Synthesis, Characterization and Protonation Behavior of Quinoxaline-Fused Porphycenes
Abstract
:1. Introduction
2. Results and Discussion
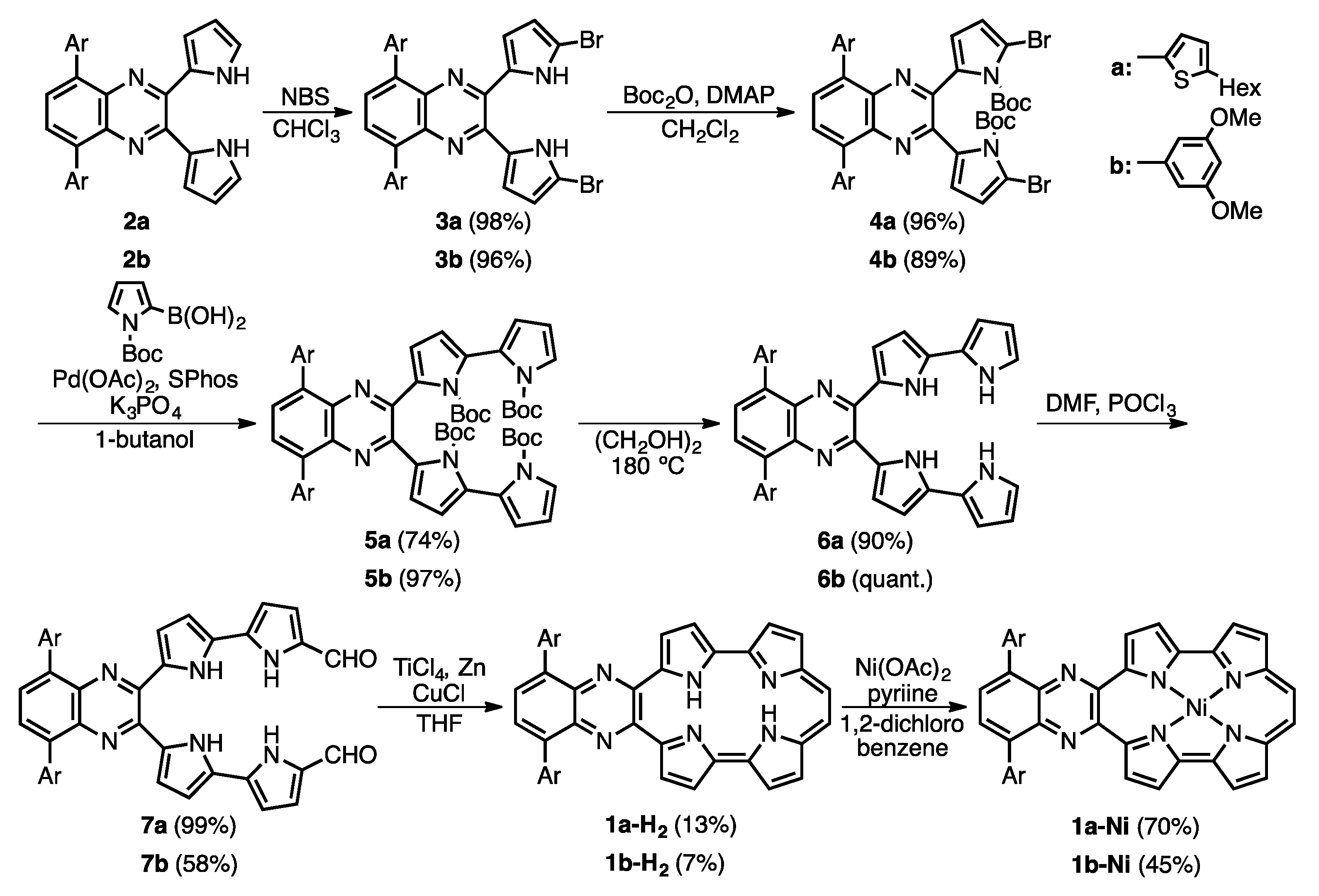
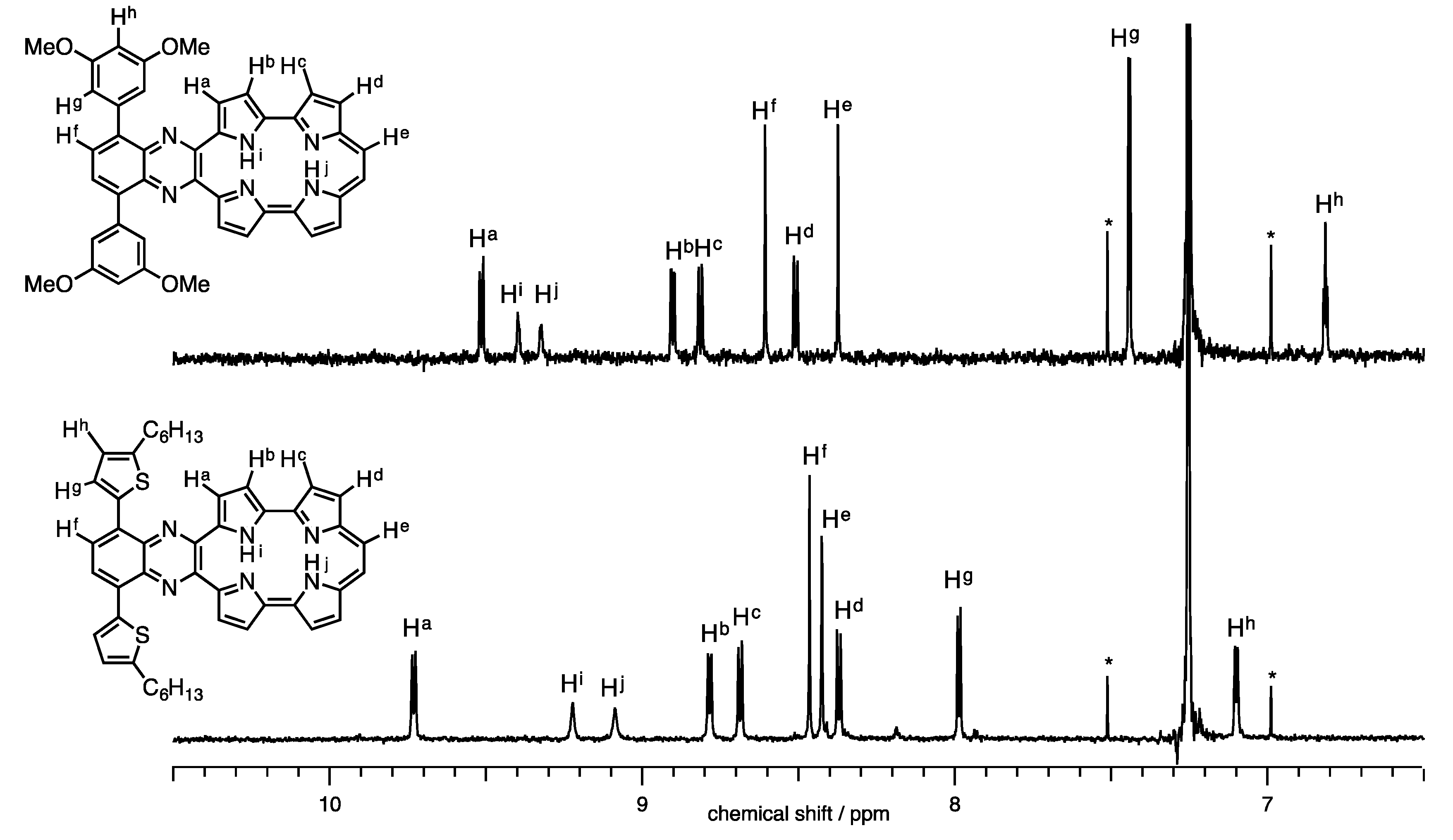
2.1. Synthesis and Characterization of Quinoxaline-Fused Porphycenes and Their Nickel Complexes
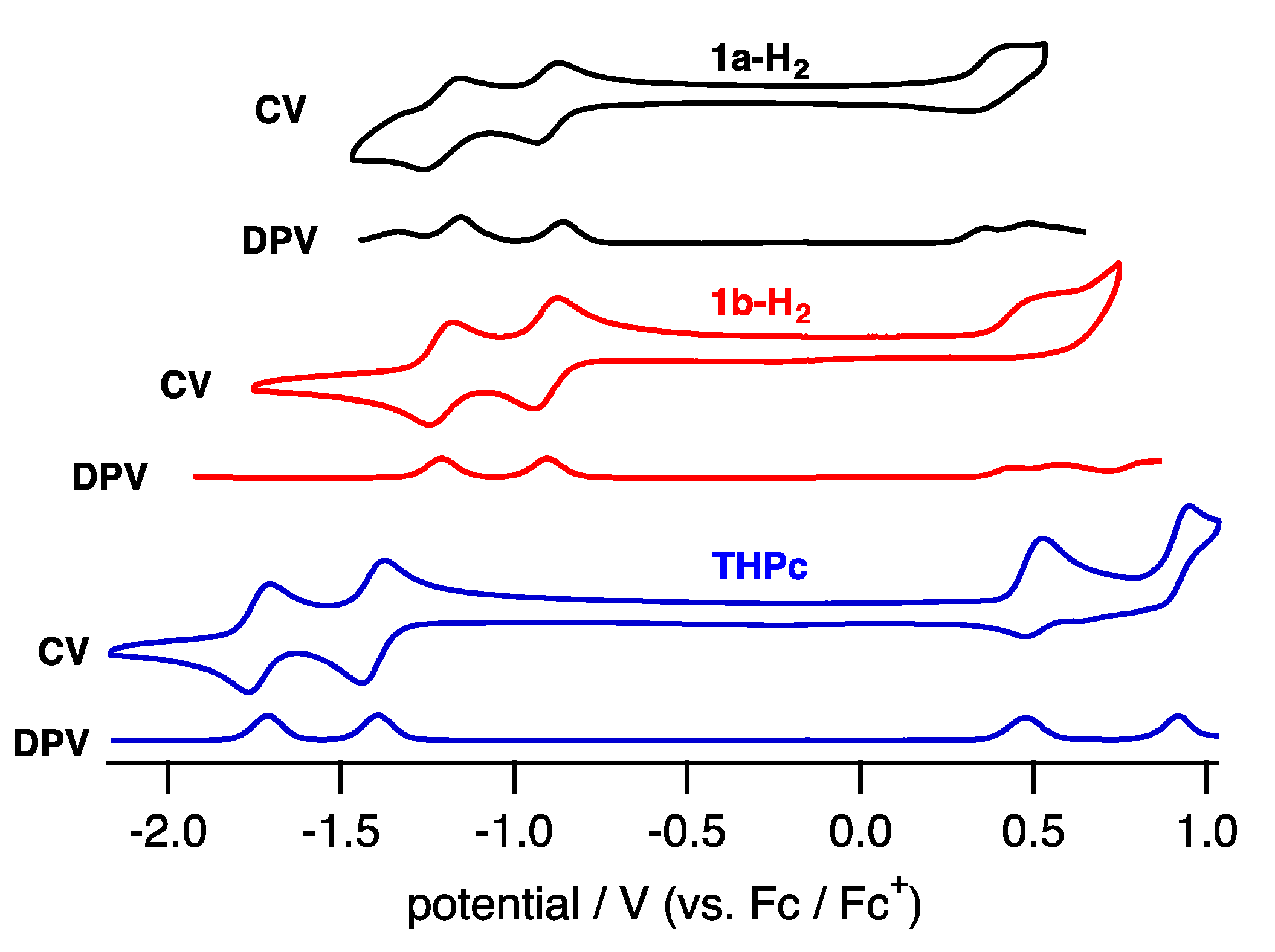
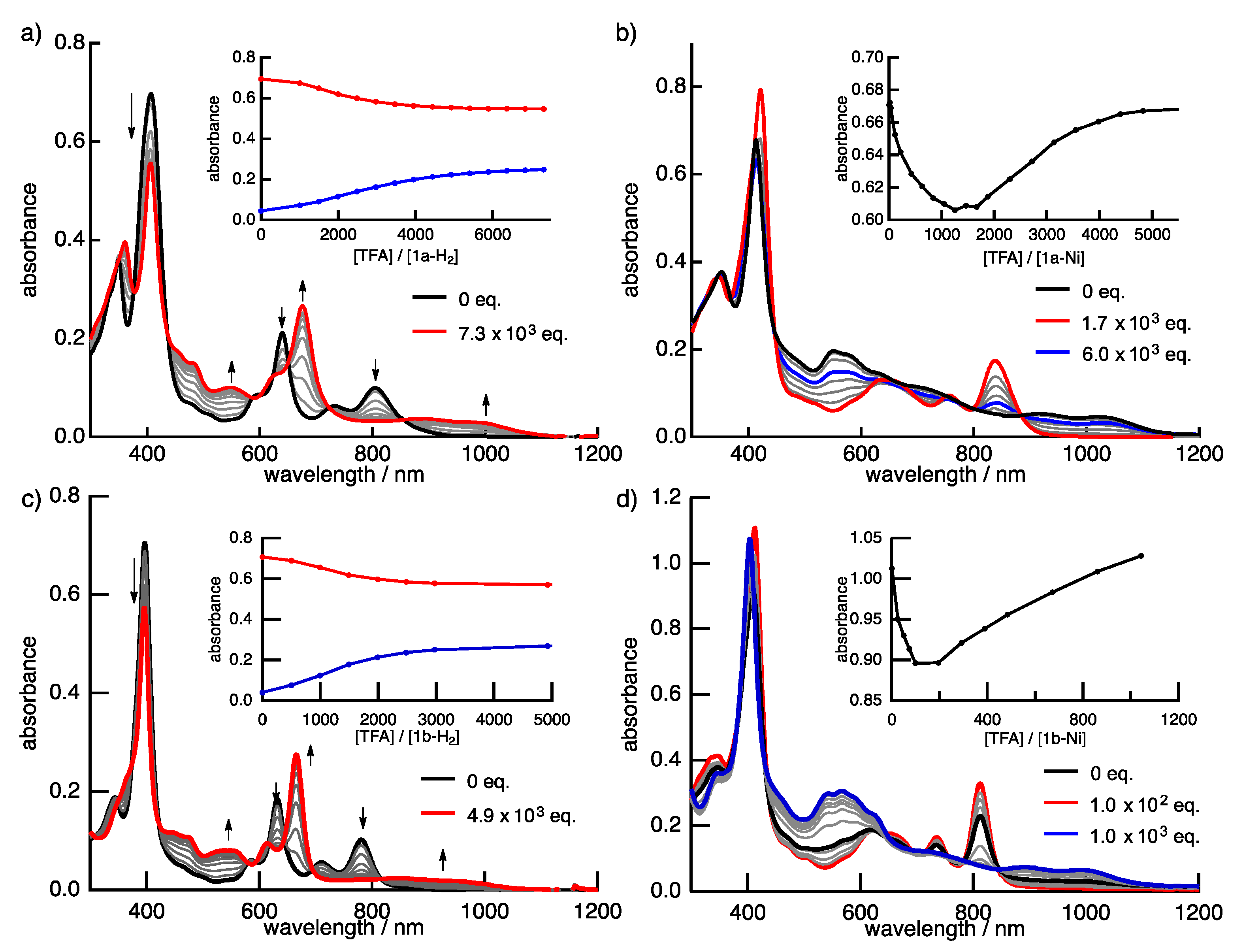
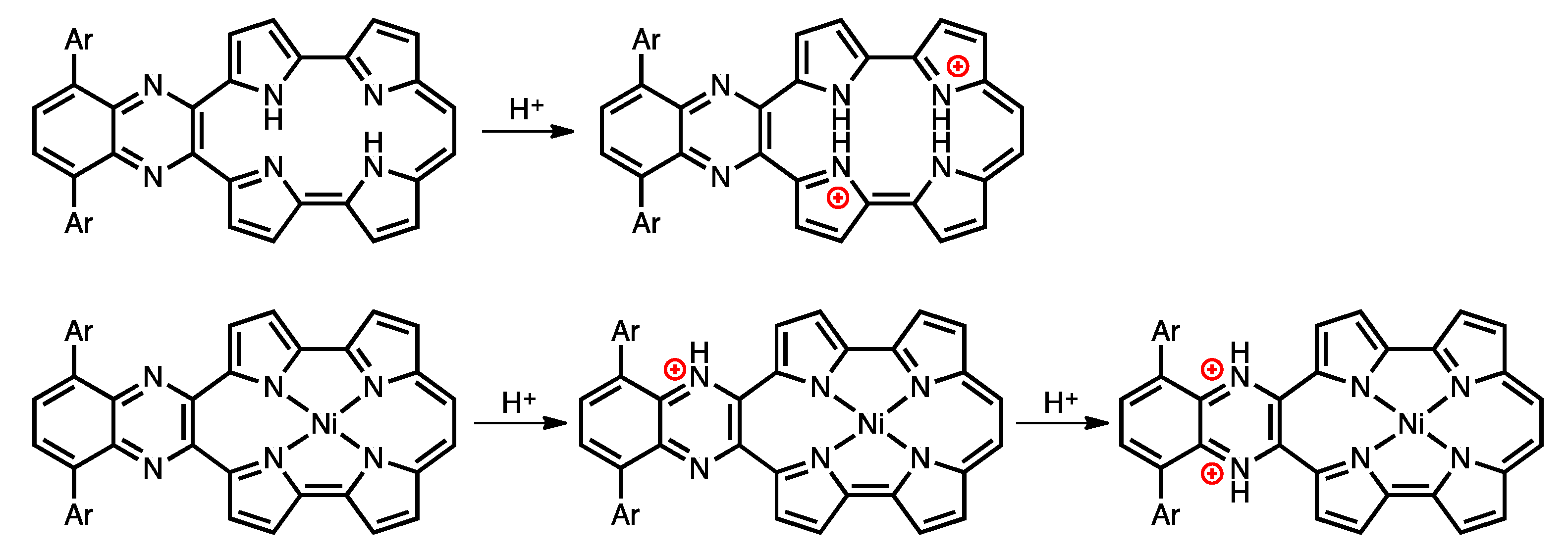
2.2. Protonation of Quinoxaline-Fused Porphycenes and Their Nickel Complexes
3. Materials and Methods
3.1. General Information
3.2. X-ray Crystallography
3.3. Cyclic Voltammetry and Differential Pulse Voltammetry Measurements
3.4. DFT Calculations
3.5. Synthesis of 5,8-Bis(5-hexylthiophen-2-yl)-2,3-di(1H-pyrrol-2-yl)quinoxaline (2a)
3.6. Synthesis of 2,3-Bis(5-bromo-1H-pyrrol-2-yl)-5,8-bis(5-hexylthiophen-2-yl)quinoxaline (3a)
3.7. Synthesis of Di-tert-butyl 5,5′-(5,8-bis(5-hexylthiophen-2-yl)quinoxaline-2,3-diyl) bis(2-bromo-1H-pyrrole-1-carboxylate) (4a)
3.8. Synthesis of Tetra-tert-butyl 5,5″-(5,8-bis(5-hexylthiophen-2-yl)quinoxaline-2,3-diyl) bis(1H,1′H-[2,2′-bipyrrole]-1,1′-dicarboxylate) (5a)
3.9. Synthesis of 2,3-Di(1H,1′H-[2,2′-bipyrrol]-5-yl)-5,8-bis(5-hexylthiophen-2-yl)quinoxaline (6a)
3.10. Synthesis of 5′,5‴-(5,8-Bis(5-hexylthiophen-2-yl)quinoxaline-2,3-diyl)-bis((1H,1′H-[2,2′-bipyrrole]-5-carbaldehyde)) (7a)
3.11. Synthesis of Porphycene 1a-H2
3.12. Synthesis of Porphycene 1a-Ni
3.13. Synthesis of 2,3-Bis(5-bromo-1H-pyrrol-2-yl)-5,8-bis(3,5-dimethoxyphenyl)quinoxaline (3b)
3.14. Synthesis of Di-tert-butyl 5,5′-(5,8-bis(3,5-dimethoxyphenyl)quinoxaline-2,3-diyl) bis(2-bromo-1H-pyrrole-1-carboxylate) (4b)
3.15. Synthesis of Di-tert-butyl 5,5′-(5,8-bis(3,5-dimethoxyphenyl)quinoxaline-2,3-diyl)-bis(2-bromo-1H-pyrrole-1-carboxylate) (5b)
3.16. Synthesis of 2,3-Di(1H,1′H-[2,2′-bipyrrol]-5-yl)-5,8-bis(3,5-dimethoxyphenyl)quinoxaline (6b)
3.17. Synthesis of 5′,5‴-(5,8-Bis(5-hexylthiophen-2-yl)quinoxaline-2,3-diyl) bis((1H,1′H-[2,2′-bipyrrole]-5-carbaldehyde)) (7b)
3.18. Synthesis of Porphycene 1b-H2
3.19. Synthesis of Porphycene 1b-Ni
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vogel, E.; Köcher, M.; Schmickler, H.; Lex, J. Porphycene—A novel porphin isomer. Angew. Chem. Int. Ed. 1986, 25, 257–259. [Google Scholar] [CrossRef]
- Waluk, J. Structure, Spectroscopy, Photophysics, and Tautomerism of Free-Base Porphycenes and Other Porphyrin Isomers. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K., Guilard, R., Eds.; World Scientific: Singapore, 2010; Volume 7, pp. 359–435. [Google Scholar]
- Shimakoshi, H.; Baba, T.; Iseki, Y.; Aritome, I.; Endo, A.; Adachi, C.; Hisaeda, Y. Photophysical and photosensitizing properties of brominated porphycenes. Chem. Commun. 2008, 2882–2884. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Panda, P.K. β-Octamethoxyporphycenes. Org. Lett. 2014, 16, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Braslavsky, S.; Muller, M.; Mártire, D.; Mártire, D.; Pörting, S.; Pörting, S.; Bertolotti, S.; Bertolotti, S.; Chakravorti, S.; Chakravorti, S.; et al. Photophysical properties of porphycene derivatives (18π porphyrinoids). J. Photochem. Photobiol. B 1997, 40, 191–198. [Google Scholar] [CrossRef]
- Stockert, J.C.; Cañete, M.; Juarranz, A.; Villanueva, A.; Horobin, R.W.; Borrell, J.I.; Teixidó, J.; Nonell, S. Porphycenes: Facts and prospects in photodynamic therapy of cancer. Curr. Med. Chem. 2007, 14, 997–1026. [Google Scholar] [CrossRef] [PubMed]
- Arnbjerg, J.; Jiménez-Banzo, A.; Paterson, M.J.; Nonell, S.; Borrell, J.I.; Christiansen, O.; Ogilby, P.R. Two-Photon Absorption in Tetraphenylporphycenes: Are Porphycenes Better Candidates than Porphyrins for Providing Optimal Optical Properties for Two-Photon Photodynamic Therapy? J. Am. Chem. Soc. 2007, 129, 5188–5199. [Google Scholar] [CrossRef] [PubMed]
- Sarma, T.; Panda, P.K.; Anusha, P.T.; Rao, S.V. Dinaphthoporphycenes: Synthesis and Nonlinear Optical Studies. Org. Lett. 2011, 13, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.V.; Shuvan Prashant, T. Two-photon and three-photon absorption in dinaphthoporphycenes. Chem. Phys. Lett. 2011, 514, 98–103. [Google Scholar]
- Kim, K.S.; Sung, Y.M.; Matsuo, T.; Hayashi, T.; Kim, D. Investigation of Aromaticity and Photophysical Properties in [18]/[20]π Porphycene Derivatives. Chem. Eur. J. 2011, 17, 7882–7889. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Lee, S.; Kim, D.; Panda, P.K. β-Octakis(methylthio)porphycenes: Synthesis, characterisation and third order nonlinear optical studies. Chem. Commun. 2015, 51, 7705–7708. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Yamada, H.; Okujima, T. Synthesis of Porphyrins Fused with Aromatic Rings. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2010; Volume 2, pp. 1–102. [Google Scholar]
- Kuzuhara, D.; Mack, J.; Yamada, H.; Okujima, T.; Ono, N.; Kobayashi, N. Synthesis, Structures, and Optical and Electrochemical Properties of Benzoporphycenes. Chem. Eur. J. 2009, 15, 10060–10069. [Google Scholar] [CrossRef] [PubMed]
- Kuzuhara, D.; Yamada, H.; Mori, S.; Okujima, T.; Uno, H. Synthesis, structures and properties of benzoporphycenes and naphthoporphycenes. J. Porphyr. Phthalocya. 2011, 15, 930–942. [Google Scholar] [CrossRef]
- Saeki, H.; Kurimoto, O.; Nakaoka, H.; Misaki, M.; Kuzuhara, D.; Yamada, H.; Ishida, K.; Ueda, Y. Effect of crystallinity in small molecular weight organic heterojunction solar cells. J. Mater. Chem. C 2014, 2, 5357–5364. [Google Scholar] [CrossRef]
- Saeki, H.; Misaki, M.; Kuzuhara, D.; Yamada, H.; Ueda, Y. Fabrication of Phase-Separated Benzoporphycene/[6,6]-Phenyl-C61-Butyric Acid Methyl Ester Films for Use in Organic Photovoltaic Cells. Jpn. J. Appl. Phys. 2013, 52, 111601. [Google Scholar] [CrossRef]
- Saeki, H.; Kurimoto, O.; Misaki, M.; Kuzuhara, D.; Yamada, H.; Ueda, Y. Thermal Conversion Behavior and Morphology Control of Benzoporphycene from a Novel Soluble Precursor. Appl. Phys. Express 2013, 6, 035601. [Google Scholar] [CrossRef]
- Roznyatovskiy, V.; Lynch, V.; Sessler, J.L. Dinaphthoporphycenes. Org. Lett. 2010, 12, 4424–4427. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, D.; Sessler, J.L. Porphycenes: Synthesis and derivatives. Chem. Soc. Rev. 2008, 37, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Oohora, K.; Ogawa, A.; Fukuda, T.; Onoda, A.; Hasegawa, J.-Y.; Hayashi, T. meso-Dibenzoporphycene has a Large Bathochromic Shift and a Porphycene Framework with an Unusual cis Tautomeric Form. Angew. Chem. Int. Ed. 2015, 54, 6227–6230. [Google Scholar] [CrossRef] [PubMed]
- Planas, O.; Gallavardin, T.; Nonell, S. A novel fluoro-chromogenic click reaction for the labelling of proteins and nanoparticles with near-IR theranostic agents. Chem. Commun. 2015, 51, 5586–5589. [Google Scholar] [CrossRef] [PubMed]
- Aldakov, D.; Anzenbacher, P., Jr. Dipyrrolyl quinoxalines with extended chromophores are efficient fluorimetric sensors for pyrophosphate. Chem. Commun. 2003, 1394–1395. [Google Scholar] [CrossRef]
- Anzenbacher, P., Jr.; Jursikova, K.; Aldakov, D.; Marquezb, M.; Pohl, R. Materials chemistry approach to anion-sensor design. Tetrahedron 2004, 60, 11163–11168. [Google Scholar] [CrossRef]
- Billingsley, K.; Buchwald, S.L. Highly Efficient Monophosphine-Based Catalyst for the Palladium-Catalyzed Suzuki−Miyaura Reaction of Heteroaryl Halides and Heteroaryl Boronic Acids and Esters. J. Am. Chem. Soc. 2007, 129, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
- Kuzuhara, D.; Yamada, H.; Yano, K.; Okujima, T.; Mori, S.; Uno, H. First Synthesis of Dodecasubstituted Porphycenes. Chem. Eur. J. 2011, 17, 3376–3383. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, P.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, N.J.R.E. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Peng, X.; Song, F.; Lu, E.; Wang, Y.; Zhou, W.; Fan, J.; Gao, Y. Heptamethine cyanine dyes with a large stokes shift and strong fluorescence: A paradigm for excited-state intramolecular charge transfer. J. Am. Chem. Soc. 2005, 127, 4170–4171. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Stone, A.; Fleischer, E.B. The molecular and crystal structure of porphyrin diacids. J. Am. Chem. Soc. 1968, 90, 2735–2748. [Google Scholar] [CrossRef]
- Honda, T.; Kojima, T.; Kobayashi, N.; Fukuzumi, S. Crystal Structures and Electronic Properties of Saddle-Distorted and Protonated Phthalocyanines. Angew. Chem. Int. Ed. 2011, 50, 2725–2728. [Google Scholar] [CrossRef] [PubMed]
- Waluk, J.; Muller, M.; Swiderek, P.; Köcher, M.; Vogel, E.; Hohlneicher, G.; Michl, J. Electronic states of porphycenes. J. Am. Chem. Soc. 1991, 113, 5511–5527. [Google Scholar] [CrossRef]
- Sessler, J.L.; Brucker, E.A.; Lynch, V.; Choe, M.; Sorey, S.; Vogel, E. Solution Phase and Single Crystal Diffraction X-ray Analyses of Diprotonated Porphyrin Isomers—Etioporphyrin, Etioporphycene, and Etiocorrphycene Bishydroperchlorate Salts. Chem. Eur. J. 1996, 2, 1527–1532. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112. [Google Scholar] [CrossRef] [PubMed]
- Aldakov, D.; Palacios, M.A.; Anzenbacher, P. Benzothiadiazoles and Dipyrrolyl Quinoxalines with Extended Conjugated Chromophores-Fluorophores and Anion Sensors. Chem. Mater. 2005, 17, 5238–5241. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available. |


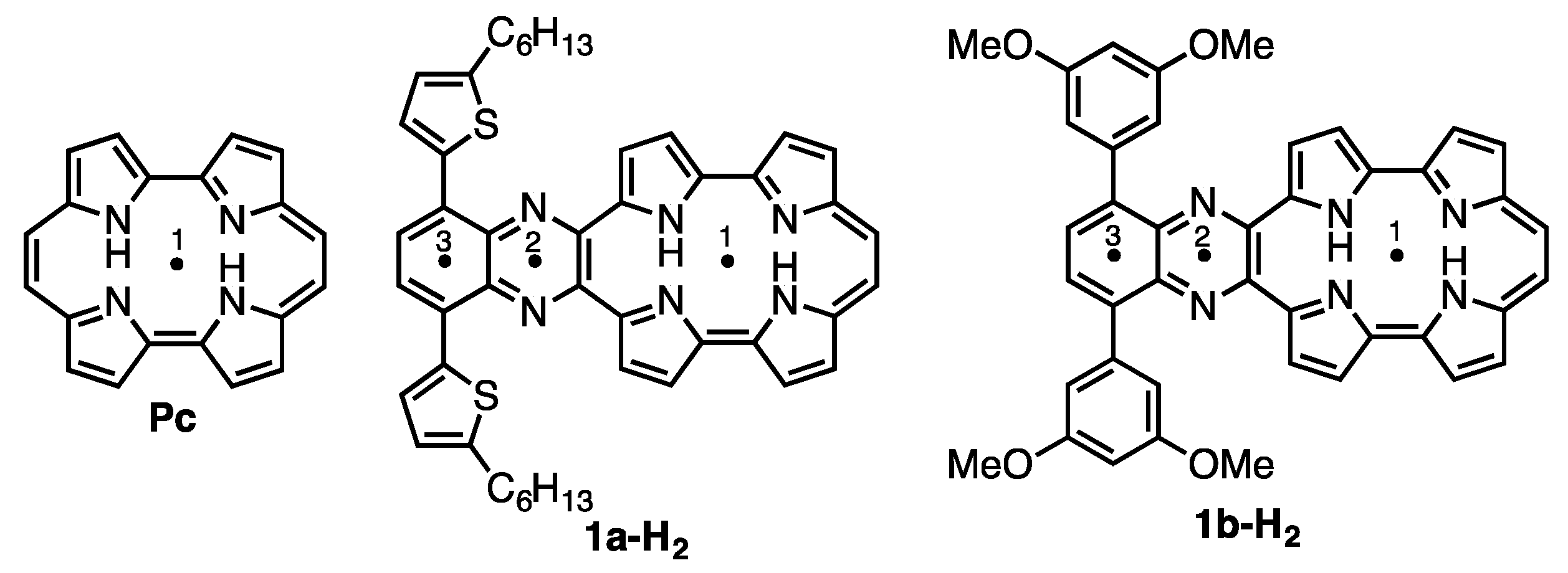
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Pc | –15.62 | – | – |
| 1a-H2 | –8.51 | –12.56 | –8.57 |
| 1b-H2 | –8.57 | –12.67 | –9.03 |


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzuhara, D.; Sakaguchi, M.; Furukawa, W.; Okabe, T.; Aratani, N.; Yamada, H. Synthesis, Characterization and Protonation Behavior of Quinoxaline-Fused Porphycenes. Molecules 2017, 22, 908. https://doi.org/10.3390/molecules22060908
Kuzuhara D, Sakaguchi M, Furukawa W, Okabe T, Aratani N, Yamada H. Synthesis, Characterization and Protonation Behavior of Quinoxaline-Fused Porphycenes. Molecules. 2017; 22(6):908. https://doi.org/10.3390/molecules22060908
Chicago/Turabian StyleKuzuhara, Daiki, Mika Sakaguchi, Wataru Furukawa, Takuya Okabe, Naoki Aratani, and Hiroko Yamada. 2017. "Synthesis, Characterization and Protonation Behavior of Quinoxaline-Fused Porphycenes" Molecules 22, no. 6: 908. https://doi.org/10.3390/molecules22060908
APA StyleKuzuhara, D., Sakaguchi, M., Furukawa, W., Okabe, T., Aratani, N., & Yamada, H. (2017). Synthesis, Characterization and Protonation Behavior of Quinoxaline-Fused Porphycenes. Molecules, 22(6), 908. https://doi.org/10.3390/molecules22060908





