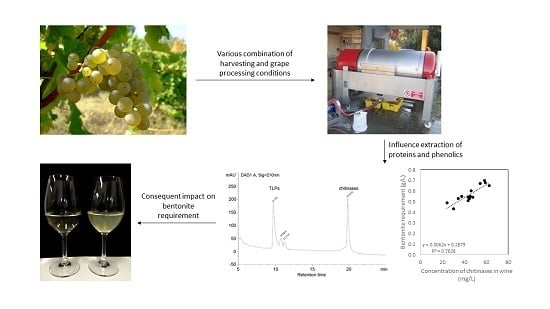
Extraction of Pathogenesis-Related Proteins and Phenolics in Sauvignon Blanc as Affected by Grape Harvesting and Processing Conditions
Abstract
:1. Introduction
2. Results
2.1. Analysis of Total Soluble Solids and Acidity in Grapes Harvested in Three Blocks
2.2. Analysis of Protein and Phenolic Content in Juices from Three Blocks
2.3. Analysis of Juices and Wines from the Separate Pressing Fractions in 2012DP
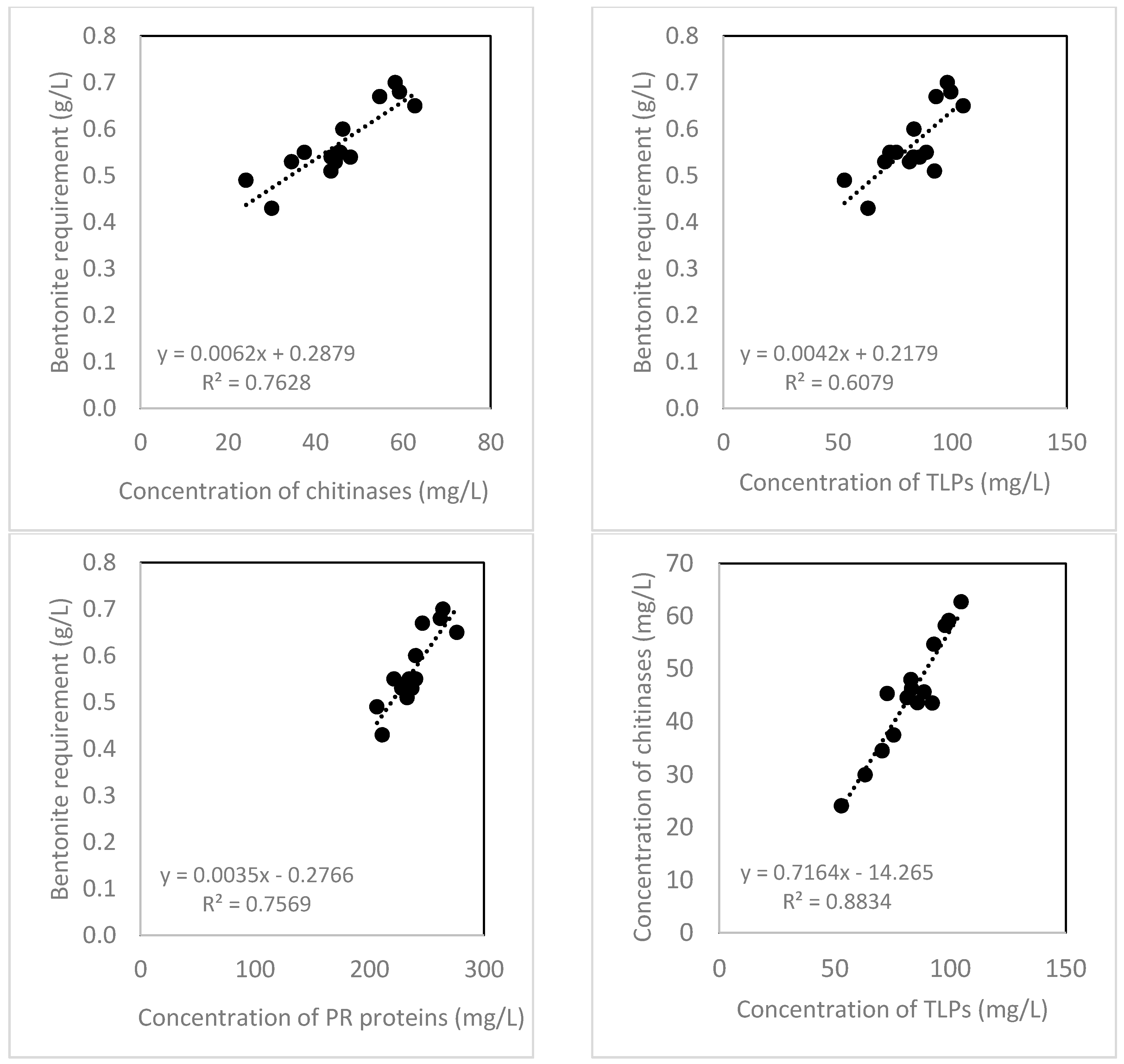
2.4. Bentonite Requirement
3. Discussion
4. Materials and Methods
4.1. Source of Grapes and Corresponding Juice and Wine Samples
4.2. Analysis of Total Soluble Solids and Acidity
4.3. Determination of Total Proteins
4.4. Determination of Total Phenolics
4.5. Pathogenesis-Related Proteins Analysis by RP-HPLC
4.6. Bentonite Requirement
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Sluyter, S.C.; McRae, J.M.; Falconer, R.J.; Smith, P.A.; Bacic, A.; Waters, E.J.; Marangon, M. Wine protein haze: Mechanisms of formation and advances in prevention. J. Agric. Food Chem. 2015, 63, 4020–4030. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.J.; Colby, C.B. Proteins. In Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2009; pp. 213–230. [Google Scholar]
- Monteiro, S.; Barakat, M.; Piçarra-Pereira, M.A.; Teixeira, A.R.; Ferreira, R.B. Osmotin and thaumatin from grape: A putative general defense mechanism against pathogenic fungi. Phytopathology 2003, 93, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Girbau, T.; Stummer, B.E.; Pocock, K.F.; Baldock, G.A.; Scott, E.S.; Waters, E.J. The effect of uncinula necator (powdery mildew) and botrytis cinerea infection of grapes on the levels of haze-forming pathogenesis-related proteins in grape juice and wine. Aust. J. Grape Wine Res. 2004, 10, 125–133. [Google Scholar] [CrossRef]
- Tian, B.; Harrison, R.; Jaspers, M.; Morton, J. Influence of ultraviolet exclusion and of powdery mildew infection on sauvignon blanc grape composition and on extraction of pathogenesis-related proteins into juice. Aust. J. Grape Wine Res. 2015, 21, 417–424. [Google Scholar] [CrossRef]
- Marchal, R.; Berthier, L.; Legendre, L.; Marchal-Delahaut, L.; Jeandet, P.; Maujean, A. Effects of botrytis cinerea infection on the must protein electrophoretic characteristics. J. Agric. Food Chem. 1998, 46, 4945–4949. [Google Scholar] [CrossRef]
- Waters, E.J.; Alexander, G.; Muhlack, R.; Pocock, K.F.; Colby, C.; O’Neill, B.K.; Hoj, P.B.; Jones, P. Preventing protein haze in bottled white wine. Aust. J. Grape Wine Res. 2005, 11, 215–225. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Picarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R. The wine proteins. Trends Food Sci. Technol. 2002, 12, 230–239. [Google Scholar] [CrossRef]
- Tian, B.; Harrison, R.; Morton, J.; Deb-Choudhury, S. Proteomic analysis of sauvignon blanc grape skin, pulp and seed and relative quantification of pathogenesis-related proteins. PLoS ONE 2015, 10, e0130132. [Google Scholar] [CrossRef] [PubMed]
- Deytieux, C.; Geny, L.; Lapaillerie, D.; Claverol, S.; Bonneu, M.; Doneche, B. Proteome analysis of grape skins during ripening. J. Exp. Bot. 2007, 58, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Fukui, M.; Takayanagi, T.; Yokotsuka, K. Characterization of major stable proteins in chardonnay wine. Food Sci. Technol. Res. 2006, 12, 131–136. [Google Scholar] [CrossRef]
- Jellouli, N.; Ben Jouira, H.; Skouri, H.; Ghorbel, A.; Gourgouri, A.; Mliki, A. Proteomic analysis of tunisian grapevine cultivar razegui under salt stress. J. Plant Physiol. 2008, 165, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Pocock, K.F.; Hayasaka, Y.; McCarthy, M.G.; Waters, E.J. Thaumatin-like proteins and chitinases, the haze-forming proteins of wine, accumulate during ripening of grape (vitis vinifera) berries and drought stress does not affect the final levels per berry at maturity. J. Agric. Food Chem. 2000, 48, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.B.; Monteiro, S.S.; Piçarra-Pereira, M.A.; Teixeira, A.R. Engineering grapevine for increased resistance to fungal pathogens without compromising wine stability. TRENDS Biotechnol. 2004, 22, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Linthorst, H.J.M.; Van Loon, L.C. Pathogenesis-related proteins of plants. Crit. Rev. Plant Sci. 1991, 10, 123–150. [Google Scholar] [CrossRef]
- Marangon, M.; van Sluyter, S.C.; Neilson, K.A.; Chan, C.; Haynes, P.A.; Waters, E.J.; Falconer, R.J. Roles of grape thaumatin-like protein and chitinase in white wine haze formation. J. Agric. Food Chem. 2011, 59, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Pocock, K.F.; Alexander, G.M.; Hayasaka, Y.; Jones, P.R.; Waters, E.J. Sulfate-a candidate for the missing essential factor that is required for the formation of protein haze in white wine. J. Agric. Food Chem. 2007, 55, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Esteruelas, M.; Kontoudakis, N.; Gil, M.; Fort, M.F.; Canals, J.M.; Zamora, F. Phenolic compounds present in natural haze protein of sauvignon white wine. Food Res. Int. 2011, 44, 77–83. [Google Scholar] [CrossRef]
- Batista, L.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R.; Ferreira, R.B. Protein haze formation in wines revisited. The stabilising effect of organic acids. Food Chem. 2010, 122, 1067–1075. [Google Scholar] [CrossRef]
- Siebert, K.J. Effects of protein-polyphenol interactions on beverage haze, stabilization, and analysis. J. Agric. Food Chem. 1999, 47, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Siebert, K.J.; Carrasco, A.; Lynn, P.Y. Formation of protein- polyphenol haze in beverages. J. Agric. Food Chem. 1996, 44, 1997–2005. [Google Scholar] [CrossRef]
- Waters, E.J.; Peng, Z.; Pocock, K.F.; Williams, P.J. Proteins in white wine, I: Procyanidin occurrence in soluble proteins and insoluble protein hazes and its relationship to protein instability. Aust. J. Grape Wine Res. 1995, 1, 86–93. [Google Scholar] [CrossRef]
- Gazzola, D.; van sluyter, S.; Curioni, A.; Waters, E.J.; Marangon, M. Roles of proteins, polysaccharides and phenolics on haze formation in white wine via reconstitution experiments. J. Agric. Food Chem. 2012, 60, 10666–10673. [Google Scholar] [CrossRef] [PubMed]
- Marangon, M.; Van Sluyter, S.C.; Waters, E.J.; Menz, R.I. Structure of haze forming proteins in white wines: Vitis vinifera thaumatin-like proteins. PLoS ONE 2014, 9, e113757. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.C.; Ough, C.S.; Kasimatis, A.N. Effect of leaf content and mechanical harvest on wine “quality”. Am. J. Enol. Vitic. 1975, 26, 158–163. [Google Scholar]
- Marais, J. Effect of grape temperature, oxidation and skin contact on sauvignon blanc juice and wine composition and wine quality. S. Afr. J. Enol. Vitic. 1998, 19, 10–16. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; González-Miret, M.L.; Hernanz, D.; Fernández, M.; Vicario, I.M.; Heredia, F.J. Effects of prefermentative skin contact conditions on colour and phenolic content of white wines. J. Food Eng. 2007, 78, 238–245. [Google Scholar] [CrossRef]
- Darias-Martín, J.; Díaz-González, D.; Díaz-Romero, C. Influence of two pressing processes on the quality of must in white wine production. J. Food Eng. 2004, 63, 335–340. [Google Scholar] [CrossRef]
- Pocock, K.F.; Hayasaka, Y.; Peng, Z.; Williams, P.J.; Waters, E.J. The effect of mechanical harvesting and long-distance transport on the concentration of haze-forming proteins in grape juice. Aust. J. Grape Wine Res. 1998, 4, 23–29. [Google Scholar] [CrossRef]
- Pocock, K.F.; Waters, E.J. The effect of mechanical harvesting and transport of grapes, and juice oxidation, on the protein stability of wines. Aust. J. Grape Wine Res. 1998, 4, 136–139. [Google Scholar] [CrossRef]
- Tian, B. Pathogenesis-Related Proteins in Sauvignon Blanc Grapes and the Influence of Their Extraction on Resultant Juice Composition and Wine Protein Stability. Doctoral Thesis, Lincoln University, Lincoln, New Zealand, August 2014. Available online: https://researcharchive.lincoln.ac.nz/handle/10182/6320 (accessed on 27 August 2014).
- Le Bourse, D.; Conreux, A.; Villaume, S.; Lameiras, P.; Nuzillard, J.M.; Jeandet, P. Quantification of chitinase and thaumatin-like proteins in grape juices and wines. Anal. Bioanal. Chem. 2011, 401, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Marangon, M.; Van Sluyter, S.C.; Haynes, P.A.; Waters, E.J. Grape and wine proteins: Their fractionation by hydrophobic interaction chromatography and identification by chromatographic and proteomic analysis. J. Agric. Food Chem. 2009, 57, 4415–4425. [Google Scholar] [CrossRef] [PubMed]
- Manteau, S.; Lambert, B.; Jeandet, P.; Legendre, L. Changes in chitinase and thaumatin-like pathogenesis-related proteins of grape berries during the champagne winemaking process. Am. J. Enol. Vitic. 2003, 54, 267. [Google Scholar]
- Marangon, M.; van Sluyter, S.; Waters, E.J.; Herderich, M.J.; Pretorius, I.S. Recent advances help us understand protein haze more clearly. Aust. N. Z. Wine Ind. J. 2010, 25, 24–27. [Google Scholar]
- Pineau, B.; Trought, M.C.T.; Stronge, K.; Beresford, M.K.; Wohlers, M.W.; Jaeger, S.R. Influence of fruit ripeness and juice chaptalisation on the sensory properties and degree of typicality expressed by sauvignon blanc wines from marlborough, new zealand. Aust. J. Grape Wine Res. 2011, 17, 358–367. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, I1.1.1–I1.1.8. [Google Scholar]
- Tattersall, D.B.; Van Heeswijck, R.; Hoj, P.B. Identification and characterization of a fruit-specific, thaumatin-like protein that accumulates at very high levels in conjunction with the onset of sugar accumulation and berry softening in grapes. Plant Physiol. 1997, 114, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.J.; Wallace, W.; Williams, P.J. Identification of heat-unstable wine proteins and their resistance to peptidases. J. Agric. Food Chem. 1992, 40, 1514–1519. [Google Scholar] [CrossRef]
- Pocock, K.F.; Rankine, B.C. Heat test for detecting protein instability in wine. Aust. Wine Brew. Spirit Rev. 1973, 91, 42–43. [Google Scholar]
- Pocock, K.F.; Waters, E.J. Protein haze in bottled white wines: How well do stability tests and bentonite fining trials predict haze formation during storage and transport? Aust. J. Grape Wine Res. 2006, 12, 212–220. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Process | Grape Weight | Harvesting Method | Grape Processing | Skin Contact |
|---|---|---|---|---|
| H-WB | 300 kg | Hand | Whole bunch press | 0 h |
| H-DC-3 | 300 kg | Hand | Destemmed and crushed | 3 h |
| M-DC-3 | 400 kg | Machine | Destemmed and crushed | 3 h |
| Block | Process | °Brix | pH | TA (g/L) * |
|---|---|---|---|---|
| 2011DP | H-WB | 19.2 ± 0.23 | 2.95 ± 0.02 | 12.2 ± 0.02 |
| H-DC-3 | 19.6 ± 0.25 | 3.06 ± 0.02 | 11.6 ± 0.41 | |
| M-DC-3 | 20.7 ± 0.29 | 3.08 ± 0.01 | 11.1 ± 0.30 | |
| 2012DP | H-WB | 19.6 ± 0.32 | 2.98 ± 0.01 | 14.6 ± 0.24 |
| H-DC-3 | 19.6 ± 0.25 | 3.06 ± 0.02 | 13.1 ± 0.13 | |
| M-DC-3 | 19.9 ± 0.23 | 3.08 ± 0.01 | 13.0 ± 0.05 | |
| 2012BM | H-WB | 21.6 ± 0.23 | 2.94 ± 0.01 | 12.3 ± 0.36 |
| H-DC-3 | 21.4 ± 0.23 | 3.03 ± 0.02 | 11.9 ± 0.17 | |
| M-DC-3 | 21.7 ± 0.32 | 3.07 ± 0.04 | 11.4 ± 0.58 |
| Source of Variation | Juice Yield (%) | Concentration of Each Component in Juice (mg/L) | Extraction of Each Component in Juice (mg/kg of Grapes) 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Proteins * | Phenolics # | TLPs † | Chitinases † | Proteins * | Phenolics # | TLPs † | Chitinases † | ||
| Process | |||||||||
| H-WB | 39.3 a | 131.5 ab | 204.2 a | 106.6 b | 75.9 a | 52.1 a | 78.4 a | 41.8 a | 30.3 a |
| H-DC-3 | 59.0 b | 151.3 b | 246.3 b | 107.3 b | 83.2 b | 88.6 b | 144.6 b | 63.1 b | 48.9 b |
| M-DC-3 | 64.5 c | 120.0 a | 235.5 b | 99.0 a | 76.7 ab | 76.5 b | 151.9 b | 63.7 b | 49.3 b |
| Pressure | |||||||||
| 0–0.4 MPa | 39.4 a | 139.3 a | 239.6 a | 104.3 ab | 76.5 a | 56.4 a | 97.1 a | 41.2 a | 31.0 a |
| 0–0.8 MPa | 57.0 b | 133.5 a | 227.6 a | 107.6 b | 81.2 a | 74.9 b | 131.7 b | 60.5 b | 45.9 b |
| 0–1.6 MPa | 66.3 c | 130.0 a | 218.9 a | 101.0 a | 78.1 a | 85.8 b | 146.1 b | 66.8 b | 51.6 b |
| Process * Pressure | |||||||||
| H-WB 0–0.4 MPa | 15.3 a | 123.5 a | 217.3 a | 102.4 a | 68.7 a | 18.5 a | 32.9 a | 15.4 a | 10.3 a |
| H-WB 0–0.8 MPa | 42.6 b | 139.6 a | 199.0 a | 114.5 a | 81.5 a | 59.3 ab | 84.7 b | 48.6 b | 34.6 b |
| H-WB 0–1.6 MPa | 59.9 d | 131.3 a | 196.4 a | 102.9 a | 77.4 a | 78.4 b | 117.6 bc | 61.5 bc | 46.1 bc |
| H-DC 3 0–0.4 MPa | 49.0 c | 162.2 a | 257.9 a | 109.5 a | 83.7 a | 80.0 b | 126.5 bcd | 53.8 bc | 41.2 bc |
| H-DC 3 0–0.8 MPa | 60.8 d | 148.8 a | 252.1 a | 109.6 a | 85.2 a | 90.3 b | 153.3 cd | 66.6 bc | 51.7 bc |
| H-DC 3–1.6 MPa | 67.1 e | 142.9 a | 228.8 a | 102.7 a | 80.7 a | 95.4 b | 154.1 cd | 68.9 bc | 53.8 bc |
| M-DC 3–0.4 MPa | 54.0 c | 132.1 a | 243.4 a | 100.9 a | 77.1 a | 70.9 b | 131.8 cd | 54.4 bc | 41.5 bc |
| M-DC 3 0–0.8 MPa | 67.6 e | 112.2 a | 231.7 a | 98.8 a | 76.8 a | 75.3 b | 157.1 cd | 66.4 bc | 51.5 bc |
| M-DC 3 0–1.6 MPa | 71.9 e | 115.8 a | 231.5 a | 97.2 a | 76.1 a | 83.5 b | 166.6 d | 70.1 c | 54.9 c |
| Source of Variation | Concentration of Each Component in Juice (mg/L) | Concentration of Each Component in Wine (mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| Proteins * | Phenolics # | TLPs † | Chitinases † | Proteins * | Phenolics # | TLPs † | Chitinases † | |
| Process | ||||||||
| H-WB | 173.0 a | 234.5 a | 140.9 a | 102.7 a | 99.4 a | 206.5 a | 89.9 a | 50.5 a |
| H-DC-3 | 180.0 a | 278.3 b | 133.9 a | 103.6 a | 94.9 a | 232.1 a | 80.8 a | 44.2 a |
| M-DC-3 | 101.0 a | 277.0 b | 123.1 a | 102.0 a | 79.4 a | 238.1 a | 70.7 a | 34.7 a |
| Pressure | ||||||||
| 0–0.4 MPa | 170.0 a | 257.1 a | 137.6 ab | 99.2 a | 97.9 a | 220.9 a | 87.5 ab | 44.6 a |
| 0.4–0.8 MPa | 173.0 a | 249.0 a | 144.3 b | 112.0 a | 103.3 a | 214.6 a | 91.1 b | 51.8 a |
| 0.8–1.6 MPa | 111.0 a | 283.6 b | 116.0 a | 97.0 a | 72.5 a | 241.1 a | 63.0 a | 33.1 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, B.; Harrison, R.; Morton, J.; Jaspers, M.; Hodge, S.; Grose, C.; Trought, M. Extraction of Pathogenesis-Related Proteins and Phenolics in Sauvignon Blanc as Affected by Grape Harvesting and Processing Conditions. Molecules 2017, 22, 1164. https://doi.org/10.3390/molecules22071164
Tian B, Harrison R, Morton J, Jaspers M, Hodge S, Grose C, Trought M. Extraction of Pathogenesis-Related Proteins and Phenolics in Sauvignon Blanc as Affected by Grape Harvesting and Processing Conditions. Molecules. 2017; 22(7):1164. https://doi.org/10.3390/molecules22071164
Chicago/Turabian StyleTian, Bin, Roland Harrison, James Morton, Marlene Jaspers, Simon Hodge, Claire Grose, and Mike Trought. 2017. "Extraction of Pathogenesis-Related Proteins and Phenolics in Sauvignon Blanc as Affected by Grape Harvesting and Processing Conditions" Molecules 22, no. 7: 1164. https://doi.org/10.3390/molecules22071164
APA StyleTian, B., Harrison, R., Morton, J., Jaspers, M., Hodge, S., Grose, C., & Trought, M. (2017). Extraction of Pathogenesis-Related Proteins and Phenolics in Sauvignon Blanc as Affected by Grape Harvesting and Processing Conditions. Molecules, 22(7), 1164. https://doi.org/10.3390/molecules22071164