Current Study of the Mechanism of Action of the Potential Anti-Epileptic Agent Q808
Abstract
:1. Introduction
2. Results
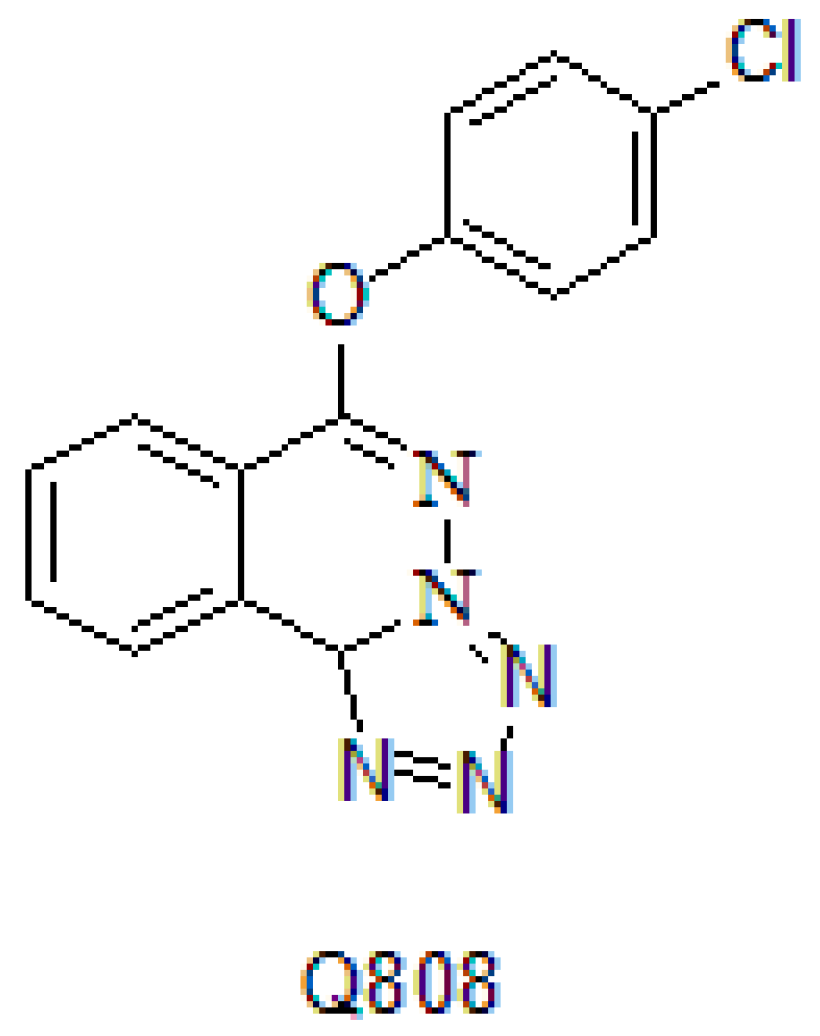
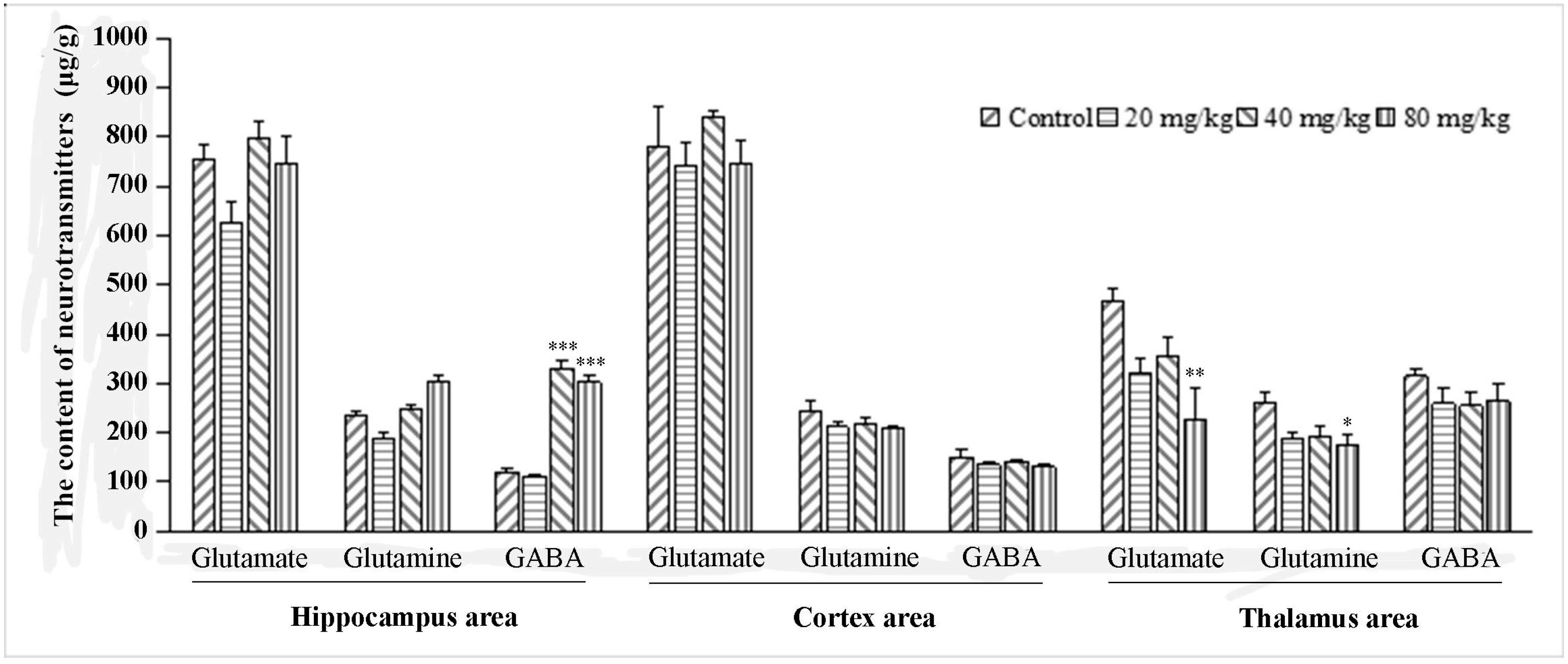
2.1. The Effect of Q808 on Neurotransmitters in the Brain
2.2. The Effect of Q808 on Electrophysiology in a Seizure Model
2.2.1. The Influence of Q808 on Bicuculline-Induced Epileptiform Bursts
2.2.2. The Influence of Q808 on High-K+-Induced Epileptiform Activity
2.2.3. The Influence of Q808 on Epileptiform Activity Induced by Mg2+ Depletion
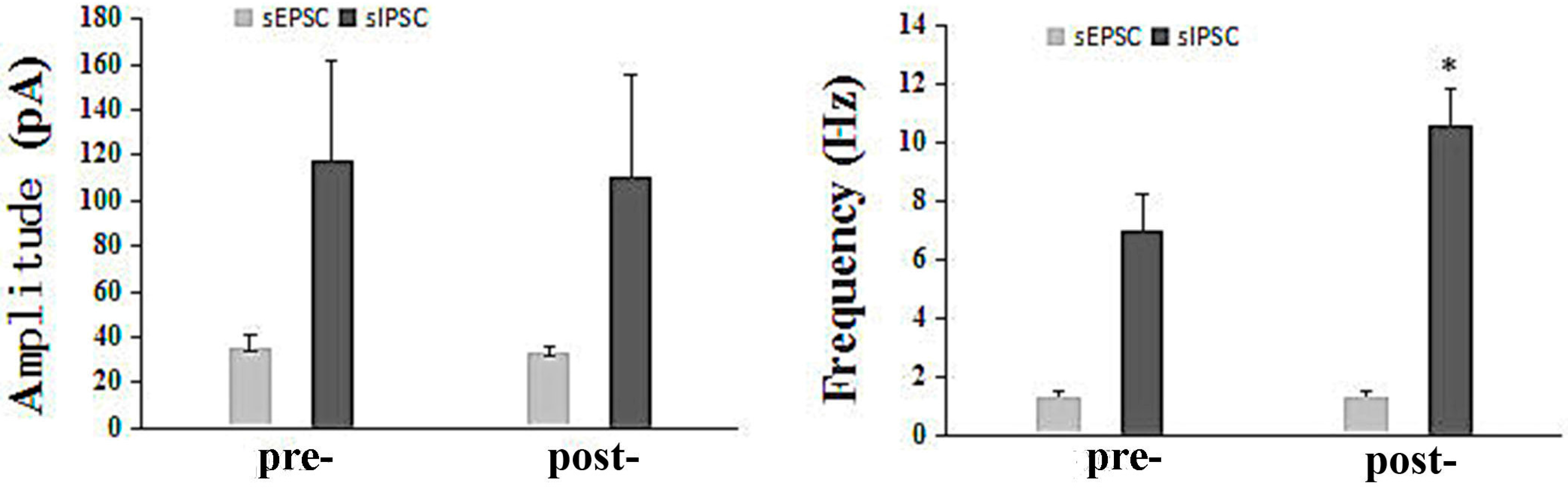
2.2.4. The Influence of Q808 on sEPSCs and sIPSCs in the CA1 Region
2.3. The effect of Q808 on GABA Receptor-Mediated Current Characteristics
3. Discussion
4. Materials and Methods
4.1. Animals and Drugs
4.2. The Maximal Electroshock Mouse Model of Epilepsy
4.3. Measurement of Neurotransmitter Content of the Brain
4.4. Electrophysiological Model
4.5. Evaluation of the Effect of Q808 on GABA Receptor-Mediated Current Characteristics
4.6. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Strine, T.W.; Kobau, R.; Chapman, D.P.; Thurman, D.J.; Price, P.; Balluz, L.S. Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: Results from the 2002 National Health Interview Survey. Epilepsia 2005, 46, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Ionov, I.D. Self-reinforcing loop mechanism in epilepsy. Med. Hypotheses 2009, 73, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Current status and future directions in the pharmacotherapy of epilepsy. Trends Pharmacol. Sci. 2002, 23, 113–118. [Google Scholar] [CrossRef]
- Dzhala, V.I.; Talos, D.M.; Sdrulla, D.A.; Brumback, A.C.; Mathews, G.C.; Benke, T.A.; Delpire, E.; Jensen, F.E.; Staley, K.J. NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 2005, 11, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Rheims, S.; Minlebaev, M.; Ivanov, A.; Represa, A.; Khazipov, R.; Holmes, G.L.; Ben-Ari, Y.; Zilberter, Y. Excitatory GABA in rodent developing neocortex in vitro. J. Neurophysiol. 2008, 100, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Khalilov, I.; Kahle, K.T.; Cherubini, E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 2012, 18, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Puskarjov, M.; Kaila, K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology 2013, 69, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.A.; Hill, A.J.; Smith, I.; Bevan, S.A.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J. Pharmacol. Exp. Ther. 2010, 332, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.B.; Wood, P.L.; Wieland, S.; Hawkinson, J.E.; Belelli, D.; Lambert, J.J.; White, H.S.; Wolf, H.H.; Mirsadeghi, S.; Tahir, S.H.; et al. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. J. Pharmacol. Exp. Ther. 1997, 280, 1284–1295. [Google Scholar] [PubMed]
- Tang, X.C.; Han, Y.F. Pharmacological profile of huperzine A, a novel acetylcholinesterase inhibitor from Chinese herb. CNS Drug Rev. 2009, 5, 281–300. [Google Scholar] [CrossRef]
- Sun, X.Y.; Wei, C.X.; Deng, X.Q.; Sun, Z.G.; Quan, Z.S. Evaluation of the anticonvulsant activity of 6-(4-chlorophenyoxy)-tetrazolo[5,1-a]phthalazine in various experimental seizure models in mice. Pharmacol. Rep. 2010, 62, 273–277. [Google Scholar] [CrossRef]
- Okada, R.; Negishi, N.; Nagaya, H. The role of the nigrotegmental GABAergic pathway in the propagation of pentylenetetrazol-induced seizures. Brain Res. 1989, 480, 383–387. [Google Scholar] [CrossRef]
- Olsen, RW. GABA-benzodiazepine-barbiturate receptor interactions. J. Neurochem. 1981, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. 3-Mercaptopropionic acid: Convulsant properties, effects on enzymes of the gamma-aminobutyrate system in mouse brain and antagonism by certain anticonvulsant drugs, aminooxyacetic acid and gabaculine. Biochem. Pharmacol. 1979, 28, 1397–1407. [Google Scholar] [CrossRef]
- Sun, H.L.; Zhu, W.; Zhang, Y.R.; Pan, X.H.; Zhang, J.R.; Chen, X.M.; Liu, Y.X.; Li, S.C.; Wang, Q.Y.; Deng, D.P. Altered glutamate metabolism contributes to antiepileptogenic effects in the progression from focal seizure to generalized seizure by low-frequency stimulation in the ventral hippocampus. Epilepsy Behav. 2017, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kanamori, K. Disinhibition reduces extracellular glutamine and elevates extracellular glutamate in rat hippocampus in vivo. Epilepsy Res. 2015, 114, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Van der Hel, W.S.; Hessel, E.V.; Bos, I.W.; Mulder, S.D.; Verlinde, S.A.; van Eijsden, P.; de Graan, P.N. Persistent reduction of hippocampal glutamine synthetase expression after status epilepticus in immature rats. Eur. J. Neurosci. 2014, 40, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- DiNuzzo, M.; Mangia, S.; Maraviglia, B.; Giove, F. Physiological bases of the K+ and the glutamate/GABA hypotheses of epilepsy. Epilepsy Res. 2014, 108, 995–1012. [Google Scholar] [CrossRef] [PubMed]
- Galzigna, L.; Garbin, L.; Bianchi, M.; Marzotto, A. Properties of two derivatives of gamma-aminobutyric acid (GABA) capable of abolishing Cardiazol− and bicuculline-induced convulsions in the rat. Arch. Int. Pharmacodyn. Ther. 1978, 235, 73–85. [Google Scholar] [PubMed]
- Lu, H.C.; Chang, W.J.; Chang, W.P.; Shyu, B.C. Direct-current Stimulation and Multi-electrode Array Recording of Seizure-like Activity in Mice Brain Slice Preparation. J. Vis. Exp. 2016, 112, e53709. [Google Scholar] [CrossRef] [PubMed]
- Yechikhov, S.; Shchipakina, T.; Savina, T.; Kalemenev, S.; Levin, S.; Godukhin, O. The role of Ca2+/calmodulin-dependent protein kinase II in mechanisms underlying neuronal hyperexcitability induced by repeated, brief exposure to hypoxia or high K+ in rat hippocampal slices. Neurosci. Lett. 2002, 335, 21–24. [Google Scholar] [CrossRef]
- Liu, J.S.; Li, J.B.; Gong, X.W.; Gong, H.Q.; Zhang, P.M.; Liang, P.J.; Lu, Q.C. Spatiotemporal dynamics of high-K+-induced epileptiform discharges in hippocampal slice and the effects of valproate. Neurosci. Bull. 2013, 29, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Noriega, L.E.; Stevens, C.F. Increased transmitter release at excitatory synapses produced by direct activation of adenylate cyclase in rat hippocampal slices. J. Neurosci. 1994, 14, 310–317. [Google Scholar] [PubMed]
- Vignes, M. Regulation of spontaneous inhibitory synaptic transmission by endogenous glutamate via non-NMDA receptors in cultured rat hippocampal neurons. Neuropharmacology 2001, 40, 737–748. [Google Scholar] [CrossRef]
- Tao, W.; Higgs, M.H.; Spain, W.J.; Ransom, C.B. Postsynaptic GABAB receptors enhance extrasynaptic GABAA receptor function in dentate gyrus granule cells. J. Neurosci. 2013, 33, 3738–3743. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.L.; Yang, L.X.; Wu, X.H.; Li, Q.; Ding, M.P.; Fan, Y.Y.; Zhang, W.P.; Luo, J.H.; Chen, Z. Effects of carnosine on amygdaloid-kindled seizures in Sprague-Dawley rats. Neuroscience 2005, 135, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.; Nguyen, P.V.; Baker, G.B. Phenylethylidenehydrazine, a novel GABA-transaminase inhibitor, reduces epileptiform activity in rat hippocampal slices. Neuroscience 2004, 126, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Semyanov, A.; Godukhin, O. Epileptiform activity and EPSP-spike potentiation induced in rat hippocampal CA1 slices by repeated high-K(+): Involvement of ionotropic glutamate receptors and Ca(2+)/calmodulin-dependent protein kinase II. Neuropharmacology 2001, 40, 203–211. [Google Scholar] [CrossRef]
- Bikson, M.; Id Bihi, R.; Vreugdenhil, M.; Kohling, R.; Fox, J.E.; Jefferys, J.G. Quinine suppresses extracellular potassium transients and ictal epileptiform activity without decreasing neuronal excitability in vitro. Neuroscience 2002, 115, 251–261. [Google Scholar] [CrossRef]
- Jones, R.S. Ictal epileptiform events induced by removal of extracellular magnesium in slices of entorhinal cortex are blocked by baclofen. Exp. Neurol. 1989, 104, 155–161. [Google Scholar] [CrossRef]
- Sharopov, S.; Moser, J.; Chen, R.; Kolbaev, S.N.; Bernedo, V.E.; Werhahn, K.J.; Luhmann, H.J.; Kilb, W. Dopaminergic modulation of low-Mg(2)(+)-induced epileptiform activity in the intact hippocampus of the newborn mouse in vitro. J. Neurosci. Res. 2012, 90, 2020–2033. [Google Scholar] [CrossRef] [PubMed]
- Huettner, J.E.; Baughman, R.W. Primary culture of identified neurons from the visual cortex of postnatal rats. J. Neurosci. 1986, 6, 3044–3060. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Groups | Control | Phenytoin | Q808 | |||
|---|---|---|---|---|---|---|
| Concentration | - (n = 5) a | 10−4 M (n = 6) | 10−6 M (n = 8) | 10−5 M (n = 10) | 10−4 M (n = 9) | |
| Frequency (Hz) | pre- b | 4.247 ± 1.063 | 1.424 ± 0.539 | 1.646 ± 0.243 | 3.400 ± 0.875 | 2.169 ± 0.754 |
| post- b | 4.194 ± 1.437 | 0.008 ± 0.008 * | 0.385 ± 0.116 ** | 0.337 ± 0.148 ** | 0.947 ± 0.291 * | |
| Amplitude (mV) | pre- b | 27.09 ± 0.833 | 25.614 c | 34.070 ± 5.288 | 34.310 ± 3.937 | 44.140 ± 5.232 |
| post- b | 29.85 ± 4.161 | 28.514 c | 33.980 ± 5.431 | 22.590 ± 7.328 | 33.000 ± 5.169 | |
| Frequency rate of decrement | 0.088 ± 0.089 | 0.987 ± 0.013 ### | 0.696 ± 0.111 ### | 0.829 ± 0.0729 ## | 0.590 ± 0.071 ### | |
| Groups | Control | Phenytoin | Q808 | |||
|---|---|---|---|---|---|---|
| Concentration | - (n = 5) | 10−4 M (n = 7) | 10−6 M (n = 9) | 10−5 M (n = 9) | 10−4 M (n = 10) | |
| Frequency (Hz) | pre- | 2.663 ± 0.943 | 0.579 ± 0.155 | 0.821 ± 0.190 | 1.462 ± 0.377 | 0.794 ± 0.169 |
| post- | 1.800 ± 0.416 | 0.05 ± 0.024 ** | 0.336 ± 0.148 ** | 0.139 ± 0.031 ** | 0.270 ± 0.110 | |
| Amplitude (mV) | pre- | 34.84 ± 1.290 | 57.60 ± 11.260 | 55.370 ± 6.842 | 58.980 ± 6.259 | 51.060 ± 6.335 |
| post- | 34.61 ± 1.741 | 67.400 ± 18.00 | 53.46 ± 5.747 | 52.14 ± 6.728 | 49.71 ± 11.39 | |
| Frequency rate of decrement | 0.183 ± 0.127 | 0.921 ± 0.030 | 0.665 ± 0.096 | 0.817 ± 0.078 | 0.283 ± 0.345 | |
| Groups | Control | Phenytoin | Q808 | |||
|---|---|---|---|---|---|---|
| Concentration | - (n = 6) | 10−4 M (n = 9) | 10−6 M (n = 8) | 10−5 M (n = 8) | 10−4 M (n = 9) | |
| Frequency (Hz) | pre- | 1.583 ± 0.391 | 1.003 ± 0.229 | 1.233 ± 0.409 | 1.726 ± 0.398 | 1.810 ± 0.465 |
| post- | 1.527 ± 0.519 | 0.372 ± 0.241 ** | 0.441 ± 0.180 | 0.6703 ± 0.257 * | 0.576 ± 0.241 * | |
| Amplitude (mV) | pre- | 35.34 ± 0.960 | 37.850 ± 0.167 | 43.530 ± 5.220 | 37.730 ± 3.874 | 34.110 ± 3.490 |
| post- | 40.53 ± 0.060 | 32.810 ± 1.510 | 34.380 ± 9.550 | 35.750 ± 2.062 | 31.750 ± 3.360 | |
| Frequency rate of decrement | 0.060 ± 0.145 | 0.183 ± 0.127 | 0.513 ± 0.122 # | 0.659 ± 0.106 # | 0.802 ± 0.112 ## | |
| Groups | Control | Q808 |
|---|---|---|
| Amplitude (pA) | 227.3 ± 62.00 | 219.5 ± 52.55 |
| The time to half-decay (↑ a, ms) | 661.0 ± 47.91 | 575.4 ± 38.86 |
| The time to half-decay (↓ b, ms) | 11220 ± 2767 | 10360 ± 1882 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, H.-J.; Wang, Q.; Zhang, D.-W.; Wu, D.; Li, W.; Quan, Z.-S. Current Study of the Mechanism of Action of the Potential Anti-Epileptic Agent Q808. Molecules 2017, 22, 1134. https://doi.org/10.3390/molecules22071134
Li X, Zhang H-J, Wang Q, Zhang D-W, Wu D, Li W, Quan Z-S. Current Study of the Mechanism of Action of the Potential Anti-Epileptic Agent Q808. Molecules. 2017; 22(7):1134. https://doi.org/10.3390/molecules22071134
Chicago/Turabian StyleLi, Xiang, Hong-Jian Zhang, Qing Wang, Dian-Wen Zhang, Di Wu, Wei Li, and Zhe-Shan Quan. 2017. "Current Study of the Mechanism of Action of the Potential Anti-Epileptic Agent Q808" Molecules 22, no. 7: 1134. https://doi.org/10.3390/molecules22071134




