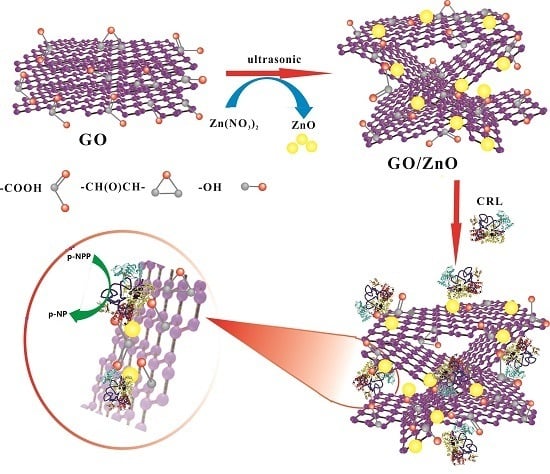
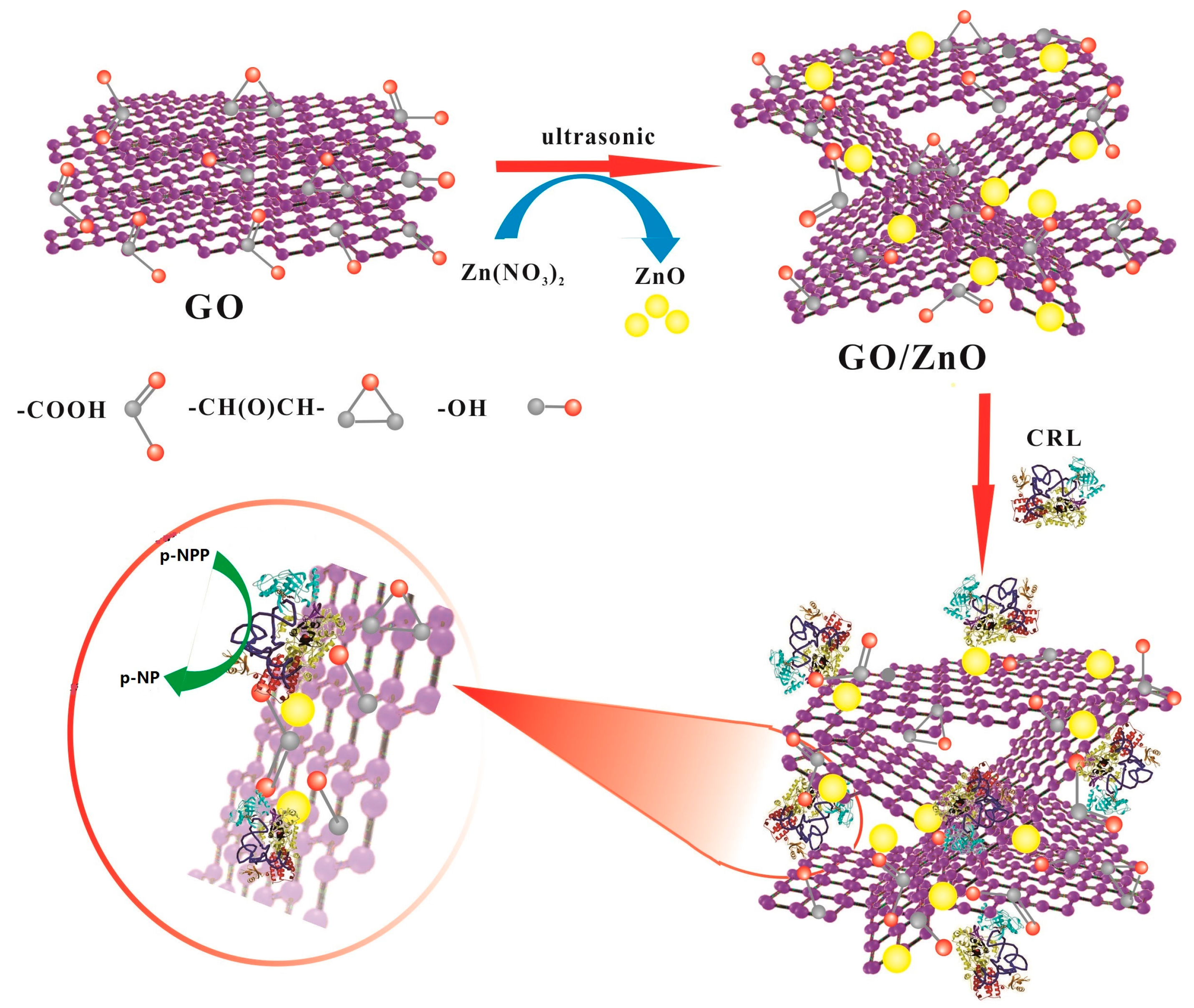
Preparation of Carriers Based on ZnO Nanoparticles Decorated on Graphene Oxide (GO) Nanosheets for Efficient Immobilization of Lipase from Candida rugosa
Abstract
:1. Introduction
2. Results and Discussion
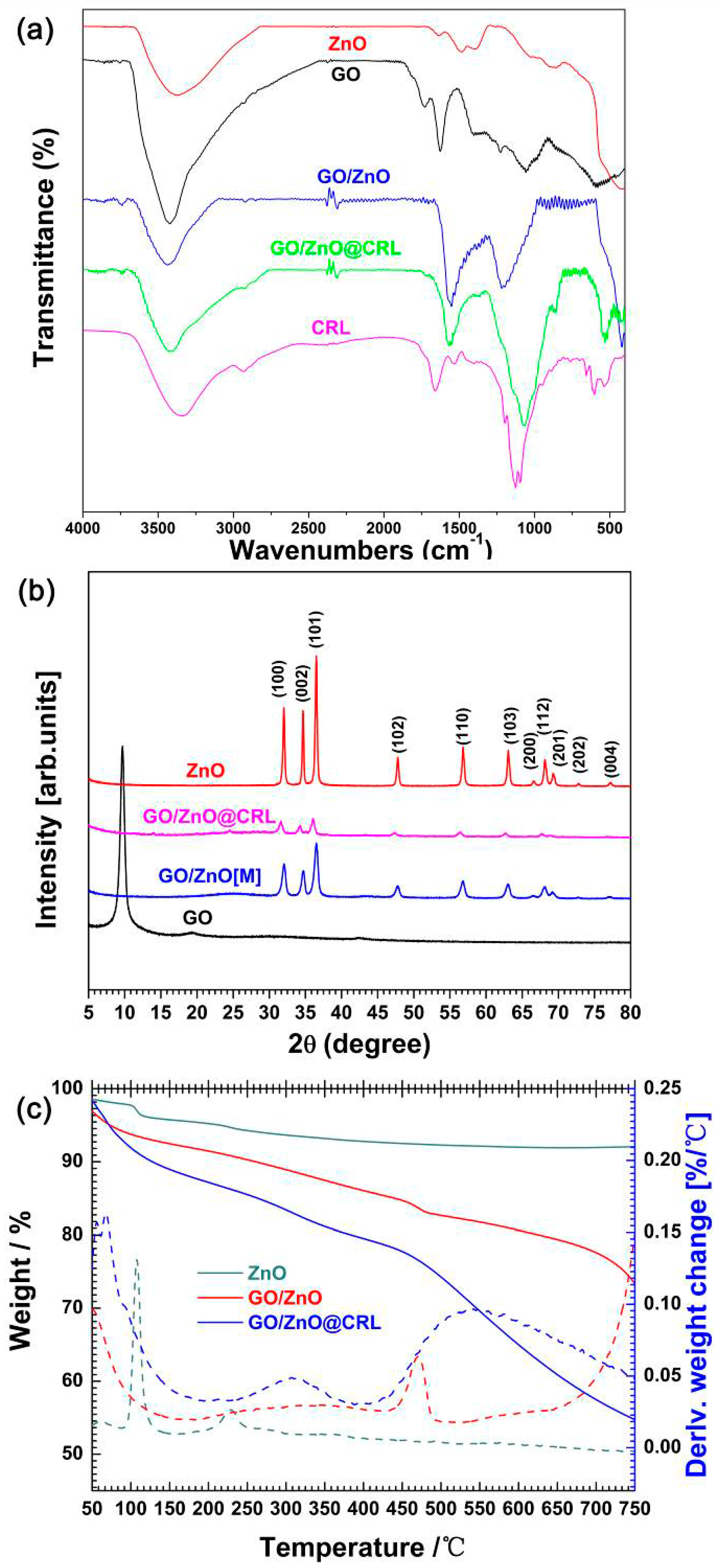
2.1. Design, Synthesis and Structural Characterization of GO/ZnO Composites
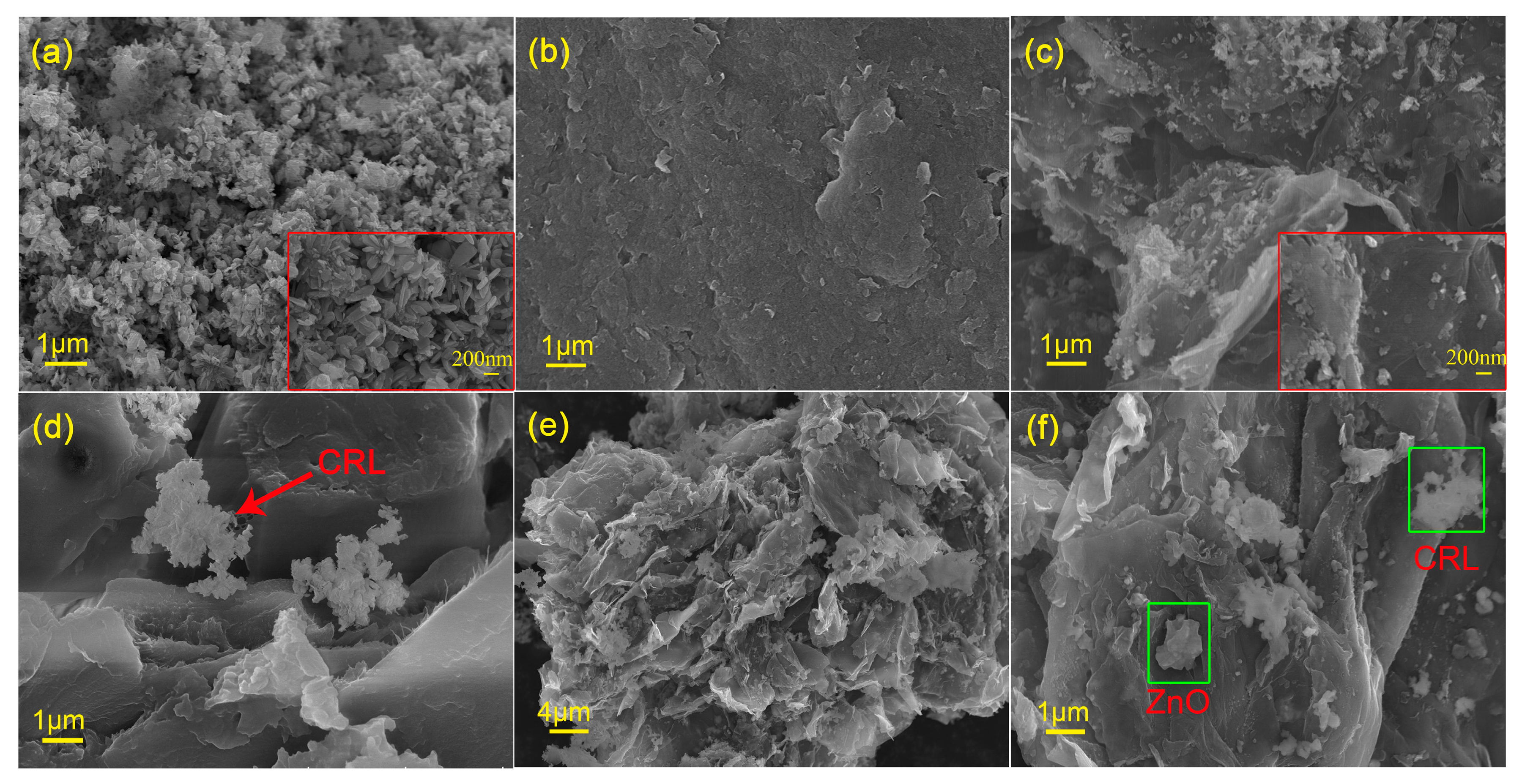
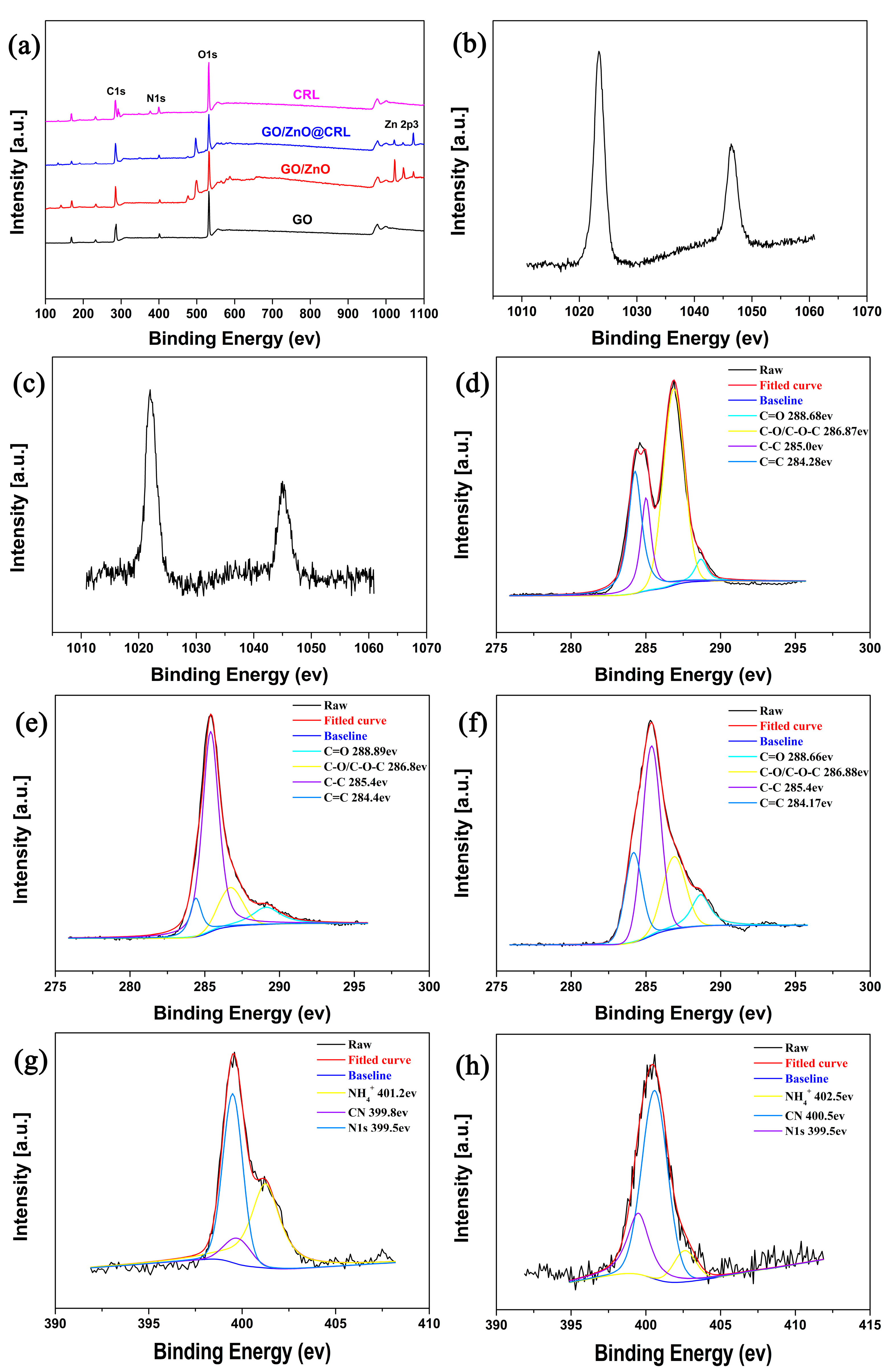
2.2. Materials Characterizations
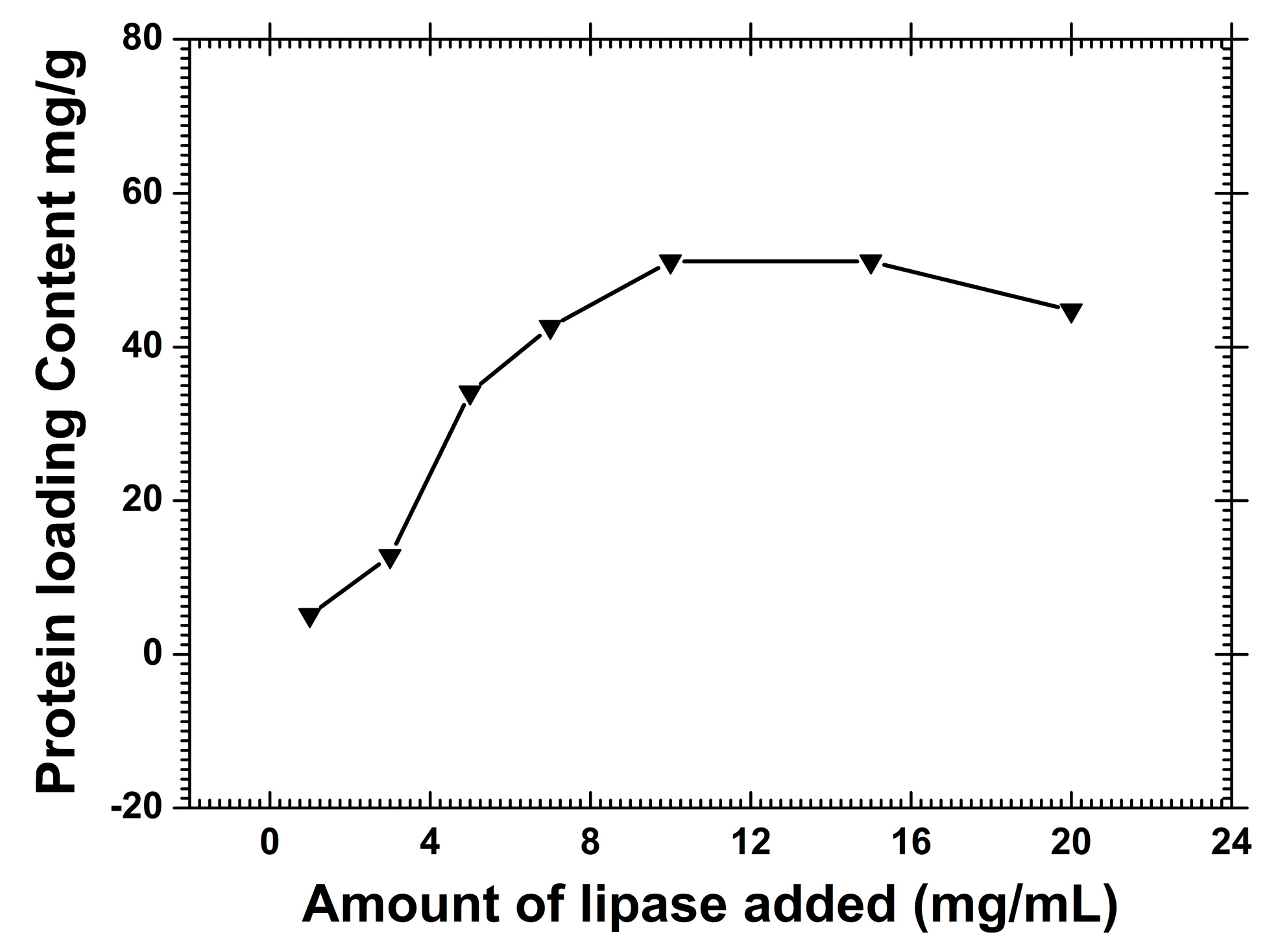
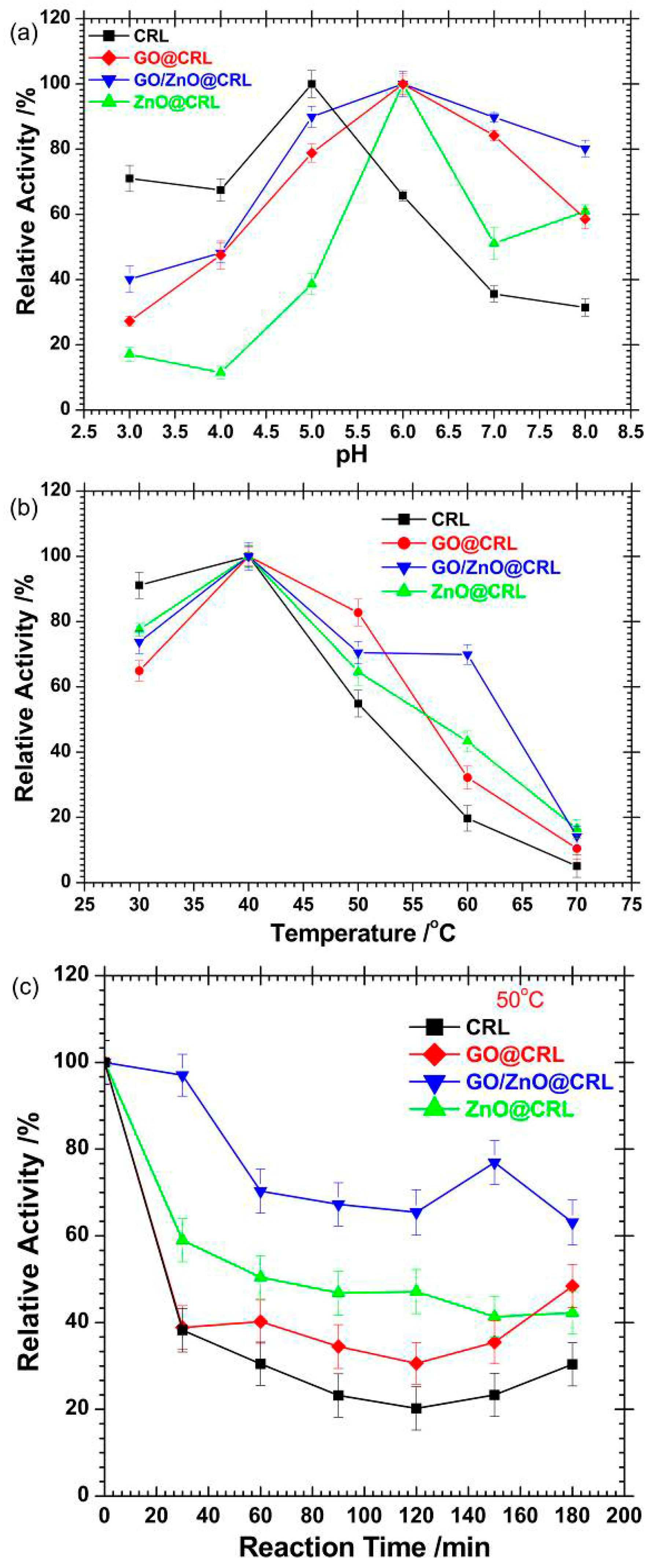
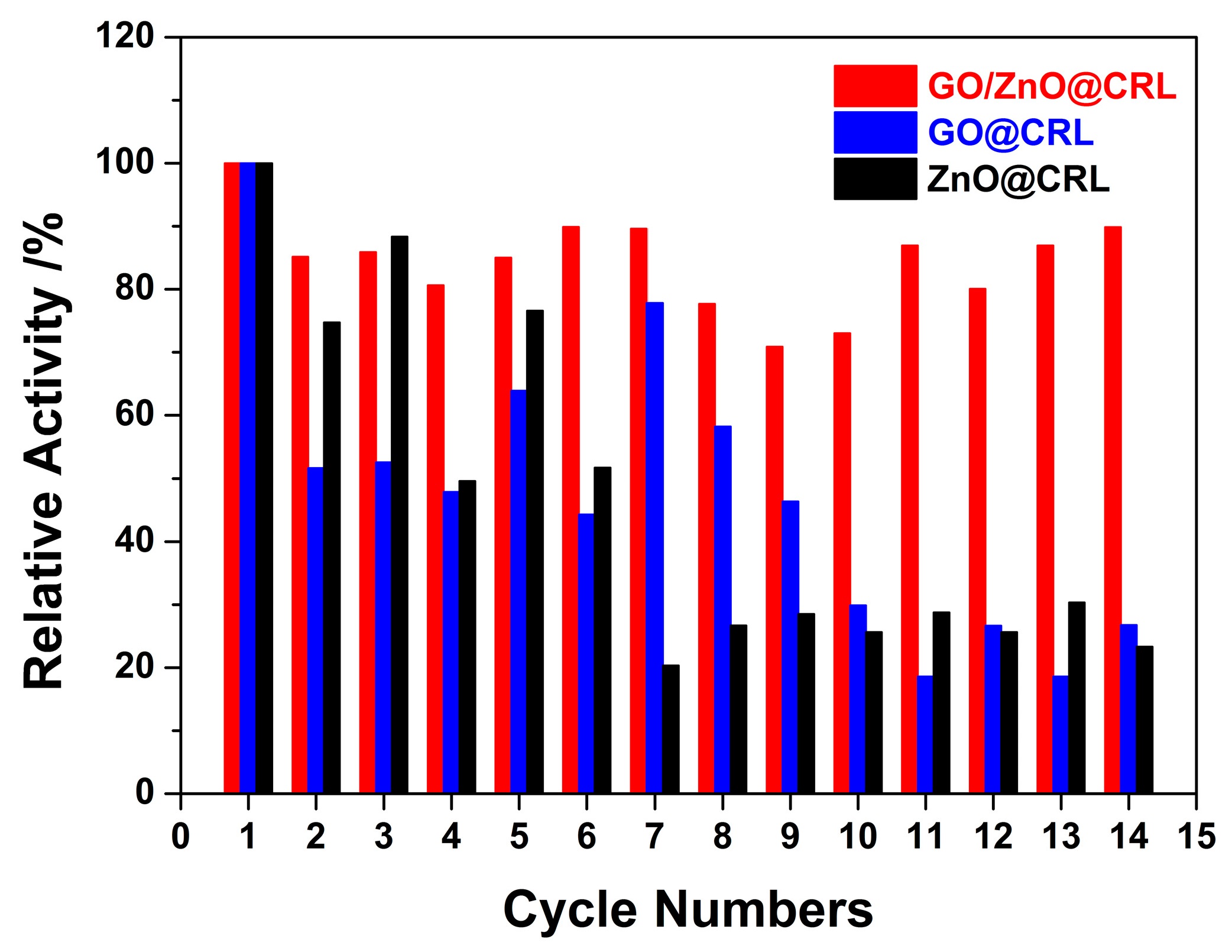
2.3. Relative Activity of the Immobilized CRL
3. Materials and Methods
3.1. Materials
3.2. Preparation of GO
3.3. Synthesis of ZnO and GO/ZnO Materials
3.4. Immobilization of Lipase
3.5. Lipase Activity Assay
3.6. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Markiton, M.; Boncel, S.; Janas, D.; Chrobok, A. Highly active nanobiocatalyst from lipase noncovalently immobilized on multiwalled carbon nanotubes for Baeyer-villiger synthesis of lactones. ACS Sustain. Chem. Eng. 2017, 5, 1685–1691. [Google Scholar] [CrossRef]
- De Souza, T.C.; de S. Fonseca, T.; da Costa, J.A.; Rocha, M.V.P.; de Mattos, M.C.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; dos Santos, J.C.S. Cashew apple bagasse as a support for the immobilization of lipase B from Candida antarctica: Application to the chemoenzymatic production of (R)-indanol. J. Mol. Catal. B Enzym. 2016, 130, 58–69. [Google Scholar] [CrossRef]
- Lage, F.A.; Bassi, J.J.; Corradini, M.C.; Todero, L.M.; Luiz, J.H.; Mendes, A.A. Preparation of a biocatalyst via physical adsorption of lipase from thermomyces lanuginosus on hydrophobic support to catalyze biolubricant synthesis by esterification reaction in a solvent-free system. Enzym. Microb. Technol. 2016, 84, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kralovec, J.A.; Wang, W.J.; Barrow, C.J. Production of omega-3 triacylglycerol concentrates using a new food grade immobilized Candida antarctica lipase B. Aust. J. Chem. 2010, 63, 922–928. [Google Scholar] [CrossRef]
- Magadum, D.B.; Yadav, G.D. Enantioselective resolution of (R,S)-alpha-methyl-4-pyridinemethanol using immobilized biocatalyst: Optimization and kinetic modeling. Biochem. Eng. J. 2017, 122, 152–158. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, Y.X.; Jiang, T.Y.; Meng, J.J.; Sheng, B.B.; Yang, C.Y.; Gao, C.; Xu, P.; Ma, C.Q. A novel biocatalyst for efficient production of 2-oxo-carboxylates using glycerol as the cost-effective carbon source. Biotechnol. Biofuels 2015, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, S.; Pandey, A. Candida rugosa lipases: Molecular biology and versatility in biotechnology. Yeast 1998, 14, 1069–1087. [Google Scholar] [CrossRef]
- Pollard, D.J.; Woodley, J.M. Biocatalysis for pharmaceutical intermediates: The future is now. Trends Biotechnol. 2007, 25, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Dordick, J.S.; Hauer, B.; Kiener, A.; Wubbolts, M.; Witholt, B. Industrial biocatalysis today and tomorrow. Nature 2001, 409, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Rios, N.S.; Pinheiro, M.P.; dos Santos, J.C.S.; de S. Fonseca, T.; Lima, L.D.; de Mattos, M.C.; Freire, D.M.G.; da Silva Júnior, I.J.; Rodríguez-Aguado, E.; Gonçalves, L.R.B. Strategies of covalent immobilization of a recombinant Candida antarctica lipase B on pore-expanded SBA-15 and its application in the kinetic resolution of (R,S)-phenylethyl acetate. J. Mol. Catal. B Enzym. 2016, 133, 246–258. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Bonazza, H.L.; de Matos, L.; Carneiro, E.A.; Barbosa, O.; Fernandez-Lafuente, R.; Goncalves, L.R.B.; de Sant’ Ana, H.B.; Santiago-Aguiar, R.S. Immobilization of CALB on activated chitosan: Application to enzymatic synthesis in supercritical and near-critical carbon dioxide. Biotechnol. Rep. 2017, 14, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Grate, J.W.; Wang, P. Nanostructures for enzyme stabilization. Chem. Eng. Sci. 2006, 61, 1017–1026. [Google Scholar] [CrossRef]
- Huang, X.J.; Yu, A.G.; Jiang, J.; Pan, C.; Qian, J.; Xu, Z.K. Surface modification of nanofibrous poly(acrylonitrile-co-acrylic acid) membrane with biomacromolecules for lipase immobilization. J. Mol. Catal. B Enzym. 2009, 57, 250–256. [Google Scholar] [CrossRef]
- Rueda, N.; Santos, J.C.S.D.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R.; Torres, R. Chemical amination of lipases improves their immobilization on octyl-glyoxyl agarose beads. Catal. Today 2016, 259, 107–118. [Google Scholar] [CrossRef]
- Albuquerque, T.L.D.; Rueda, N.; dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Binay, B.; Özdemir, E.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Easy stabilization of interfacially activated lipases using heterofunctional divinyl sulfone activated-octyl agarose beads. Modulation of the immobilized enzymes by altering their nanoenvironment. Process Biochem. 2016, 51, 865–874. [Google Scholar] [CrossRef]
- Manoel, E.A.; Pinto, M.; dos Santos, J.C.S.; Tacias-Pascacio, V.G.; Freire, D.M.G.; Pinto, J.C.; Fernandez-Lafuente, R. Design of a core-shell support to improve lipase features by immobilization. RSC Adv. 2016, 6, 62814–62824. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Rueda, N.; Torres, R.; Barbosa, O.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Evaluation of divinylsulfone activated agarose to immobilize lipases and to tune their catalytic properties. Process Biochem. 2015, 50, 918–927. [Google Scholar] [CrossRef]
- Min, K.; Yoo, Y.J. Recent progress in nanobiocatalysis for enzyme immobilization and its application. Biotechnol. Bioprocess Eng. 2014, 19, 553–567. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Yan, B.; Ma, H.; Li, N.; Hu, Y.; Ye, M. Covalent attaching protein to graphene oxide via diimide-activated amidation. Colloids Surf. B Biointerfaces 2010, 81, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Mathesh, M.; Luan, B.; Akanbi, T.O.; Weber, J.K.; Liu, J.; Barrow, C.J.; Zhou, R.; Yang, W. Opening lids: Modulation of lipase immobilization by graphene oxides. ACS Catal. 2016, 6, 4760–4768. [Google Scholar] [CrossRef]
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Jing, T.; Tian, J.; Zheng, Y. Graphene oxide-based Fe3O4 nanoparticles as a novel scaffold for the immobilization of porcine pancreatic lipase. RSC Adv. 2015, 5, 103943–103955. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ashjari, M.; Garmroodi, M.; Yousefi, M.; Karkhane, A.A. The use of isocyanide-based multicomponent reaction for covalent immobilization of Rhizomucormiehei lipase on multiwall carbon nanotubes and grapheme nanosheets. RSC Adv. 2016, 6, 72275–72285. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhao, G.H.; Jing, L.Y.; Peng, X.M.; Li, Y.F. Facile self-assembly of magnetite nanoparticles on three-dimensional graphene oxide-chitosan composite for lipase immobilization. Biochem. Eng. J. 2015, 98, 75–83. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, C.S.; Rodriguez, M.D.; Albuquerque, T.L.; Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Reversible immobilization of lipases on octyl-glutamic agarose beads: A mixed adsorption that reinforces enzyme immobilization. J. Mol. Catal. B Enzym. 2016, 128, 10–18. [Google Scholar] [CrossRef]
- Dos Santos, J.C.; Rueda, N.; Goncalves, L.R.; Fernandez-Lafuente, R. Tuning the catalytic properties of lipases immobilized on divinylsulfone activated agarose by altering its nanoenvironment. Enzym. Microb. Technol. 2015, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopeza, L.; Ruedaa, N.; Bartolome-Cabreroa, R.; Rodrigueza, M.D.; Albuquerquea, T.L.; dos Santosa, J.C.S.; Barbosae, O.; Fernandez-Lafuentea, R. Improved immobilization and stabilization of lipase from Rhizomucormiehei on octyl-glyoxyl agarose beads by using CaCl2. Process Biochem. 2016, 51, 48–52. [Google Scholar] [CrossRef]
- Shah, E.; Mahapatra, P.; Bedekar, A.V.; Soni, H.P. Immobilization of Thermomyces lanuginosus lipase on ZnO nanoparticles: Mimicking the interfacial environment. RSC Adv. 2015, 5, 26291–26300. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Derewenda, U.; Derewenda, Z.S.; Dodson, G.G.; Lawson, D.M.; Turkenburg, J.P.; Bjorkling, F.; Huge-Jensen, B.; Patkar, S.A.; Thim, L. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex. Nature 1991, 351, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Manoel, E.A.; Dos Santos, J.C.; Freire, D.M.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzym. Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.G.; Wahab, R.; Ansari, Z.A.; Kim, Y.S.; Khang, G.; Al-Hajry, A.; Shin, H.S. Effect of nanostructure on the urea sensing properties of sol-gel synthesized ZnO. Sens. Actuators B Chem. 2009, 137, 566–573. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Chen, S.M.; Kwak, C.H.; Hwang, S.K.; Huh, Y.S.; Han, Y.K. Immobilization of hemoglobin on functionalized multi-walled carbon nanotubes-poly-l-histidine-zinc oxide nanocomposites toward the detection of bromate and H2O2. Sens. Actuators B Chem. 2016, 224, 607–617. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhou, X.C.; Wang, K.; Zou, X.B.; Shi, J.Y.; Huang, X.W.; Mei, H. A novel sensor for determination of dopamine in meat based on ZnO-decorated reduced graphene oxide composites. Innov. Food Sci. Emerg. Technol. 2015, 31, 196–203. [Google Scholar] [CrossRef]
- Shang, C.Y.; Li, W.X.; Zhang, R.F. Immobilization of Candida rugosa lipase on ZnO nanowires/macroporous silica composites for biocatalytic synthesis of phytosterol esters. Mater. Res. Bull. 2015, 68, 336–342. [Google Scholar] [CrossRef]
- Costa, V.M.; Souza, M.C.M.D.; Fechine, P.B.A.; Macedo, A.C.; Gonçalves, L.R.B. Nanobiocatalytic systems based on lipase-Fe3O4 and conventional systems for isoniazid synthesis: A comparative study. Braz. J. Chem. Eng. 2016, 33, 661–673. [Google Scholar] [CrossRef]
- Patel, V.; Gajera, H.; Gupta, A.; Manocha, L.; Madamwar, D. Synthesis of ethyl caprylate in organic media using Candida rugosa lipase immobilized on exfoliated graphene oxide: Process parameters and reusability studies. Biochem. Eng. J. 2015, 95, 62–70. [Google Scholar] [CrossRef]
- Dey, R.S.; Raj, C.R. A hybrid functional nanoscaffold based on reduced graphene oxide-ZnO for the development of an amperometric biosensing platform. RSC Adv. 2013, 3, 25858–25864. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pedrero, S.G.; Lopez-Carrobles, N.; Gorines, B.C.; Virgen-Ortiz, J.J.; Fernandez-Lafuente, R. Effect of protein load on stability of immobilized enzymes. Enzym. Microb. Technol. 2017, 98, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisanand, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Chen, Z.M.; Xu, W.H.; Jin, L.; Zha, J.J.; Tao, T.X.; Lin, Y.; Wang, Z.L. Sythesis of amine-functionalized Fe3O4@C nanoparticles for lipase immobilization. J. Mater. Chem. A 2014, 2, 18339–18344. [Google Scholar] [CrossRef]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl-sepabeads) immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19–20, 279–286. [Google Scholar] [CrossRef]
- Peters, G.H.; Olsen, H.O.; Svendsen, A.; Wade, R.C. Theoretical investigation of the dynamics of the active site lid in rhizomucor miehei lipase. Biophys. J. 1996, 71, 119–129. [Google Scholar] [CrossRef]
- Wang, J.Y.; Ma, C.L.; Bao, Y.M.; Xu, P.S. Lipase entrapment in protamine-induced bio-zirconia particles: Characterization and application to the resolution of (R,S)-1-phenylethanol. Enzym. Microb. Technol. 2012, 51, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hermanová, S.; Zarevúcká, M.; Bouša, D.; Pumera, M.; Sofer, Z. Graphene dioxide immobilized enzymes show high thermal and solvent stability. Nanoscale 2015, 7, 5852–5858. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Shi, J.; Deng, Q.; Zheng, M.; Wan, C.; Zheng, C.; Li, Y.; Huang, F. Preparation of Carriers Based on ZnO Nanoparticles Decorated on Graphene Oxide (GO) Nanosheets for Efficient Immobilization of Lipase from Candida rugosa. Molecules 2017, 22, 1205. https://doi.org/10.3390/molecules22071205
Zhang S, Shi J, Deng Q, Zheng M, Wan C, Zheng C, Li Y, Huang F. Preparation of Carriers Based on ZnO Nanoparticles Decorated on Graphene Oxide (GO) Nanosheets for Efficient Immobilization of Lipase from Candida rugosa. Molecules. 2017; 22(7):1205. https://doi.org/10.3390/molecules22071205
Chicago/Turabian StyleZhang, Shan, Jie Shi, Qianchun Deng, Mingming Zheng, Chuyun Wan, Chang Zheng, Ya Li, and Fenghong Huang. 2017. "Preparation of Carriers Based on ZnO Nanoparticles Decorated on Graphene Oxide (GO) Nanosheets for Efficient Immobilization of Lipase from Candida rugosa" Molecules 22, no. 7: 1205. https://doi.org/10.3390/molecules22071205
APA StyleZhang, S., Shi, J., Deng, Q., Zheng, M., Wan, C., Zheng, C., Li, Y., & Huang, F. (2017). Preparation of Carriers Based on ZnO Nanoparticles Decorated on Graphene Oxide (GO) Nanosheets for Efficient Immobilization of Lipase from Candida rugosa. Molecules, 22(7), 1205. https://doi.org/10.3390/molecules22071205