Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review
Abstract
:1. Introduction
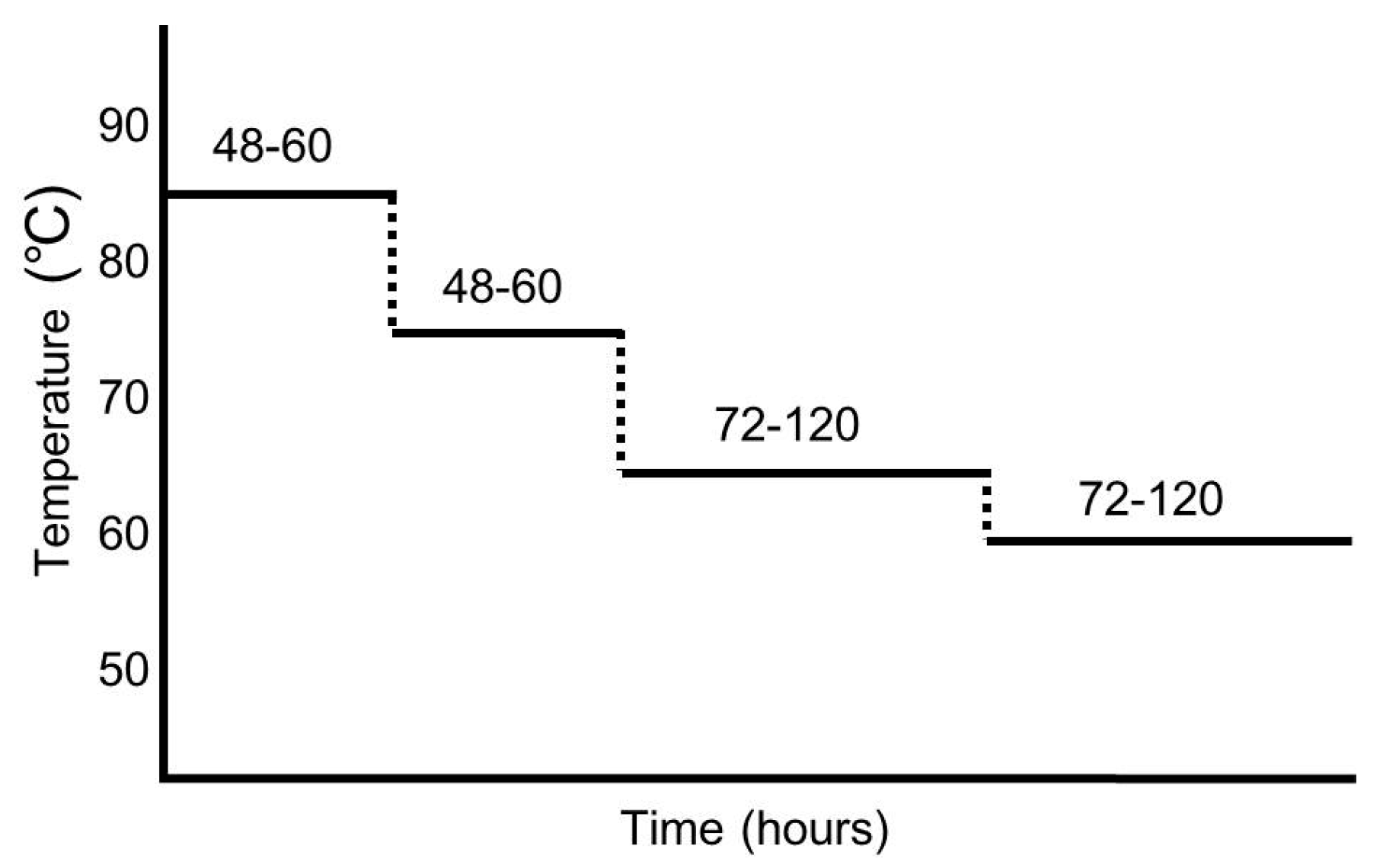
2. Preparation of ABG
Manufacturing Protocol
3. Properties of ABG
3.1. Physicochemical Properties
3.2. Antioxidant Activity, Compounds, and Therapeutic Effects
3.3. Anti-Inflammatory Activity, Compounds and Therapeutic Effects
3.4. Other Biological Effects
4. Adverse Effects and General Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Block, E. The chemistry of garlic and oniono. Sci. Am. 1985, 251, 114–119. [Google Scholar] [CrossRef]
- Augusti, K.; Mathew, P. Lipid lowering effect of allicin (diallyl disulphide-oxide) on long term feeding to normal rats. Cell. Mol. Life Sci. 1974, 30, 468–470. [Google Scholar] [CrossRef]
- Rahman, M.S. Allicin and other functional active components in garlic: Health benefits and bioavailability. Int. J. Food Prop. 2007, 10, 245–268. [Google Scholar] [CrossRef]
- Milner, J. Garlic (Allium sativum). In Encyclopedia of Dietary Supplements; Marcel Dekker: New York, NY, USA, 2005; pp. 229–240. [Google Scholar]
- Banerjee, S.; Mukherjee, P.K.; Maulik, S. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res. 2003, 17, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [PubMed]
- Tanamai, J.; Veeramanomai, S.; Indrakosas, N. The efficacy of cholesterol-lowering action and side effects of garlic enteric coated tablets in man. J. Med. Assoc. Thail. 2004, 87, 1156–1161. [Google Scholar]
- Lee, Y.-M.; Gweon, O.-C.; Seo, Y.-J.; Im, J.; Kang, M.-J.; Kim, M.-J.; Kim, J.-I. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr. Res. Pract. 2009, 3, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.Y.; Ryu, J.H.; Shin, J.-H.; Kang, M.J.; Kang, J.R.; Han, J.; Kang, D. Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules 2016, 21, 430. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Medina, M.A.; Pérez-Aparicio, J.; Moreno-Rojas, R.; Merinas-Amo, T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chem. 2016, 199, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Feng, Y.; Yan, J.; Wang, M.; Sasaki, J.; Lu, C. Black garlic (Allium sativum) extracts enhance the immune system. Med. Aromat. Plant Sci. Biotechnol. 2010, 4, 37–40. [Google Scholar]
- Lee, E.N.; Choi, Y.W.; Kim, H.K.; Park, J.K.; Kim, H.J.; Kim, M.J.; Lee, H.W.; Kim, K.H.; Bae, S.S.; Kim, B.S. Chloroform extract of aged black garlic attenuates tnf-α-induced ros generation, vcam-1 expression, nf-κb activation and adhesiveness for monocytes in human umbilical vein endothelial cells. Phytother. Res. 2011, 25, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.-J. Evaluation of melanoidins formed from black garlic after different thermal processing steps. Prev. Nutr. Food Sci. 2016, 21, 398. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.-I.; Lu, C.; Machiya, E.; Tanahashi, M.; Hamada, K. Processed black garlic (Allium sativum) extracts enhance anti-tumor potency against mouse tumors. Med. Aromat. Plant Sci. Biotechnol. 2007, 1, 278–281. [Google Scholar]
- Bae, S.E.; Cho, S.Y.; Won, Y.D.; Lee, S.H.; Park, H.J. A comparative study of the different analytical methods for analysis of s-allyl cysteine in black garlic by hplc. LWT-Food Sci. Technol. 2012, 46, 532–535. [Google Scholar] [CrossRef]
- Jang, E.-K.; Seo, J.-H.; Lee, S.-P. Physiological activity and antioxidative effects of aged black garlic (Allium sativum L.) extract. Korean J. Food Sci. Technol. 2008, 40, 443–448. [Google Scholar]
- Lee, H.-S.; Yang, S.-T.; Ryu, B.-H. Effects of aged black garlic extract on lipid improvement in rats fed with high fat-cholesterol diet. J. Life Sci. 2011, 21, 884–892. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.W.; Lee, E.N.; Park, J.K.; Kim, S.G.; Park, D.J.; Kim, B.S.; Lim, Y.T.; Yoon, S. 5-hydroxymethylfurfural from black garlic extract prevents tnfα-induced monocytic cell adhesion to huvecs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and nf-κb activation. Phytother. Res. 2011, 25, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Kang, M.J.; Hong, S.S.; Choi, Y.H.; Shin, J.H. Antiinflammatory effects of functionally active compounds isolated from aged black garlic. Phytother. Res. 2017, 31, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Park, J.K.; Choi, Y.-W.; Kim, Y.-H.; Lee, E.N.; Lee, J.-R.; Kim, H.-S.; Baek, S.-Y.; Kim, B.-S.; Lee, K.-S. Hexane extract of aged black garlic reduces cell proliferation and attenuates the expression of icam-1 and vcam-1 in tnf-α-activated human endometrial stromal cells. Int. J. Mol. Med. 2013, 32, 67–78. [Google Scholar] [PubMed]
- Seo, Y.-J.; Gweon, O.-C.; Lee, Y.; Kang, M.; Kim, J. Effect of garlic and aged black garlic on hyperglycemia and dyslipidemia in animal model of type 2 diabetes mellitus. J. Food Sci. Nutr. 2009, 14, 1–7. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [PubMed]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2000, 11, 340–346. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R. Antioxidant activity of water-soluble maillard reaction products. Food Chem. 2005, 93, 273–278. [Google Scholar] [CrossRef]
- Kang, O.-J. Physicochemical characteristics of black garlic after different thermal processing steps. Prev. Nutr. Food Sci. 2016, 21, 348. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Lee, C.W.; Oh, S.J.; Yun, J.; Kang, M.R.; Han, S.-B.; Park, H.; Jung, J.C.; Chung, Y.H.; Kang, J.S. Hepatoprotective effect of aged black garlic extract in rodents. Toxicol. Res. 2014, 30, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Lee, S.; Shin, J.; Sung, N. Antioxidant and inhibition of nitrosodimethylamine formation in marketing black garlics. J. Agric. Life Sci. 2012, 46, 151–162. [Google Scholar]
- Imai, J.; Ide, N.; Nagae, S.; Moriguchi, T.; Matsuura, H.; Itakura, Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994, 60, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Shin, J.-H.; Kang, M.-J.; Jung, W.-J.; Ryu, J.-H.; Kim, R.-J.; Sung, N.-J. Antioxidants activity of aged red garlic. J. Life Sci. 2010, 20, 775–781. [Google Scholar]
- Shin, J.-H.; Choi, D.-J.; Lee, S.-J.; Cha, J.-Y.; Sung, N.-J. Antioxidant activity of black garlic (Allium sativum L.). J. Korean Soc. Food Sci. Nutr. 2008, 37, 965–971. [Google Scholar] [CrossRef]
- Choi, D.-J.; Lee, S.-J.; Kang, M.-J.; Cho, H.-S.; Sung, N.-J.; Shin, J.-H. Physicochemical characteristics of black garlic (Allium sativum L.). J. Korean Soc. Food Sci. Nutr. 2008, 37, 465–471. [Google Scholar] [CrossRef]
- Borek, C. Antioxidant health effects of aged garlic extract. J. Nutr. 2001, 131, 1010S–1015S. [Google Scholar] [PubMed]
- Kimura, S.; Tung, Y.-C.; Pan, M.-H.; Su, N.-W.; Lai, Y.-J.; Cheng, K.-C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2016, 11, 3. [Google Scholar] [CrossRef]
- Bae, S.E.; Cho, S.Y.; Won, Y.D.; Lee, S.H.; Park, H.J. Changes in s-allyl cysteine contents and physicochemical properties of black garlic during heat treatment. LWT-Food Sci. Technol. 2014, 55, 397–402. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2015. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Choi, D.-J.; Lee, S.-J.; Cha, J.-Y.; Kim, J.-G.; Sung, N.-J. Changes of physicochemical components and antioxidant activity of garlic during its processing. J. Life Sci. 2008, 18, 1123–1131. [Google Scholar] [CrossRef]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and antioxidant properties of black garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Sohn, H. Antioxidation, antimicrobial and antithrombosis activities of aged black garlic (Allium sativum L.). Korean J. Microbiol. Biotechnol. 2014. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, S.H.; Rico, C.W.; Kang, M.Y. A comparative study on the antioxidative and anti-allergic activities of fresh and aged black garlic extracts. Int. J. Food Sci. Technol. 2012, 47, 1176–1182. [Google Scholar] [CrossRef]
- Shin, J.-H.; Lee, H.-G.; Kang, M.-J.; Lee, S.-J.; Sung, N.-J. Antioxidant activity of solvent fraction from black garlic. J. Korean Soc. Food Sci. Nutr. 2010, 39, 933–940. [Google Scholar] [CrossRef]
- Sato, E.; Kohno, M.; Hamano, H.; Niwano, Y. Increased anti-oxidative potency of garlic by spontaneous short-term fermentation. Plant Foods Hum. Nutr. 2006, 61, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecień, I.; Włodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Yoshida, J.; Ide, N.; Sasaoka, T.; Yamaguchi, H.; Ono, K. Tetrahydro-β-carboline derivatives in aged garlic extract show antioxidant properties. J. Nutr. 2006, 136, 726S–731S. [Google Scholar] [PubMed]
- Sato, E.; Kohno, M.; Niwano, Y. Increased level of tetrahydro-β-carboline derivatives in short-term fermented garlic. Plant Foods Hum. Nutr. 2006, 61, 175–178. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Gillies, D. Product of the maillard reaction in aged garlic extract are antioxidants. In Proceedings of the Nutritional and Health Benefits of Garlic as a Supplement Conference, New Beach, CA, USA, 15–17 November 1998; p. 66. [Google Scholar]
- Ryu, K.; Ide, N.; Matsuura, H.; Itakura, Y. Nα-(1-deoxy-d-fructos-1-yl)-l-arginine, an antioxidant compound identified in aged garlic extract. J. Nutr. 2001, 131, 972S–976S. [Google Scholar] [PubMed]
- Das, U.N. Pyruvate is an endogenous anti-inflammatory and anti-oxidant molecule. Med. Sci. Monit. 2006, 12, RA79–RA84. [Google Scholar] [PubMed]
- Gupta, D.; Du, Y.; Piluek, J.; Jakub, A.M.; Buela, K.A.; Abbott, A.; Schuman, J.S.; SundarRaj, N. Ethyl pyruvate ameliorates endotoxin-induced corneal inflammationanti-inflammatory effect of ep in mouse cornea. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6589–6599. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, A.M.; Puupponen-Pimiä, R.; Aarni, M.; Oksman-Caldentey, K.-M. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003, 81, 485–493. [Google Scholar] [CrossRef]
- Kim, S.H.; Jung, E.Y.; Kang, D.H.; Chang, U.J.; Hong, Y.-H.; Suh, H.J. Physical stability, antioxidative properties, and photoprotective effects of a functionalized formulation containing black garlic extract. J. Photochem. Photobiol. B Biol. 2012, 117, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, M.J.; Lee, J.H.; Han, J.I.; Kim, J.H.; Sok, D.-E.; Kim, M.R. Hepatoprotective effect of aged black garlic on chronic alcohol-induced liver injury in rats. J. Med. Food 2011, 14, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T. Effects of aged black garlic extract on ethanol induced hangover in rats. J. Life Sci. 2010, 20, 225–230. [Google Scholar] [CrossRef]
- Ha, A.W.; Ying, T.; Kim, W.K. The effects of black garlic (Allium satvium) extracts on lipid metabolism in rats fed a high fat diet. Nutr. Res. Pract. 2015, 9, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-J.; Lee, S.-J.; Shin, J.-H.; Kang, S.-K.; Kim, J.-G.; Sung, N.-J. Effect of garlic with different processing on lipid metabolism in 1% cholesterol fed rats. J. Korean Soc. Food Sci. Nutr. 2008, 37, 162–169. [Google Scholar] [CrossRef]
- Kang, M.-J.; Shin, J.-H. The effect of black garlic extract on lipid metabolism in restraint stressed rats. J. Life Sci. 2012, 22, 1529–1537. [Google Scholar] [CrossRef]
- Kim, M.J.; Yoo, Y.C.; Kim, H.J.; Shin, S.K.; Sohn, E.J.; Min, A.Y.; Sung, N.Y.; Kim, M.R. Aged black garlic exerts anti-inflammatory effects by decreasing no and proinflammatory cytokine production with less cytoxicity in lps-stimulated raw 264.7 macrophages and lps-induced septicemia mice. J. Med. Food 2014, 17, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Oh-Cheon Gweon, Y.H.C.; Kim, J.-I. Aged black garlic inhibits cyclooxygenase-2 expression and prostaglandin e2 production by phorbol 12-myristate-13-acetate through inactivation of nuclear factor-kappab. Cancer Prev. Res. 2009, 14, 161–170. [Google Scholar]
- Tak, H.-M.; Kang, M.-J.; Kim, K.M.; Kang, D.; Han, S.; Shin, J.-H. Anti-inflammatory activities of fermented black garlic. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1527–1534. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, F.; Wang, Q.-W.; Wang, J.; Yang, K.; Hu, R.-R.; Liu, H.-C.; Wang, H.-Y.; Wang, Y.-S. Aged black garlic extract induces inhibition of gastric cancer cell growth in vitro and in vivo. Mol. Med. Rep. 2012, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.-Y.; Yoon, M.-K.; Choi, Y.-W.; Gweon, O.-C.; Kim, J.-I.; Choi, T.-H.; Choi, Y.-H. Effects of aged black garlic extracts on the tight junction permeability and cell invasion in human gastric cancer cells. J. Life Sci. 2010, 20, 528–534. [Google Scholar] [CrossRef]
- Park, C.; Park, S.; Chung, Y.H.; Kim, G.-Y.; Choi, Y.W.; Kim, B.W.; Choi, Y.H. Induction of apoptosis by a hexane extract of aged black garlic in the human leukemic u937 cells. Nutr. Res. Pract. 2014, 8, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Yang, G.; Liu, H.; Liu, X.; Lin, S.; Sun, D.; Wang, Y. Aged black garlic extract inhibits ht29 colon cancer cell growth via the pi3k/akt signaling pathway. Biomed. Rep. 2014, 2, 250–254. [Google Scholar] [PubMed]
- Purev, U.; Chung, M.J.; Oh, D.-H. Individual differences on immunostimulatory activity of raw and black garlic extract in human primary immune cells. Immunopharmacol. Immunotoxicol. 2012, 34, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.-M.; Lee, S.-H.; Lee, D.-S.; You, M.-J.; Chung, I.K.; Cheon, W.H.; Kwon, Y.-S.; Lee, Y.-J.; Ku, S.-K. Fermented garlic protects diabetic, obese mice when fed a high-fat diet by antioxidant effects. Nutr. Res. 2011, 31, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yu, S.H.; Cho, Y.J.; Pan, J.H.; Cho, H.T.; Kim, J.H.; Bong, H.; Lee, Y.; Chang, M.H.; Jeong, Y.J. Preparation of s-allyl cysteine-enriched black garlic juice and its antidiabetic effects in streptozotocin-induced insulin-deficient mice. J. Agric. Food Chem. 2017, 65, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-J.; Lee, S.J.; Sung, N.J.; Shin, J.-H. The effect of extract powder from fresh and black garlic on main components in serum and organs of streptozotocin-induced diabetic rats. J. Life Sci. 2013, 23, 432–442. [Google Scholar] [CrossRef]
- Jung, E.-S.; Park, S.-H.; Choi, E.-K.; Ryu, B.-H.; Park, B.-H.; Kim, D.-S.; Kim, Y.-G.; Chae, S.-W. Reduction of blood lipid parameters by a 12-wk supplementation of aged black garlic: A randomized controlled trial. Nutrition 2014, 30, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, C.; Han, M.-H.; Kim, B.-W.; Chung, Y.-H.; Choi, Y.-H. Inhibition of adipocyte differentiation and adipogenesis by aged black garlic extracts in 3t3-l1 preadipocytes. J. Life Sci. 2011, 21, 720–728. [Google Scholar] [CrossRef]
- Yoo, J.-M.; Sok, D.-E.; Kim, M.R. Anti-allergic action of aged black garlic extract in rbl-2h3 cells and passive cutaneous anaphylaxis reaction in mice. J. Med. Food 2014, 17, 92–102. [Google Scholar] [CrossRef] [PubMed]
- García-Villalón, A.; Amor, S.; Monge, L.; Fernández, N.; Prodanov, M.; Muñoz, M.; Inarejos-García, A.; Granado, M. In vitro studies of an aged black garlic extract enriched in S-allylcysteine and polyphenols with cardioprotective effects. J. Funct. Foods 2016, 27, 189–200. [Google Scholar] [CrossRef]
- Aminuddin, M.; Partadiredja, G.; Sari, D. The effects of black garlic (Allium sativum L.) ethanol extract on the estimated total number of purkinje cells and motor coordination of male adolescent wistar rats treated with monosodium glutamate. Anat. Sci. Int. 2015, 90, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hermawati, E.; Sari, D.C.R.; Partadiredja, G. The effects of black garlic ethanol extract on the spatial memory and estimated total number of pyramidal cells of the hippocampus of monosodium glutamate-exposed adolescent male wistar rats. Anat. Sci. Int. 2015, 90, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T. Antioxidative activity of extracts of aged black garlic on oxidation of human low density lipoprotein. J. Life Sci. 2007, 17, 1330–1335. [Google Scholar] [CrossRef]
- Baek, Y.-H.; Lee, S.-H.; Han, M.-H.; Choi, Y.-H.; Kim, S.-H.; Kwak, Y.-S. Effects of black garlic supplementation and exercise on tbars, hsp 70 and cox-2 expression after high-intensity exercise. J. Life Sci. 2012, 22, 772–777. [Google Scholar] [CrossRef]
- Kim, S.-H.; Baek, Y.-H. Effects of aerobic exercise and black garlic intake on blood lipids, lipid peroxidation and bap in rats. J. Life Sci. 2011, 21, 1025–1031. [Google Scholar] [CrossRef]
- Li, M.; Yan, Y.X.; Yu, Q.T.; Deng, Y.; Wu, D.T.; Wang, Y.; Ge, Y.Z.; Li, S.P.; Zhao, J. Comparison of immunomodulatory effects of fresh garlic and black garlic polysaccharides on raw 264.7 macrophages. J. Food Sci. 2017, 82, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The pungency of garlic: Activation of trpa1 and trpv1 in response to allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Högestätt, E.D.; Julius, D.; Jordt, S.-E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel trpa1. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Saito, J.; Misa, K.; Fukuhara, N.; Fukuhara, A.; Munakata, M. A case of black garlic-induced pneumonia as an adverse reaction. Allergol. Int. 2016, 65, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.K.; Maulik, S.K. Effect of garlic on cardiovascular disorders: A review. Nutr. J. 2002, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Travica, N.; Sali, A. The effect of aged garlic extract on blood pressure and other cardiovascular risk factors in uncontrolled hypertensives: The age at heart trial. Integr. Blood Press. Control 2016, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Harauma, A.; Moriguchi, T. Aged garlic extract improves blood pressure in spontaneously hypertensive rats more safely than raw garlic. J. Nutr. 2006, 136, 769S–773S. [Google Scholar] [PubMed]
- Pérez-Torres, I.; Torres-Narváez, J.C.; Pedraza-Chaverri, J.; Rubio-Ruiz, M.E.; Díaz-Díaz, E.; del Valle-Mondragón, L.; Martínez-Memije, R.; Varela López, E.; Guarner-Lans, V. Effect of the aged garlic extract on cardiovascular function in metabolic syndrome rats. Molecules 2016, 21, 1425. [Google Scholar] [CrossRef] [PubMed]
- Numagami, Y.; Sato, S.; Ohnishi, S.T. Attenuation of rat ischemic brain damage by aged garlic extracts: A possible protecting mechanism as antioxidants. Neurochem. Int. 1996, 29, 135–143. [Google Scholar] [CrossRef]
- Colín-González, A.L.; Ortiz-Plata, A.; Villeda-Hernández, J.; Barrera, D.; Molina-Jijón, E.; Pedraza-Chaverrí, J.; Maldonado, P.D. Aged garlic extract attenuates cerebral damage and cyclooxygenase-2 induction after ischemia and reperfusion in rats. Plant Foods Hum. Nutr. 2011, 66, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, P.; Chánez-Cárdenas, M.; Ortiz-Plata, A.; León-Aparicio, D.; Barrera, D.; Espinoza-Rojo, M.; Villeda-Hernández, J.; Sánchez-García, A.; Maldonado, P. Aged garlic extract delays the appearance of infarct area in a cerebral ischemia model, an effect likely conditioned by the cellular antioxidant systems. Phytomedicine 2010, 17, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Avula, P.R.; Asdaq, S.M.; Asad, M. Effect of aged garlic extract and s-allyl cysteine and their interaction with atenolol during isoproterenol induced myocardial toxicity in rats. Indian J. Pharmacol. 2014, 46, 94. [Google Scholar] [PubMed]
- Takashima, M.; Kanamori, Y.; Kodera, Y.; Morihara, N.; Tamura, K. Aged garlic extract exerts endothelium-dependent vasorelaxant effect on rat aorta by increasing nitric oxide production. Phytomedicine 2017, 24, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Waterer, D.; Schmitz, D. Influence of variety and cultural practices on garlic yields in saskatchewan. Can. J. Plant Sci. 1994, 74, 611–614. [Google Scholar] [CrossRef]
- Chen, S.; Shen, X.; Cheng, S.; Li, P.; Du, J.; Chang, Y.; Meng, H. Evaluation of garlic cultivars for polyphenolic content and antioxidant properties. PLoS ONE 2013, 8, e79730. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-B.; Kim, R.-J.; Lee, S.-J.; Kang, M.-J.; Seo, J.-K.; Sung, N.-J. Aroma compounds and antimicrobial effect of garlic from different areas in korea. Korean J. Food Preserv. 2011, 18, 199–207. [Google Scholar] [CrossRef]
- Shin, J.-H.; Ju, J.-C.; Kwen, O.; Yang, S.-M.; Lee, S.-J.; Sung, N.-J. Physicochemical and physiological activities of garlic from different area. Korean J. Food Nutr. 2004, 17, 237–245. [Google Scholar]

| Components | Content in ABG | Content in FRG | Change in ABG Compared to FRG | Analytical Methods | References | Remarks |
|---|---|---|---|---|---|---|
| Moisture (%) | 58.2 ± 0.4 | 66.6 ± 1.3 | ↓ | AOAC 925.10 | [31] | |
| 45.3 | 66.1 | ↓ | [34] | |||
| 29.9 ± 0.5 | 64.2 ± 1.5 | ↓ | [37] | |||
| 43.1 ± 0.4 | 62.3 ± 0.6 | ↓ | AOAC 991.02 | [25] | ||
| 45.1 | 60.3 | ↓ | - | [14] | ||
| Protein (%) | 1.0 ± 0.1 | 0.7 | ↑ | AOAC 984.13 | [31] | |
| 9.1 | 8.4 | ↑ | - | [14] | ||
| Lipid (%) | 0.6 ± 0.1 | 0.2 | ↑ | AOAC 920.39 | [31] | |
| 0.3 | 0.1 | ↑ | - | [14] | ||
| Carbohydrate (%) | 47 | 28.7 | ↑ | - | [14] | |
| Ash (%) | 1.8 ± 0.1 | 0.9 ± 0.6 | - | AOAC 942.05 | [31] | |
| 0.11 | 0.07 | ↑ | [25] | |||
| 2.1 | - | ↑ | - | [14] | no detection in FRG | |
| Total sugar (%) | 6.2 | 4.5 ± 0.1 | ↑ | colorimetric method using PSA | [31] | |
| 49.2 ± 0.2 | 14.6 ± 1.3 | ↑ | [38] | |||
| Reducing sugar (%) | 1.6 | 0.2 | ↑ | colorimetric method using DNS | [37] | |
| 28.2 ± 1.2 | 4.2 ± 0.1 | ↑ | [38] | |||
| pH | 4.4 ± 0.1 | 6.8 | ↓ | by pH meter | [31] | |
| 3.7 ± 0.1 | 6.3 ± 0.1 | ↓ | [37] | |||
| 3.1 | 6.4 | ↓ | [34] | |||
| 4.2 ± 0.1 | 6.3 ± 0.1 | ↓ | [25] | |||
| Color Brightness | 22.5 ± 0.2 | 78.8 ± 0.1 | ↓ | Spectrocolori-meter color comparison | [31] | |
| 4.3 ± 2 | 68.4 ± 1.7 | ↓ | [37] | |||
| Redness | 2.9 ± 0.7 | -3.2 | ↑ | [31] | ||
| 2.7 ± 1.0 | −3.8 ± 0.5 | ↑ | [37] | |||
| Yellowness | 3.2 ± 0.7 | 21.6 ± 1.4 | ↓ | [31] | ||
| −3.9 ± 1.5 | 26.6 ± 1.8 | ↓ | [37] | |||
| Energy (kcal/100 g) | 227.1 | 138 | ↑ | - | [14] |
| Components | Content in ABG | Content in FRG | Change in ABG Compared to FRG | Analytical Methods | Basis for Comparison | References |
|---|---|---|---|---|---|---|
| Allicin (mg/100 g) | - | 362 ± 1 | ↓ | colorimetric method | allicin | [9] |
| 20 | 345 | ↓ | HPLC | [35] | ||
| Flavonoid (mg/100 g) | 0.8 | 0.1 | ↑ | colorimetric method | quercetin | [31] |
| 1570 ± 211 | 322 ± 7 | ↑ | rutin | [37] | ||
| 195 ± 8 | 125 ± 13 | ↑ | rutin | [38] | ||
| Pyruvate (mmol/100 g) | 27.8 ± 0.3 | 18.8 ± 0.3 | ↑ | Colorimetric method | [31] | |
| 245.7 ± 2.4 | 48.7 ± 1.2 | ↑ | [9] | |||
| Thiosulfate (mmol/100 g) | 9.12 ± 0.05 | 0.65 ± 0.03 | ↑ | colorimetric method | [9] | |
| 0.3 | 10.5 ± 0.4 | ↓ | [25] | |||
| 0.8 | 0.1 | ↑ | OD value | [31] | ||
| Total phenol (mg/100 g) | 1.6 ± 0.1 | 0.6 ± 0.1 | ↑ | Colorimetric method | caffeic acid | [31] |
| 4835 ± 114 | 1391 ± 162 | ↑ | garlic acid | [37] | ||
| 1000 ± 100 | 367 ± 22 | ↑ | garlic acid | [16] | ||
| 22.3 ± 0.8 | 3.7 ± 0.2 | ↑ | garlic acid | [39] | ||
| 1023 ± 19 | 255 ± 12 | ↑ | tannic acid | [38] | ||
| SAC (mg/100 g) | 8.5 ± 0.1 | 2 | ↑ | HPLC-FLD | [34] | |
| 19.4 | 2.4 | ↑ | HPLC | [14] | ||
| 9.8 ± 0.2 | 2.2 | ↑ | HPLC-FLD | [15] | ||
| 11.4 ± 0.9 | 2.3 | ↑ | HPLC | [15] |
| Components | Content in ABG | Content in FRG | Change in ABG Compared to FRG | References | Remarks | |
|---|---|---|---|---|---|---|
| Free sugars (mg/100 g) | Arabinose | 1.6 ± 0.3 | - | ↑ | [31] | no detection in FRG |
| 114.5 ± 15.6 | 51.1 ± 6 | ↑ | [25] | |||
| Galactose | 13.1 ± 1.7 | - | ↑ | [31] | no detection in FRG | |
| Glucose | 181.7 ± 8.8 | 91.6 ± 2.7 | ↑ | [31] | ||
| 210 ± 5 | - | ↑ | [9] | no detection in FRG | ||
| 221.9 ± 11.5 | 16.7 ± 0.7 | ↑ | [25] | |||
| Fructose | 2043.7 ± 5 | 63.9 ± 3.4 | ↑ | [31] | ||
| 4002 ± 71 | 707 ± 8 | ↑ | [9] | |||
| 3383.2 ± 44.0 | 31.4 ± 1.0 | ↑ | [25] | |||
| Sucrose | 119.1 ± 3.5 | 76.3 ± 0.1 | ↑ | [31] | ||
| 242.9 ± 18.1 | 181.7 ± 1.6 | ↑ | [25] | |||
| Maltose | 7.8 ± 0.4 | 1.7 | ↑ | [31] | ||
| 48.2 ± 4.4 | 11.7 ± 0.8 | ↑ | [25] | |||
| Minerals (mg/100 g) | Al | 25.4 ± 0.8 | 0.5 | ↑ | [31] | |
| Ca | 13.1 ± 0.4 | 7.5 | ↑ | [31] | ||
| 15.2 ± 0.4 | 12.2 ± 0.3 | ↑ | [25] | |||
| Cu | 2 ± 0.1 | 1.7 | ↑ | [31] | ||
| 0.27 | 0.32 | ↑ | [25] | |||
| Fe | 4.4 ± 0.2 | 3.3 ± 0.2 | ↑ | [31] | ||
| 2.3 ± 0.5 | 1.6 | ↑ | [25] | |||
| K | 738.2 ± 11.2 | 434.9 ± 4.2 | ↑ | [31] | ||
| 1018.4 ± 23.2 | 907.4 ± 20.6 | ↑ | [25] | |||
| Mg | 27.2 ± 0.8 | 15.7 ± 0.1 | ↑ | [31] | ||
| 48.7 ± 1.1 | 43.2 ± 1.0 | ↑ | [25] | |||
| Mn | 0.7 | 0.5 | ↑ | [31] | ||
| 0.5 | 0.4 | ↑ | [25] | |||
| Na | 13.0 ± 0.1 | 9.1 | ↑ | [31] | ||
| 35.5 ± 0.8 | 22.0 ± 0.5 | ↑ | [25] | |||
| Zn | 1.6 ± 0.1 | 1.5 | - | [31] | ||
| 1.3 | 1.2 | ↑ | [25] | |||
| P | 143.7 ± 5.5 | 93.3 ± 0.3 | ↑ | [31] | ||
| 2.6 ± 0.1 | 2.2 ± 0.1 | ↑ | [25] | |||
| Se | 0.13 | 0.05 | ↑ | [25] | ||
| S | 189.7 ± 4.3 | 183.1 ± 4.2 | - | [25] | ||
| Biological Activities | Measurement | Content in ABG | Content in FRG | Change in ABG Compared to FRG | Basis for Comparison | References |
|---|---|---|---|---|---|---|
| Antioxidant activity | DPPH radical scavenging activity | 0.1 mg/mL | 0.4 mg/mL | ↑ | IC50 | [9] |
| 4.1 mg/mL | 114.9 mg/mL | ↑ | IC50 (Japanese garlic) | [14] | ||
| 7.3 mg/mL | 88.5 mg/mL | ↑ | IC50 (Chinese garlic) | [14] | ||
| 97% | 10.4% | ↑ | 900 μg/mL | [16] | ||
| 67.4 ± 0.2% | 35.7 ± 0.6% | ↑ | 1 mg/mL | [36] | ||
| 1.3 mg/mL | 0.7 mg/mL | ↓ | IC50 | [38] | ||
| 82.5 ± 0.5% | 35.1 ± 0.7% | ↑ | 2 mg/mL | [39] | ||
| ABTS radical scavenging activity | 0.2 mg/mL | 0.3 mg/mL | ↑ | IC50 | [9] | |
| 1 mg/mL | 1.1 mg/mL | ↑ | IC50 | [38] | ||
| Hydroxy radical scavenging activity | 75 ± 0.7% | 60.7 ± 0.2% | ↑ | 2 mg/mL | [39] | |
| Nitrite radical scavenging activity | 32.9% | 55.2% | ↓ | 5 mg/mL | [16] | |
| 0.1 mg/mL | 0.2 mg/mL | ↑ | IC50 | [38] | ||
| Fe2+-chelating activity | 18.2 ± 0.7% | 30.6 ± 1.4% | ↓ | 2 mg/mL | [39] | |
| Reducing power | 2.6 | 0.4 | ↑ | OD | [39] | |
| SOD activity | 64.4% | 16.8% | ↑ | 100 mg/mL | [16] | |
| Anti-inflammatory activity | Inhibition of COX-2 activity | 39.1 ± 3.8% | 80.5 ± 7.8% | ↓ | 250 μg/mL | [9] |
| Inhibition of 5-LO activity | 29.5 ± 2.1% | 97.4 ± 9.5% | ↓ | 250 μg/mL | [9] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.H.; Kang, D. Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules 2017, 22, 919. https://doi.org/10.3390/molecules22060919
Ryu JH, Kang D. Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules. 2017; 22(6):919. https://doi.org/10.3390/molecules22060919
Chicago/Turabian StyleRyu, Ji Hyeon, and Dawon Kang. 2017. "Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review" Molecules 22, no. 6: 919. https://doi.org/10.3390/molecules22060919
APA StyleRyu, J. H., & Kang, D. (2017). Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules, 22(6), 919. https://doi.org/10.3390/molecules22060919







