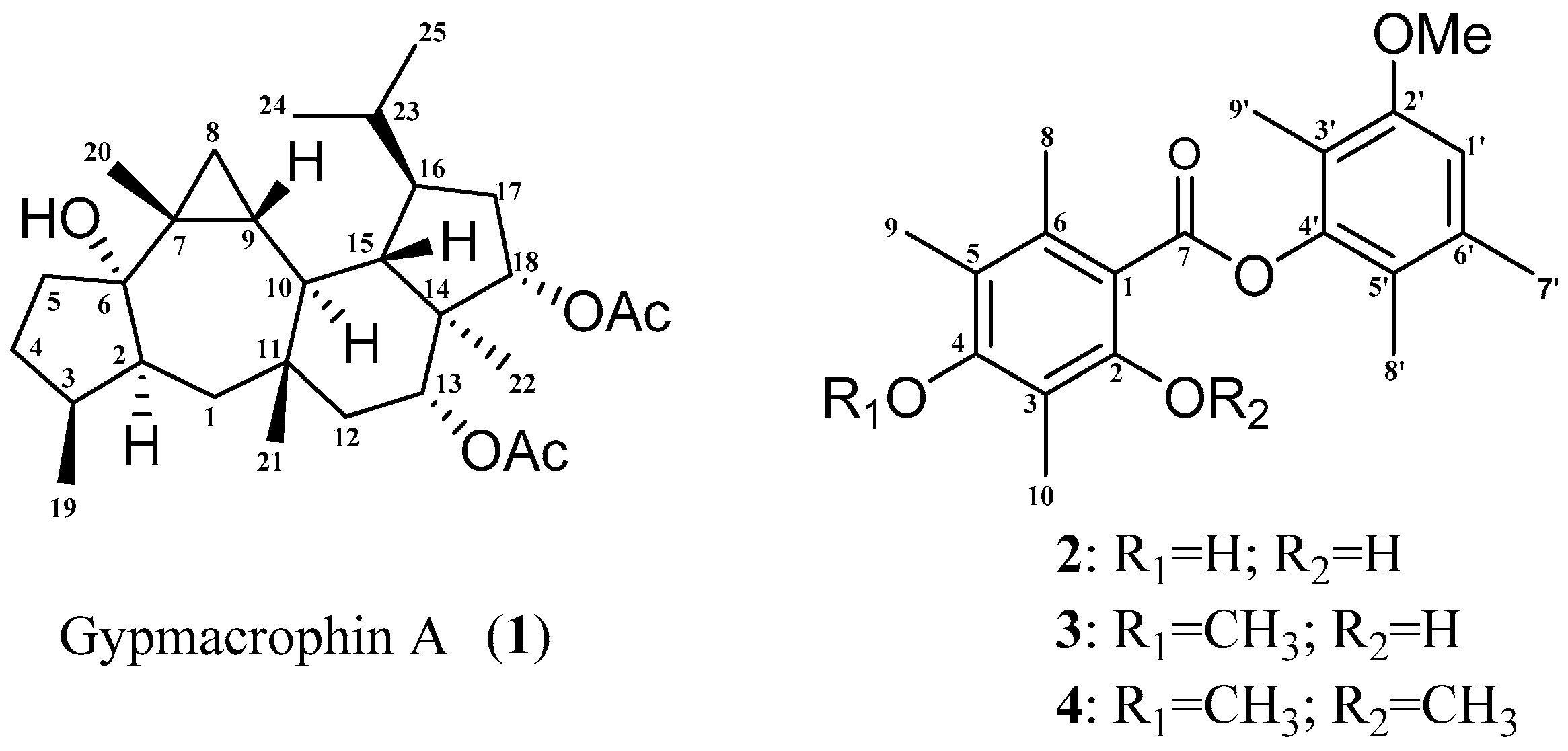
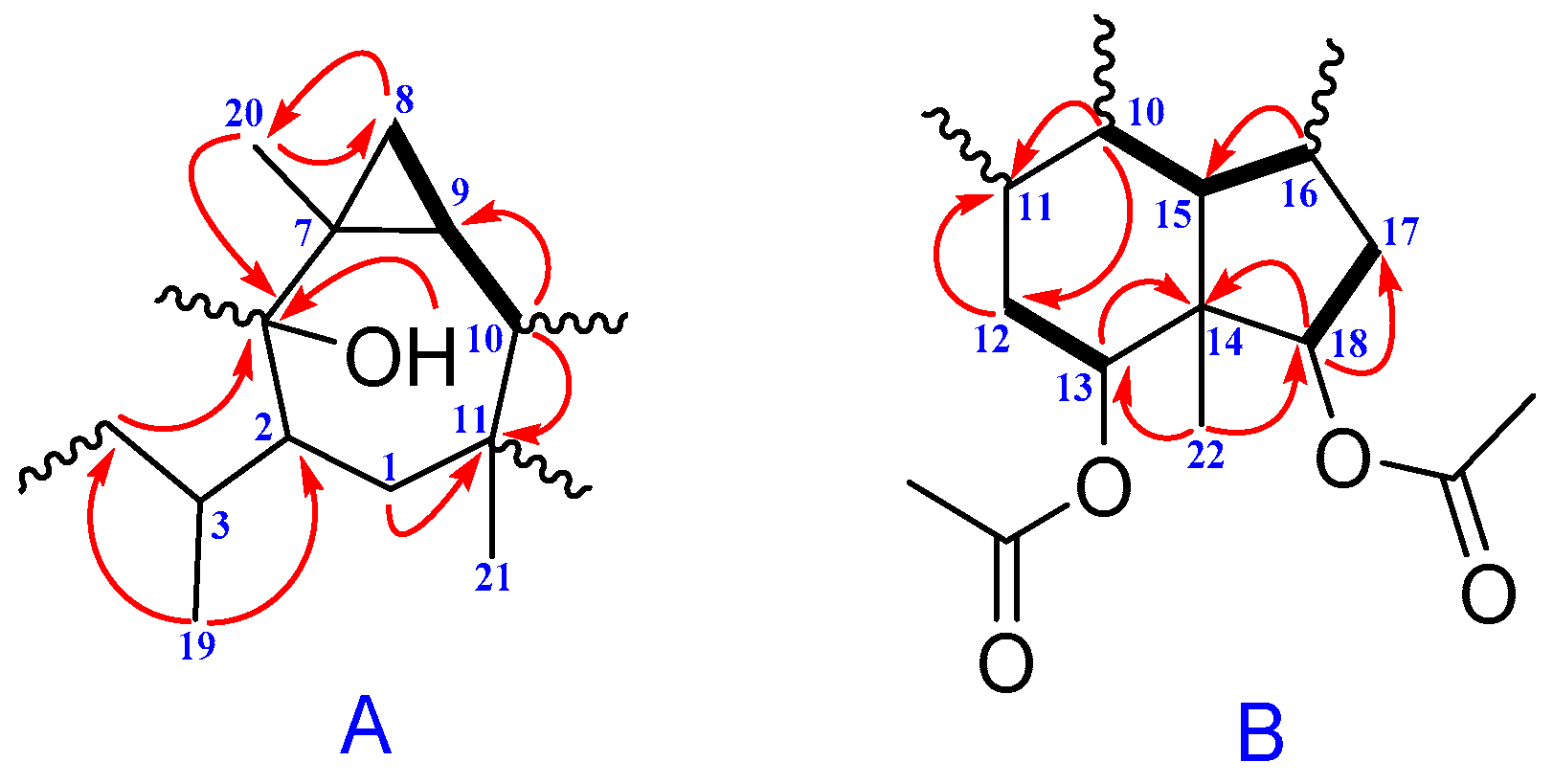
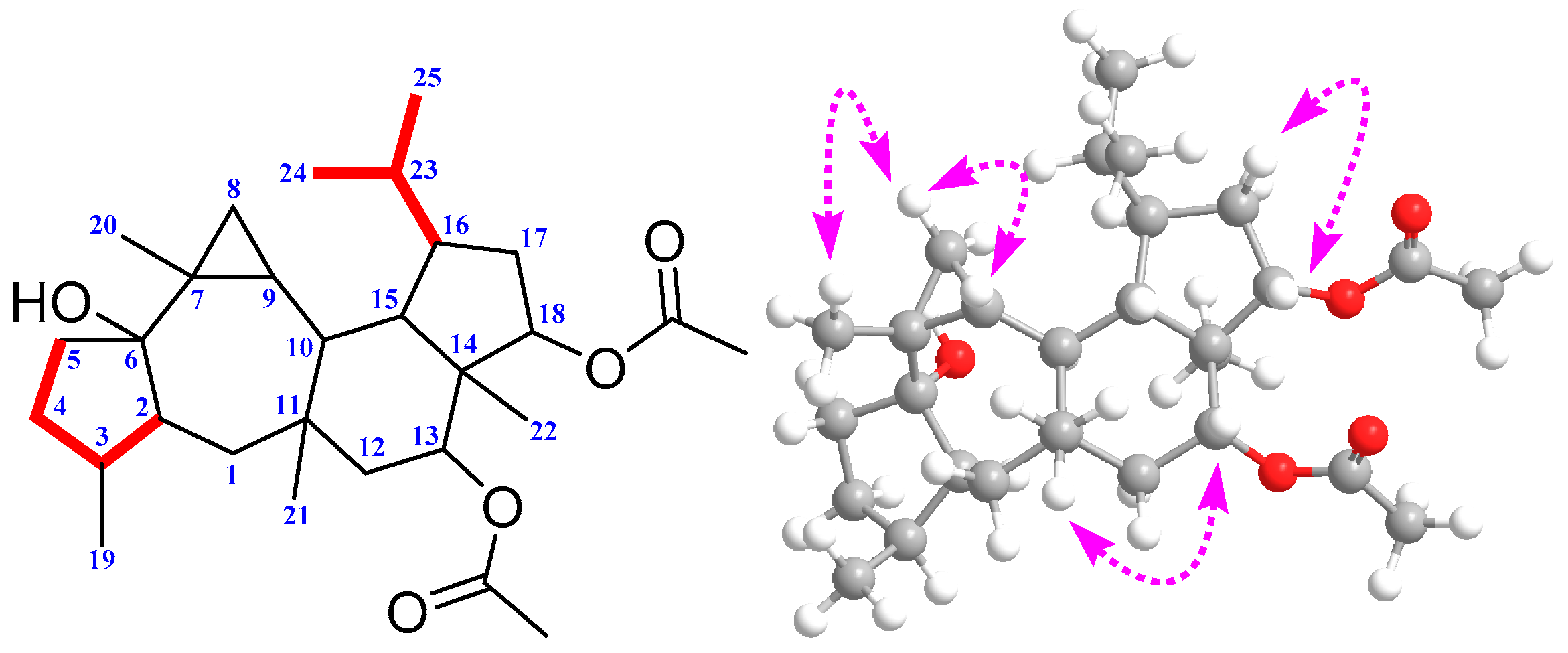
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Collection and Identification of Biological Materials
3.3. Extraction and Isolation
3.4. 13C-NMR and OR Calculations
3.5. Bioactive Assay of Gypmacrophin A
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tian, W.; Deng, Z.; Hong, K. The biological activities of sesterterpenoid-Type ophiobolins. Mar. Drugs 2017, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Kornienko, A.; Lefranc, F.; Cimmino, A.; Dasari, R.; Evidente, M.; Mathieu, V.; Kiss, R. Sesterterpenoids with anticancer activity. Curr. Med. Chem. 2015, 22, 3502–3522. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Coque, T.M.; de la Cruz, F. Ecology and evolution as targets: The need for novel eco-evo drugs and strategies to fight antibiotic resistance. Antimicrob. Agents Chemother. 2011, 55, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Bocanegra-García, V.; Palma-Nicolás, J.P.; Rivera, G. Recent advances in antitubercular natural products. Eur. J. Med. Chem. 2012, 49, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Stowe, S.D.; Richards, J.J.; Tucker, A.T.; Thompson, R.; Melander, C.; Cavanagh, J. Anti-Biofilm compounds derived from marine sponges. Mar. Drugs 2011, 9, 2010–2035. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, B.; Lin, X.P.; Zhou, X.F.; Liu, Y. Sesterterpenoids. Nat. Prod. Rep. 2013, 30, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Y.; Chen, S.; Liu, Y.; Lu, Y.; Chen, D.; Lin, Y.; Huang, X.; She, Z. Aspterpenacids A and B, two sesterterpenoids from a mangrove endophytic fungus Aspergillus terreus H010. Org. Lett. 2016, 18, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. The sesterterpenoids. Nat. Prod. Rep. 1992, 9, 481–489. [Google Scholar] [CrossRef]
- Okada, M.; Matsuda, Y.; Mitsuhashi, T.; Hoshino, S.; Mori, T.; Nakagawa, K.; Quan, Z.; Qin, B.; Zhang, H.; Hayashi, F.; et al. Genome-based discovery of an unprecedented cyclization mode in fungal sesterterpenoid biosynthesis. J. Am. Chem. Soc. 2016, 138, 10011–10018. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Mitsuhashi, T.; Lee, S.; Hoshino, M.; Mori, T.; Okada, M.; Zhang, H.P.; Hayashi, F.; Fujita, M.; Abe, I. Astellifadiene: Structure determination by NMR spectroscopy and crystalline sponge method, and elucidation of its biosynthesis. Angew. Chem. Int. Ed. 2016, 55, 5785–5788. [Google Scholar] [CrossRef] [PubMed]
- Lutzoni, F.; Pagel, M.; Reeb, V. Major fungal lineages are derived from lichen symbiotic ancestors. Nature 2001, 411, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, K.V.; Schmitt, N.; Volk, P.L.; Suárez, P.J.; Beck, A.; Eisenreich, W. Metabolic profiling of alpine and ecuadorian lichens. Molecules 2015, 20, 18047–18065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Wang, X.Y.; Liu, D.; Shi, H.X.; Ye, X.; Yang, M.X.; Wang, L.S. The genus Bulbothrix (Parmeliaceae) in China. Lichenologist 2016, 48, 121–133. [Google Scholar] [CrossRef]
- Wang, X.Y.; Goffinet, B.; Liu, D.; Liang, M.M.; Shi, H.X.; Zhang, Y.Y.; Zhang, J.; Wang, L.S. Taxonomic study of the genus Anzia (Lecanorales, lichenized Ascomycota) from Hengduan Mountains, China. Lichenologist 2015, 47, 99–115. [Google Scholar] [CrossRef]
- Timdal, E. Gypsoplacaceae and Gypsoplaca, a new family and genus of squamiform lichens. Bibl. Lichenol. 1990, 38, 419–427. [Google Scholar]
- Abdulla, A.; Hurnisa, X.; Reyim, M.; Adilijiang, A. A new record of lichen family from Xinjiang. Arid Zone Res. 2015, 32, 509–511. [Google Scholar]
- Zhao, Z.-Z.; Chen, H.-P.; Wu, B.; Zhang, L.; Li, Z.-H.; Feng, T.; Liu, J.-K. Matsutakone and Matsutoic Acid, Two (Nor)steroids with unusual skeletons from the edible mushroom Tricholoma matsutake. J. Org. Chem. 2017, 82, 7974–7979. [Google Scholar] [CrossRef] [PubMed]
- Elix, J.A.; Barclay, C.E.; David, F.; Griffin, F.K.; Hill, A.M.; Mcconnell, D.B.; Wardlaw, J.H. Synthesis of further lichen depsides. Aust. J. Chem. 1993, 46, 301–313. [Google Scholar] [CrossRef]
- Seo, C.; HanYim, J.; Kum Lee, H.; Oh, H. PTP1B inhibitory secondary metabolites from the Antarctic lichen Lecidella carpathica. Mycology 2011, 2, 18–23. [Google Scholar] [CrossRef]
- Huang, X.; Huang, H.; Li, H.; Sun, X.; Huang, H.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Asperterpenoid A, a new sesterterpenoid as an inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B from the culture of Aspergillus sp. 16–5c. Org. Lett. 2013, 15, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Position | δH-exp a | δC-exp b | δC-cal c |
|---|---|---|---|
| 1β | 1.41 (overlapped, 1H) | 40.9 | 40.1 |
| 1α | 1.13 (d, J = 14.1 Hz, 1H) | -- | -- |
| 2 | 1.99 (overlapped, 1H) | 49.7 | 49.8 |
| 3 | 2.28 (m, 1H) | 37.7 | 39.9 |
| 4β | 1.29 (m, 1H) | 32.4 | 33.2 |
| 4α | 1.94 (m, 1H) | -- | -- |
| 5β | 2.12 (m, 1H) | 38.8 | 40.4 |
| 5α | 1.53 (dd, J = 13.0, 5.7 Hz, 1H) | -- | -- |
| 6 | -- | 83.3 | 84.7 |
| 7 | -- | 24.2 | 27.4 |
| 8β | 0.24 (dd, J = 8.5, 4.1 Hz, 1H) | 16.4 | 16.7 |
| 8α | 1.02 (overlapped, 1H) | -- | -- |
| 9 | 0.54 (ddd, J = 11.4, 8.5, 5.5 Hz, 1H) | 24.8 | 26.3 |
| 10 | 1.76 (t, J = 11.4 Hz, 1H) | 38.8 | 40.2 |
| 11 | -- | 39.7 | 42.6 |
| 12β | 1.18 (dd, J = 12.7, 4.9 Hz, 1H) | 39.4 | 38.9 |
| 12α | 1.60 (overlapped, 1H) | -- | -- |
| 13 | 4.91 (dd, J = 11.7, 4.9 Hz, 1H) | 77.3 | 77.4 |
| 14 | -- | 49.4 | 51.9 |
| 15 | 1.60 (m, 1H) | 44.6 | 45.3 |
| 16 | 2.02 (overlapped, 1H) | 42.2 | 42.9 |
| 17β | 1.92 (overlapped, 1H) | 28.7 | 30.1 |
| 17α | 1.42 (m, 1H) | -- | -- |
| 18 | 4.65 (t, J = 9.0 Hz, 1H) | 81.1 | 81.9 |
| 19 | 0.83 (d, J = 7.4, 3H) | 15.9 | 16.2 |
| 20 | 1.07 (s, 3H) | 25.8 | 25.6 |
| 21 | 1.03 (s, 3H) | 22.8 | 22.8 |
| 22 | 0.92 (s, 3H) | 8.7 | 10.7 |
| 23 | 2.31 (m, 1H) | 28.4 | 30.6 |
| 24 | 0.85 (d, J = 7.3, 3H) | 23.2 | 23.2 |
| 25 | 0.86 (d, J = 7.3, 3H) | 15.5 | 15.4 |
| AcO-13 | -- | 170.4 | 166.9 |
| -- | 1.93 (s, 3H) | 21.2 | 22.1 |
| AcO-18 | -- | 170.2 | 166.8 |
| -- | 1.90 (s, 3H) | 20.9 | 21.8 |
| HO-6 | 2.96 (s, 1H) | -- | -- |
| Position | δH (2) a | δC (2) b | δH (3) a | δC (3) b | δH (4) a | δC (4) b |
|---|---|---|---|---|---|---|
| 1 | -- | 105.1 | -- | 123.1 | -- | 121.5 |
| 2 | -- | 161.8 | -- | 162.3 | -- | 159.8 |
| 3 | -- | 109.3 | -- | 109.8 | -- | 117.1 |
| 4 | -- | 159.8 | -- | 161.0 | -- | 155.4 |
| 5 | -- | 117.3 | -- | 117.2 | -- | 125.7 |
| 6 | -- | 138.4 | -- | 138.6 | -- | 136. 0 |
| 7 | -- | 171.5 | -- | 171.0 | -- | 167.1 |
| 8 | 2.66 (s, 3H) | 19.5 | 2.64 (s, 3H) | 19.3 | 2.14 (s, 3H) | 17.3 |
| 9 | 2.23 (s, 3H) | 12.4 | 2.23 (s, 3H) | 12.6 | 2.17 (s, 3H) | 12.5 |
| 10 | 2.16 (s, 3H) | 8.7 | 2.16 (s, 3H) | 9.3 | 2.31 (s, 3H) | 9.8 |
| 1′ | 6.79 (s, 1H) | 110.8 | 6.80 (s, 1H) | 111.0 | 6.77 (s, 1H) | 110.7 |
| 2′ | -- | 156.8 | -- | 156.6 | -- | 156.8 |
| 3′ | -- | 116.6 | -- | 116.5 | -- | 122.9 |
| 4′ | -- | 149.2 | -- | 149.2 | -- | 149.6 |
| 5′ | -- | 136.1 | -- | 136.2 | -- | 127.1 |
| 6′ | -- | 121.0 | -- | 121.0 | -- | 133.9 |
| 7′ | 2.31 (s, 3H) | 20.2 | 2.31 (s, 3H) | 20.2 | 2.23 (s, 3H) | 20.3 |
| 8′ | 2.02 (s, 3H) | 12.6 | 2.02 (s, 3H) | 12.7 | 2.27 (s, 3H) | 12.7 |
| 9′ | 1.99 (s, 3H) | 9.7 | 1.99 (s, 3H) | 9.8 | 2.34 (s, 3H) | 9.9 |
| CH3O-2′ | 3.85 (s, 3H) | 56.0 | 3.80 (s, 3H) | 56.1 | 3.74 (s, 3H) | 56.0 |
| R2O-2 | 11.56 (s, 1H) | 11.02 (s, 1H) | 3.84 (s, 3H) | 62.1 | ||
| R1O-4 | 8.18 (s, 1H) | 3.74 (s, 3H) | 60.3 | 3.81 (s, 3H) | 60.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.-F.; Shi, H.-X.; Hu, K.; Tang, J.-W.; Li, X.-R.; Du, X.; Sun, H.-D.; Wang, L.-S.; Pu, J.-X. Gypmacrophin A, a Rare Pentacyclic Sesterterpenoid, Together with Three Depsides, Functioned as New Chemical Evidence for Gypsoplaca macrophylla (Zahlbr.) Timdal Identification. Molecules 2017, 22, 1675. https://doi.org/10.3390/molecules22101675
Zhou Y-F, Shi H-X, Hu K, Tang J-W, Li X-R, Du X, Sun H-D, Wang L-S, Pu J-X. Gypmacrophin A, a Rare Pentacyclic Sesterterpenoid, Together with Three Depsides, Functioned as New Chemical Evidence for Gypsoplaca macrophylla (Zahlbr.) Timdal Identification. Molecules. 2017; 22(10):1675. https://doi.org/10.3390/molecules22101675
Chicago/Turabian StyleZhou, Yuan-Fei, Hai-Xia Shi, Kun Hu, Jian-Wei Tang, Xing-Ren Li, Xue Du, Han-Dong Sun, Li-Song Wang, and Jian-Xin Pu. 2017. "Gypmacrophin A, a Rare Pentacyclic Sesterterpenoid, Together with Three Depsides, Functioned as New Chemical Evidence for Gypsoplaca macrophylla (Zahlbr.) Timdal Identification" Molecules 22, no. 10: 1675. https://doi.org/10.3390/molecules22101675
APA StyleZhou, Y.-F., Shi, H.-X., Hu, K., Tang, J.-W., Li, X.-R., Du, X., Sun, H.-D., Wang, L.-S., & Pu, J.-X. (2017). Gypmacrophin A, a Rare Pentacyclic Sesterterpenoid, Together with Three Depsides, Functioned as New Chemical Evidence for Gypsoplaca macrophylla (Zahlbr.) Timdal Identification. Molecules, 22(10), 1675. https://doi.org/10.3390/molecules22101675